Abstract
The BRIGHT-2 study (NCT05077449) is a randomized, double-blind, placebo-controlled, phase 3 trial evaluating the efficacy and safety of bireociclib plus fulvestrant (BF) vs. placebo plus fulvestrant (F) in Chinese female patients with hormone receptor-positive (HR+)/HER2-negative (HER2-) advanced breast cancer (ABC) who had progressed on or after prior endocrine therapy (ET). Interim results were analyzed after 70% of progression-free survival (PFS) events across 64 centers in China between December 8, 2021, and March 28, 2023. Patients were randomized (2:1) to receive BF or F, with stratification based on visceral involvement (yes/no) and resistance to prior primary or secondary ET. As the primary outcome, PFS was significantly prolonged in the BF group (n = 204) (12.94 months; 95% CI: 11.07–not reached) compared to 7.29 months (95% CI: 5.45–11.04) in the F group (n = 101) (hazard ratio, 0.56; 95% CI: 0.39–0.80; p = 0.001). The objective response rate in the BF group was 39.7% in the intention-to-treat population. Grade ≥3 adverse events were more frequent in the BF group (64.7%) than in the F group (18.8%), with neutropenia, leukopenia, and anemia being the most common. These findings suggest that BF is a promising therapeutic option for patients with HR+/HER2- ABC following ET failure.
Similar content being viewed by others
Introduction
In 2022, the incidence of female breast cancer (BC) ranked 2nd for all newly diagnosed cancers, accounting for 2,308,897 cases worldwide1, of which ~357,200 (15.6%) occurred in Chinese females2. Among hospital admissions for BC in China, the peak diagnosed age is between 40 and 59 years old, which is substantially younger and also presents with a higher risk of recurrence than females in Europe and the United States3. Compared with Westerners, Asians have a higher proportion of luminal B BC, which has a worse prognosis and is more prone to resistance to endocrine therapy (ET)4. Despite optimal ET, a certain percentage of patients failed to respond to treatment due to primary or secondary drug resistance.
Cyclin-dependent kinases 4 and 6 (CDK4/6) inhibitors block the phosphorylation of the retinoblastoma tumor suppressor and control the transition from G0/G1 phase to S phase of the cell cycle. These inhibitors have become the preferred regimen for first-line and second-line treatment of hormone receptor positive (HR+)/human epidermal growth factor receptor 2 negative (HER2−) advanced breast cancer (ABC), in conjunction with aromatase inhibitors or fulvestrant5,6,7,8,9,10,11,12. Since palbociclib has been marketed in China in 2018, three other selective CDK4/6 inhibitors (ribociclib, abemaciclib and dalpiciclib) have been approved by the National Medical Products Administration. Improved clinical outcomes have been reported for CDK4/6 inhibitor treatment of Chinese patients13,14, however, the four inhibitors exhibit different selectivity against CDK4 and CDK6 as well as the other spectrum of kinases15. Abemaciclib has been shown to have the most efficient inhibitory capacity and selectivity against CDK4, inhibiting CDK4 14 times more potently than CDK616 which may be associated with its lower hematologic toxicity, supporting continuous clinical administration17. However, abemaciclib exhibits higher gastrointestinal toxicity18. Bireociclib is a highly selective CDK4/6 inhibitor, with similar characteristics to abemaciclib, exhibiting a significantly stronger selective inhibitory effect on CDK4 than on CDK6, but demonstrating additional inhibitory activity against CDK2 and CDK9. In a previous phase 2 clinical trial (NCT04539496), bireociclib (480 mg administered orally on a continuous schedule twice a day) achieved an objective response rate (ORR) of 29.0% and progression-free survival (PFS) of 11.0 months in patients with metastatic HR+/HER2− ABC who had progressed after previous ET and chemotherapy regimens19,20,21.
In this work, we conduct the phase 3, randomized, double-blind, and placebo-controlled clinical trial (BRIGHT-2 study) to evaluate the efficacy and safety of bireociclib plus fulvestrant (BF) in HR+/HER2− ABC patients who have progressed on or after prior ET. We present the planned interim results, comparing median PFS (assessed by both investigator and blinded independent central review [BICR]) between BF and placebo plus fulvestrant (F) groups, along with ORR, adverse events (AEs), and additional exploratory subgroup analysis, etc.
Results
Patients and disposition
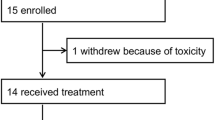
Between December 8, 2021, and October 24, 2022, 404 patients were screened in 64 hospitals, with 305 eligible female HR+/HER2− ABC patients finally being enrolled and randomized into BF (n = 204) or F (n = 101) groups (Fig. 1). The baseline characteristics between the BF group and the F group patients were generally well-balanced (Table 1). The median age of patients was 55 years, with 63.0% being postmenopausal and 68.2% having visceral metastasis. The proportion of patients who exhibited primary and secondary endocrine resistances was 25.6% and 74.4%, respectively, of whom 23.9% had been treated with chemotherapy in the advanced setting. A total of 279 (91.5%) patients had disease progression (PD) during ET, among them 194 (63.6%) experienced PD while receiving (neo)adjuvant ET (Supplementary Table 1).
As of data cut-off on March 28, 2023 for the planned interim analysis, the median follow-up time from randomization was 8.7 months and 84 (41.2%) and 56 (55.4%) of patients in the BF and F groups discontinued treatment, respectively. A total of 25 patients (8.2%) died during the study, with the majority of deaths occurring 30 days after end of treatment (18, 5.9%).
Efficacy
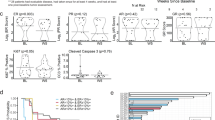
According to investigator assessment, a total of 127 PFS events were observed, with 72 (35.3%) in the BF group and 55 (54.5%) in the F group. The median PFS of the BF group was 12.94 months (95% confidence interval [CI]: 11.07–not reached), was significantly prolonged compared to the F group of 7.29 months (95% CI: 5.45–11.04) (hazard ratio [HR], 0.56; 95% CI: 0.39–0.80, p = 0.001) (Fig. 2a). Furthermore, the BICR results revealed that the HR for PD or death during the study period was 0.46 (95% CI: 0.31–0.68) for the BF group compared to the F group (p < 0.001). The median PFS for the BF group has not yet been reached, while the median PFS for the F group was 7.46 months (95% CI: 5.49–not reached) (Fig. 2b).
Improvement in PFS with BF was consistent across most subgroups whatever the investigator or BICR assessment (Fig. 3, Supplementary Fig. S1). Per investigators assessment, the most significant effects were revealed for patients with primary resistance to ET (HR, 0.25; 95% CI: 0.13–0.50), bone only metastases (HR, 0.23; 95% CI: 0.07–0.79) and those with liver metastases (HR, 0.38; 95% CI: 0.23–0.64) (Fig. 3). Sensitivity analysis supports the robustness of the PFS finding (Supplementary Table 2).
Data are presented as HRs for the BF group vs. the F group, with error bars representing 95% CIs, indicated by crossing horizontal lines. HRs are unstratified except the overall PFS, overall PFS estimates were stratified by visceral metastatic and ET resistance. BF bireociclib plus fulvestrant, CI confidence interval, ECOG Eastern Cooperative Oncology Group, ET endocrine therapy, F placebo plus fulvestrant, HR hazard ratio, mPFS median progression-free survival, No. numbers, PFS progression-free survival, PR progesterone receptor.
The ORR in the intention-to-treat (ITT) population based on investigator assessment for BF treated patients was 39.7% (95% CI: 32.9–46.8%) and was significantly greater than for F patients (13.9% [95% CI: 7.8–22.2%], p < 0.0001). In addition, the disease control rate (DCR) and clinical benefit rate (CBR) were superior in the BF group compared to the F group (Table. 2). In patients with measurable disease, the ORR assessed by the investigator was 43.8% (95% CI: 36.5–51.3%) in the BF group and 15.6% (95% CI: 8.8–24.7%) for the F group (p < 0.0001) (Table 2). Tumor responses per BICR for the ITT population or with measurable disease are shown in Supplementary Table 3 and exhibited similar superiority in the BF group compared to the F group.
Safety
During the treatment period, the median exposure durations to bireociclib and placebo were 225 days and 184 days, with median relative dose intensities of 90.7% and 98.8%. The median relative dose exposure intensity of fulvestrant was 100% for both groups (Supplementary Table 4). All grades and grade ≥3 treatment-emergent adverse events (TEAEs) occurred in 204 (100%) and 132 (64.7%) patients in the BF group and 94 (93.1%) and 19 (18.8%) patients in the F group, respectively. The most common TEAEs of any grade in the BF group were diarrhea, neutropenia, leukopenia, anemia, nausea, alanine aminotransferase increased, vomiting, aspartate aminotransferase increased and blood creatinine increased. The severity of these TEAE were predominately grade 1 or 2. The most common grade 3 or 4 TEAEs included neutropenia (31.4% in the BF group vs. 2.0% in F group), leukopenia (18.6% vs. 2.0%), anemia (10.8% vs. 0), hypokalemia (8.3% vs. 1.0%), diarrhea (5.4% vs. 0) and thrombocytopenia (3.9% vs. 0) (Table 3). The profile of the treatment-related adverse events (TRAEs) was basically consistent with the TEAEs (Table 3, Supplementary Table 5). Dose interruptions due to TEAEs occurred in 143 (70.1%) patients in the BF group and 34 (33.7%) in the F group, respectively. The primary reason for the interruptions was the impact of COVID-19, with 92 (45.1%) and 27 (26.7%) of patients affected in the two groups. During the COVID-19 pandemic, proactive measures including mailing the study drugs ensured that the majority of patients received their drugs in a timely manner with only two patients having short treatment interruptions due to transportation restrictions. A total of 68 (33.3%) patients received dose reductions due to TEAEs in the BF group while only 1.0% in the F group. The most frequent reasons for dose reduction of bireociclib were neutropenia (7.8%), leukopenia (3.9%) and diarrhea (4.4%).
Although diarrhea was the most common TEAE in the BF group, grade 3 diarrhea occurred in only 11 (5.4%) patients and no grade 4 diarrhea occurred. No patient discontinued the study treatment due to this TEAE. Diarrhea occurred mainly in the early stage of treatment, decreasing gradually with prolonged medication from the 3rd cycle (Supplementary Fig. 2). The median time to the onset of the first diarrhea event and its duration was 2 days, respectively. Most diarrhea was resolved with supportive care or dose adjustment of bireociclib. Only one patient was hospitalized due to grade 3 diarrhea. The most frequent grade ≥3 TEAE in BF group was neutropenia (64, 31.4%), including 1 patient with grade 3 febrile neutropenia. The median time until the first neutropenia incidence was 15 days, with an overall median duration of 29 days. Two cases of neutropenia occurred as serious adverse events (SAE), but no patient died or discontinued the study treatment due to neutropenia. A prolonged QT occurred in 5 patients (2.5%), with only 1 case being grade 3 (0.5%), and none of the prolonged QT events led to dose reduction or discontinuation of the study treatment.
The treatment-related serious adverse events (TRSAEs) that occurred more frequently in the BF group were thrombocytopenia (4, 2.0%) and abnormal hepatic functions (4, 2.0%) (Supplementary Table 6). Two cases of interstitial pneumonia (1.0%) occurred as SAEs and led to temporary dose interruption and 2 patients (1.0%) with grade 3 thrombotic events were reported in the BF group. Four patients died within 30 days after end of treatment in the BF group, due to PD (1, 0.5%), AEs (2, 1.0%), including 1 sudden cardiac death and 1 infectious pneumonia, both of which were considered unrelated to the study treatment per investigator, and one death due to other reasons (1, 0.5%, the exact reasons remain undetermined at present).
Discussion
In the present study, we found that HR+/HER2− ABC patients who had previously exhibited PD on or after ET, experienced a significant reduction in the risk of PD or death when treated with the BF regimen. The median PFS in the BF group was 12.94 months (95% CI: 11.07–not reached), compared to 7.29 months (95% CI: 5.45–11.04) in the F group (HR, 0.56; 95% CI: 0.39–0.80, p = 0.001), with no new significant safety issues identified.
The introduction of CDK4/6 inhibitors into clinical practice has revolutionized the treatment landscape for HR+/HER2− ABC, with several agents, including palbociclib, ribociclib, abemaciclib and dalpiciclib, producing significant improvements in PFS when combined with ET. For patients with HR+/HER2− who had experienced recurrence or progression after ET, combining a CDK4/6 inhibitor (palbociclib22, ribociclib23, abemaciclib9, or dalpiciclib24) with fulvestrant offers significant efficacy advantages over fulvestrant monotherapy as second-line therapy, with a median PFS ranging from 9.5 months to 16.6 months and an HR between 0.46 and 0.57. The ORR ranged from 19% to 35.7% in the ITT population.
Building on these findings, the BRIGHT-2 study was designed to better reflect the characteristics of the Chinese BC population3,4, which was typically diagnosed at a younger age, with later- stage disease, a higher proportion of luminal B BC, increased chemotherapy applications in advanced stages, and an elevated risk of recurrence. The results of our study found that the investigator-assessed median PFS was 12.94 months with HR of 0.56 compared to fulvestrant monotherapy per investigator while 0.46 per BICR.
The investigator-assessed ORR was 39.7% in the BF group and indicated that the addition of bireociclib to fulvestrant yields strong and durable tumor shrinkage in the BRIGHT-2 study although 91.5% patients experienced PD while receiving prior ET, similar to the results observed in the BRIGHT-121 study (NCT04539496) in which bireociclib was administered as monotherapy in heavily pre-treated ABC patients. The tumor shrinkage effects may help alleviate tumor related symptoms and potentially translate into survival benefits9.
Additionally, due to the differing characteristics of the study populations in various reported CDK4/6 inhibitor clinical trials, caution is needed when interpreting the efficacy of different drugs. For example, MONARCH-29 and PALOMA-322 studies included patients with both primary and secondary ET resistance, while the DAWNA-1 study14 only included patients with secondary ET resistance. The MONALEESA-310 study enrolled patients who were receiving ribociclib plus fulvestrant as first-line or second-line therapy. Neither MONALEESA-310 nor MONARCH-29 allowed chemotherapy, whereas PALOMA-322 and DAWNA-114 studies allowed patients to receive first-line chemotherapy. Furthermore, only two large phase III clinical trials have been conducted in the Chinese population (MONARCH plus13 with about 80% Chinese patients participating, and DAWNA-114). Therefore, the efficacy and safety of the promising CDK4/6 inhibitor bireociclib in the Chinese population are highly clinically relevant.
In this study, the proportion of primary and secondary endocrine resistances was 25.6% and 74.4%, respectively. Of these patients, 23.9% had received chemotherapy in the advanced setting. With early diagnosis and prolonged adjuvant ET, more patients experienced PD during adjuvant ET9,25. In the BRIGHT-2 study, 63.6% patients had progressed while receiving (neo)adjuvant ET, irrespective of primary resistance and secondary resistance to ET. It is noteworthy that in the subgroup analyses, the median PFS was improved in the BF group compared to the F group across most subgroups including patients with primary resistance to ET, patients with liver metastases or who had received previous chemotherapies. In patients with primary ET resistance, BF exhibited the outstanding efficacy compared to the control group (HR = 0.25), lower than other CDK4/6 inhibitors such as MONARCH-29 (HR = 0.454), MONARCHplus13 (HR = 0.348) and PALOMA-314 (HR = 0.64). These results suggest that, in a population consistent with the characteristics of Chinese BC patients, bireociclib may be the suitable option, especially preferred for patients with primary resistance. However, to determine clearly the comparative advantage of bireociclib over other CDK4/6 inhibitors, further analysis through direct comparative clinical trials or large-scale real-world studies is needed.
In previous studies, palbociclib, ribociclib and dalpiciclib therapy elicited high incidences of hematological toxicity, mainly neutropenia, while abemaciclib was linked to a high rate of gastrointestinal toxicity, notably diarrhea18. The highest incidence of grade ≥3 TEAEs during the BF regimen was neutropenia (31.4%), and similar to the incidence reported in the MONARCH plus (29.8%) study with abemaciclib13. The lower hematologic toxicity of bireociclib in comparison to other CDK4/6 inhibitors may be related to its greater selectivity for CDK4 over CDK6, since in preclinical studies, bireociclib was 18-fold more potent in inhibiting CDK4 compared to CDK621.
Diarrhea was the most common TEAE elicited by bireociclib, but the majority were grades 1 or 2, with only 5.4% being grade 3, which was lower than the incidence of this condition reported in MONARCH-29. A higher gastrointestinal toxicity induced by abemaciclib compared to other CDK4/6 inhibitors might be connected with its activity on glycogen synthase kinase 3β (GSK3β), CDK9 and calcium/calmodulin-dependent kinase II (CAMKII)26,27, among which bireociclib and abemaciclib showed a similar inhibition against CDK9 and CAMKII. However, bireociclib is 10 times less effective at inhibiting GSK3β than abemaciclib, which may have contributed to the lower incidence of grade 3 and above diarrhea during bireociclib treatment.
Comparing to the AEs reported in PALOMA-325, MONALEESA-310, and MONARCH-29, the incidence of any grade alopecia, cough, back pain, headache, hot flush, constipation, pain in extremity, stomatitis, dyspnea, and anxiety were all below 10% in BF group, with the most occurrence rates being similar to those in the control group. The grade 3 AEs of back pain, headache, constipation and stomatitis were not experienced in the BF group of our study.
Although the use of CDK4/6 inhibitors in combination with ET in China may lag behind the United States, the application of CDK4/6 inhibitors in first-line treatment is gradually becoming more widespread5,7,28,29. The BRIGHT-3 study (NCT05257395) designed to evaluate the efficacy and safety of bireociclib in combination with letrozole or anastrozole is ongoing. For patients who failed to respond to CDK4/6 inhibitors combined with ET, the postMONARCH30 study provides insight: the findings revealed that abemaciclib combined with fulvestrant still offered an advantage over fulvestrant alone (HR = 0.73, 95% CI: 0.57–0.95). Therefore, the efficacy of bireociclib combined with ET in patients who have previously failed to respond to CDK4/6 inhibitors is also being actively explored. In addition, CDK4/6 inhibitors have also continuously achieved success in clinical trials including among populations beyond HR+/HER2− ABC31,32, and the exploration of more combination treatment strategies involving bireociclib in the future is expected.
As the current data are based on an interim analysis of the BRIGHT-2 study, with 54.1% of patients still receiving treatment, the OS data is not yet mature and therefore not presented in this interim report.
To sum up, the present phase 3 clinical trial showed that bireociclib combined with fulvestrant was effective in improving PFS and reducing the tumor burden, and produced tolerable and manageable safety profiles in Chinese women with HR+/HER2− ABC. These findings support BF as a promising option for the treatment of these patients.
Methods
Study design and patients
BRIGHT-2 (NCT05077449) is an ongoing phase 3, randomized, double-blind clinical trial of bireociclib or placebo combined with fulvestrant to treat patients with HR+/HER2− ABC. The trial was conducted in 64 hospitals across China and was given approval by the ethics committees of each participating center. The study was carried out in accordance with the Declaration of Helsinki and the Good Clinical Practice guidelines. The principal investigators and participating hospitals are listed in Supplementary Table 7. All patients provided written informed consents before enrollment.
Any menstrual condition women aged ≥18 and ≤75 years old with confirmed locally advanced, recurrent, or metastatic HR+/HER2− BC who had progressed on or after prior ET were included in the trial. Women who were premenopausal or perimenopausal were given a gonadotropin-releasing hormone analog (ideally goserelin) for the duration of the trial. Eligible women had at least one measurable lesion or only bone metastasis according to the Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST v1.1) and an Eastern Cooperative Oncology Group (ECOG) performance status score of 0 or 1. Patients were given no more than one chemotherapy regimen in the advanced setting. Patients must have had a relapse or PD during (neo)adjuvant ET or <12 months after adjuvant ET or first-line ET for advanced settings. The key exclusion criteria included visceral crisis, inflammatory BC, symptomatic brain metastasis without any radiotherapy or surgery, meningeal metastasis, previous therapy with mechanistic targets of rapamycin or CDK4/6 inhibitors, fulvestrant, or similar drugs. See protocol (available in the Supplementary Note 1) for full eligibility criteria.
Randomization and masking
An interactive web response system was employed to randomly allocate patients in a 2:1 ratio to be given bireociclib combined with fulvestrant or placebo combined with fulvestrant. Randomization was stratified based on prior ET resistance (primary vs. secondary) and visceral metastases (yes vs. no). Primary ET resistance was defined as patients who experienced PD during the first 2 years of (neo)adjuvant ET or PD within first 6 months of first-line ET for metastatic BC. Patients who did not meet the criteria for primary ET resistance were classified as having secondary ET resistance, defined as recurrence while on neoadjuvant or adjuvant ET but after the first 2 years, or a recurrence within 12 months of completing adjuvant ET; or PD ≥ 6 months after initiating ET for metastatic BC while on ET.
During the treatment period, investigators, patients and everybody who participated in trial analyses were blinded to the treatment allocations.
Treatment and procedures
Patients were injected intramuscularly with 500 mg of fulvestrant on days 1 and 15 of the initial cycle, as well as on day 1 of the following cycles. During each 28-day cycle, they were given bireociclib or placebo (360 mg) two times a day. Treatment continued until progression of disease, unacceptable toxicity, withdrawal of consent, a decision by patients/investigators or death.
Dose adjustments, interruptions or discontinuations of bireociclib were allowed in the case of TEAEs. No dose adjustment was allowed for fulvestrant, but delays in fulvestrant administration were permitted due to fulvestrant-related toxicity.
Patients underwent imaging (CT/MRI) every 8 weeks during the screening and study treatment periods for the first 64 weeks, followed by imaging every 12 weeks, with tumor assessments according to RECIST v1.1. AEs were monitored from the time of the patients signed informed consent until at least 30 days after the last dose, including vital signs, physical examinations, echocardiograms, electrocardiograms and laboratory examinations, with AE grading assessments according to Common Terminology Criteria for Adverse Events version 5.0 (CTCAE v5.0).
Outcomes
The primary endpoint was investigator-assessed PFS following RECIST v1.1 and defined from the randomization date until PD or death from any cause, whichever occurred first. Secondary endpoints were PFS assessed by the BICR and OS, OS rates at 1–5 years, DoR, DCR, CBR, ORR and safety. AEs, including TEAEs, TRAEs and TRSAEs, were evaluated and graded following the CTCAE v5.0.
Statistical analysis
The BF and F groups were randomly assigned in a 2:1 ratio, assuming a median PFS of 9 months for the F group and 15 months for the BF group. To achieve a one-sided type 1 error of 0.025 and 90% statistical power, 178 events needed to be collected during the trial. Upon reaching 70% of PFS, a protocol-prespecified interim analysis in the original study design was conducted as authorized by an independent data monitoring committee consisting of experts not affiliated with the study investigators. For the final analysis, 255 patients were required with an assumed 15% dropout rate, and a minimum enrollment of 300 patients being needed (200 in the BF group and 100 in the F group). This group sequential design was based on the OBF type 1 error spending function. A predefined one-sided p-value for the interim analysis was 0.008.
The data collection was conducted using Medidata Rave EDC 2021.1.4 (EDC system, Medidata, NY, USA). The efficacy analyses were performed in the ITT population, which included all randomized patients per ITT definition. Based on the treatment assigned during randomization and the stratification factors, the analysis of PFS was carried out. A sensitivity analysis was performed using a multiple Cox regression model based on the stratification factors of visceral metastases, ET resistance, age, menopausal status, progesterone receptor status, ECOG performance status score and bone-only metastases. The Cox regression model was employed to estimate the HR for treatment effects and 95% CIs. A logistic model was used to compare the rates of binary endpoints. All statistical tests were conducted at the two-sided 0.05 level unless otherwise specified, and all CIs at 95% unless stated otherwise. Safety analysis took place within the safety analysis set, encompassing all patients who had been given ≥1 dose of the study treatment. SAS ver. 9.4 was employed to carry out all statistical analyses.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The data supporting the findings of this trial, including source data, cannot be made available openly owing to their proprietary nature and that the BRIGHT-2 study is still ongoing. The source data and individual de-identified patient data within the BRIGHT-2 study will be made available two years after the publication of this article to researchers whose proposals for data use have been approved by the BRIGHT-2 Trial Management Group. The data required for the approved, specified purposes for research but not commercial use will be provided after completion of a data sharing agreement, that will be set up by the study sponsor. Please address requests for data to the corresponding author, B.H.X. at: [email protected]. It usually takes about one month to process accession requests. The study protocol, statistical analysis plan and remaining data are readily available within the article and Supplementary Notes 1 and 2.
References
Bray, F. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 74, 229–263 (2024).
Rongshou, Z. et al. Cancer incidence and mortality in China, 2022. Chin. J. Oncol. 46, 221–231 (2024).
Li, T., Mello-Thoms, C. & Brennan, P. C. Descriptive epidemiology of breast cancer in China: incidence, mortality, survival and prevalence. Breast Cancer Res. Treat. 159, 395–406 (2016).
Park, Y. H. et al. Pan-Asian adapted ESMO Clinical Practice Guidelines for the Management of Patients with Early Breast Cancer: a KSMO-ESMO initiative endorsed by CSCO, ISMPO, JSMO, MOS, SSO and TOS. Ann. Oncol. 31, 451–469 (2020).
Finn, R. S. et al. Palbociclib and letrozole in advanced breast cancer. N. Engl. J. Med. 375, 1925–1936 (2016).
Hortobagyi, G. N. et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N. Engl. J. Med. 375, 1738–1748 (2016).
Goetz, M. P. et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J. Clin. Oncol. 35, 3638–3646 (2017).
Turner, N. C. et al. Palbociclib in hormone-receptor-positive advanced breast cancer. N. Engl. J. Med. 373, 209–219 (2015).
Sledge, G. W. Jr. et al. MONARCH 2: Abemaciclib in combination with fulvestrant in women with HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J. Clin. Oncol. 35, 2875–2884 (2017).
Slamon, D. J. et al. Phase III randomized study of ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: MONALEESA-3. J. Clin. Oncol. 36, 2465–2472 (2018).
Ashai, N. & Swain, S. M. Post-CDK 4/6 inhibitor therapy: current agents and novel targets. Cancers (Basel) 15, 1855 (2023).
Sammons, S. L., Topping, D. L. & Blackwell, K. L. HR+, HER2− advanced breast cancer and CDK4/6 inhibitors: mode of action, clinical activity, and safety profiles. Curr. Cancer Drug Targets 17, 637–649 (2017).
Zhang, Q. Y. et al. MONARCH plus: abemaciclib plus endocrine therapy in women with HR+/HER2− advanced breast cancer: the multinational randomized phase III study. Ther. Adv. Med. Oncol. 12, 1758835920963925 (2020).
Xu, B. et al. Dalpiciclib or placebo plus fulvestrant in hormone receptor-positive and HER2-negative advanced breast cancer: a randomized, phase 3 trial. Nat. Med. 27, 1904–1909 (2021).
Johnston, S. et al. Cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitors: existing and emerging differences. JNCI Cancer Spectr. 7, pkad045 (2023).
Torres-Guzmán, R. et al. Preclinical characterization of abemaciclib in hormone receptor positive breast cancer. Oncotarget 8, 69493–69507 (2017).
Patnaik, A. et al. Efficacy and safety of abemaciclib, an inhibitor of CDK4 and CDK6, for patients with breast cancer, non-small cell lung cancer, and other solid tumors. Cancer Discov. 6, 740–753 (2016).
Onesti, C. E. & Jerusalem, G. CDK4/6 inhibitors in breast cancer: differences in toxicity profiles and impact on agent choice. A systematic review and meta-analysis. Expert Rev. Anticancer Ther. 21, 283–298 (2021).
Wang, J. et al. A multicenter, single-arm, open-label trial of birociclib, a CDK4/6 inhibitor, as a single agent, in patients with refractory HR+/HER2− metastatic breast cancer. J. Clin. Oncol. 41, 1072–1072 (2023).
Wang, J. et al. 404P A multicenter, single-arm, open-label trial of birociclib, a CDK4/6 inhibitor, as a single agent, in patients with refractory HR+/HER2− metastatic breast cancer. Ann. Oncol. 34, S352 (2023).
Wang, J. et al. An open-label, single-arm, multicenter, phase II trial of bireociclib as monotherapy for heavily pretreated HR-positive, HER2-negative advanced breast cancer patients: BRIGHT-1 trial. Cancer Commun. (Lond.) https://doi.org/10.1002/cac2.70009 (2025).
Cristofanilli, M. et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 17, 425–439 (2016).
Slamon, D. J. et al. Overall survival with ribociclib plus fulvestrant in advanced breast cancer. N. Engl. J. Med. 382, 514–524 (2020).
Zhang, P. et al. 229P Dalpiciclib plus fulvestrant in HR+/HER2− advanced breast cancer (ABC): updated analysis from the phase III DAWNA-1 trial. Ann. Oncol. 33, S642–S643 (2022).
Loibl, S. et al. Palbociclib combined with fulvestrant in premenopausal women with advanced breast cancer and prior progression on endocrine therapy: PALOMA-3 results. Oncologist 22, 1028–1038 (2017).
Jacobs, F. et al. Sticking to the rules: outcome and success rate of guideline-based diarrhea management in metastatic breast cancer patients treated with abemaciclib. J. Clin. Med. 12, 1775 (2023).
Thibault, S. et al. Intestinal toxicity in rats following administration of CDK4/6 inhibitors is independent of primary pharmacology. Mol. Cancer Ther. 18, 257–266 (2019).
Zhang, P. et al. Dalpiciclib plus letrozole or anastrozole versus placebo plus letrozole or anastrozole as first-line treatment in patients with hormone receptor-positive, HER2-negative advanced breast cancer (DAWNA-2): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 24, 646–657 (2023).
Hortobagyi, G. N. et al. Overall survival with ribociclib plus letrozole in advanced breast cancer. N. Engl. J. Med. 386, 942–950 (2022).
Kalinsky, K. et al. Abemaciclib plus fulvestrant vs. fulvestrant alone for HR+, HER2− advanced breast cancer following progression on a prior CDK4/6 inhibitor plus endocrine therapy: primary outcome of the phase 3 postmonarch trial. J. Clin. Oncol. 42, LBA1001–LBA1001 (2024).
Loibl, S. et al. PATINA: a randomized, open label, phase III trial to evaluate the efficacy and safety of palbociclib + anti-HER2 therapy + endocrine therapy (ET) vs. anti-HER2 therapy + ET after induction treatment for hormone receptor positive (HR+)/HER2-positive metastatic breast cancer (MBC). Ann. Oncol. 29, viii121 (2018).
Turner, N. C. et al. Inavolisib-based therapy in PIK3CA-mutated advanced breast cancer. N. Engl. J. Med. 391, 1584–1596 (2024).
Acknowledgements
The study was supported by the National Major Scientific and Technological Special Project for “Significant New Drugs Development” (No. 2018ZX09711002-011-027, L.W.) and CAMS Innovation Fund for Medical Sciences (CIFMS, 2021-I2M-1-014 and 2023-12M-2-004, B.H.X.). The study was funded by Xuanzhu Biopharmaceutical Co., Ltd. and was envisioned and carried out by the principal investigator in collaboration with the sponsor. Xuanzhu Biopharmaceutical Co., Ltd. was involved in data collection, analysis, and interpretation of findings, and in writing the manuscript. We are most grateful to all patients and healthcare personnel who participated in the BRIGHT-2 study.
Author information
Authors and Affiliations
Contributions
B.H.X. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Conceptualization: B.H.X., J.Y.W., L.W. Collection and assembly of data: J.Y.W., Q.Y.Z. and H.P.L. Data analysis and its interpretation: X.H.D. Funding acquisition: B.H.X. Project administration: B.H.X. Supervision: B.H.X. Writing—original draft: J.Y.W. Provision of study material or patient enrollment, writing—review & editing, final approval of the manuscript, accountable for all aspects of the work: J.Y.W., Q.Y.Z., H.P.L., Z.S.T., Q.C.O., H.H.L., Y.E.T., B.Y.W., T.S., J.F.W., W.L., Z.F.N., H.S.L., C.G., S.W., X.S.W., X.H.W., N.L., G.H.Y., F.L., X.H.D., S.Y.W., Y.P.M., L.W. and B.H.X.
Corresponding author
Ethics declarations
Competing interests
F.L., X.H.D., S.Y.W., Y.P.M., and L.W. are employees of Xuanzhu Biopharmaceutical Co., Ltd. B.H.X. reported receiving consulting fees and payment for lectures from AstraZeneca and Novartis outside the submitted work. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, J., Zhang, Q., Li, H. et al. Bireociclib plus fulvestrant for HR+/HER2- advanced female breast cancer progressed on or after endocrine therapy: phase 3 BRIGHT-2 study interim analysis. Nat Commun 16, 3350 (2025). https://doi.org/10.1038/s41467-025-58647-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-58647-z