Abstract
The Endoplasmic/sarcoplasmic reticulum (ER/SR) is central to calcium (Ca2+) signaling, yet current genetically encoded Ca2+ indicators (GECIs) cannot detect elementary Ca2+ release events from ER/SR, particularly in muscle cells. Here, we report NEMOer, a set of organellar GECIs, to efficiently capture ER Ca2+ dynamics with increased sensitivity and responsiveness. NEMOer indicators exhibit dynamic ranges an order of magnitude larger than G-CEPIA1er, enabling 2.7-fold more sensitive detection of Ca2+ transients in both non-excitable and excitable cells. The ratiometric version further allows super-resolution monitoring of local ER Ca2+ homeostasis and dynamics. Notably, NEMOer-f enabled the inaugural detection of Ca2+ blinks, elementary Ca2+ releasing signals from the SR of cardiomyocytes, as well as in vivo spontaneous SR Ca2+ releases in zebrafish. In summary, the highly dynamic NEMOer sensors expand the repertoire of organellar Ca2+ sensors that allow real-time monitoring of intricate Ca2+ dynamics and homeostasis in live cells with high spatiotemporal resolution.
Similar content being viewed by others
Introduction
Calcium (Ca2+) signaling is indispensable for the orchestration of multiple cellular functions and physiological processes1. As one of the major Ca2+ sources and internal stores in animal cells, endoplasmic/sarcoplasmic reticulum (ER/SR) takes center stage in the choreography of calcium signaling2. Dysregulated ER homeostasis, recognized as an instigator of ER stress and apoptosis3, is associated with various diseases, including cancer, cardiovascular disorders, and neurodegenerative diseases4,5. To better unveil the roles of ER Ca2+ in health and disease, it is imperative to visualize the spatiotemporal dynamics of ER/SR Ca2+ with high precision and resolution.
By introducing Ca2+-binding site into fluorescence proteins, or via mutagenesis of Ca2+-binding-___domain of cytosolic GECIs like Cameleon6, GCaMP7,8, GECO9, or aequorin10, researchers have developed over a dozen sensors with relatively low Ca2+ affinities (Kd > 400 μM). Notable examples of ER/SR Ca2+ include YC4er, CatchER sets11,12,13, GCaMPER14, CEPIA1er based series15,16,17,18, and erGAP2/319. However, these ER-resident green GECIs among them often exhibit limited dynamic ranges (<5)13,15 or relatively slow kinetics14, thereby impeding real-time monitoring of rapid Ca2+-modulated events. To accurately capture elementary local ER Ca2+ changes20,21, such as Ca2+ blinks in cardiac muscle cells22, an ideal ER GECI should satisfy the following criteria: low Ca2+ affinity, large dynamics, and rapid kinetics.
Mostly by mutating Ca2+-binding residues in our recently developed NEMO indicators23, we present herein a set of highly dynamic and sensitive green GECIs tailored for ER/SR, termed NEMOer, along with its ratiometric version TuNer. Basal fluorescence and responses of NEMOer indicators can be one order of magnitude larger than G-CEPIA1er, reporting significantly larger Ca2+ oscillation signals. TuNer sensors enable super-resolution recording of SR/ER Ca2+ homeostasis and dynamics. With the NEMOer-f variant, we successfully detect Ca2+ blink, the elementary Ca2+ releasing signal from SR of cardiomyocytes, as well as in vivo spontaneous SR Ca2+ releasing event in zebrafish. Collectively, the highly dynamic NEMOer sensors hold high potential for a wide range of applications in monitoring ER Ca2+ dynamics and homeostasis within live cells.
Results
Engineering of low Ca2+ affinity NEMOer indicators
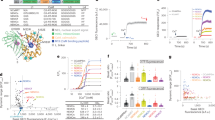
By introducing known Ca2+-affinity-reducing mutations into the calmodulin ___domain of NEMO indicators15,23 and adding ER-targeting sequences, we generated NEMO variants for monitoring ER Ca2+, and screened their performance in HEK293 cells (Fig. 1A–C, and Supplementary Tables 1-2). Basic sensor properties, including their basal fluorescence (F0), maximal (Fmax), and minimal (Fmin) fluorescence were obtained in cells transiently expressing these variants at comparable levels. Two well-known ER Ca2+ sensors G-CEPIA1er15 and ER-GCaMP6-15024 were used as controls. Briefly, after the recording of F0, Fmin was measured by depleting ER Ca2+ store with 2.5 μM ionomycin (iono; a Ca2+ ionophore) and subsequent permeabilization using 25 μM digitonin. In the end, large amount of Ca2+ (30 mM) was added to the bath containing 25 μM digitonin to induce Fmax in permeabilized cells (Fig. 1B). Fluorescence responses relative to Fmin (Fig. 1C), mean F0 and dynamic range (DR) values defined as ΔF/Fmin, or (Fmax - Fmin)/Fmin (Fig. 1D) were plotted to visualize the full capacity of these low Ca2+ affinity NEMOer variants.
A NEMOer sensors are generated by introducing amino acid substitutions in calmodulin region of NEMO indicators. Top, a diagram showing the design of NEMOer variants. Middle, key amino acids substitutions. Bottom, predicted NEMOer-s structure by I-TASSER41 (Iterative Threading ASSEmbly Refinement). Four loops of EF-hands (EF) within calmodulin ___domain of NEMOer sensors are indicated by black arrows. B–E Screening of NEMOer variants in non-excitable mammalian cells. Ca2+-imaging-based screening assay shown by typical traces from ER-GCaMP6-150-, G-CEPIA1er- (B) or NEMOer variants (C) -expressing cells. Inserts, traces with enlarged scales. After recording the basal fluorescence (F0), endoplasmic reticulum Ca2+ store was depleted with 2.5 μM ionomycin (iono) to obtain minimal GECI fluorescence (Fmin). Cells were permeablized with 30 mM Ca2+ imaging solution containing 25 μM digitonin to obtain maximal response (Fmax). The observed peak-followed-by-a-decay-to-plateau phenomenon for G-CEPIA1er and ER-GCaMP6-150, likely due to the harsh imaging protocol, remains to be elucidated. (For typical trace in (B, C), G-CEPIA1er, n = 33 cells; ER-GCaMP6-150, n = 40 cells; NEMOer-m, n = 30 cells; NEMOer-s, n = 36 cells NEMOer-f, n = 38 cells; NEMOer-c, n = 35 cells; NEMOer-b, n = 27 cell). D Scatter plot of F0–mean dynamic range (DR; (Fmax - Fmin)/Fmin) of the indicated GECIs. E In situ dose–response curves of NEMOer sensors. Top, typical traces; Bottom, statistics (G-CEPIA1er, n = 52 cells; NEMOer-m, n = 56 cells; NEMOer-s, n = 63 cells NEMOer-f, n = 46 cells; NEMOer-c, n = 57 cells; NEMOer-b, n = 52 cell). F Basal brightness of NEMOer or G-CEPIA1er viewed with YFP (top) or GFP (bottom) filters. To estimate the basal fluorescence of GECIs (FGECI), cells expressing mKate-P2A-GECI were normalized against the fluorescence of mKate, an expression marker (FmKate). (G-CEPIA1er, n = 390 cells; NEMOer-m, n = 431 cells; NEMOer-s, n = 421 cells NEMOer-f, n = 469 cells; NEMOer-c, n = 411 cells; NEMOer-b, n = 473 cell). B, C, E, F Data shown as mean ± s.e.m. B–F Three independent biological replicates, ≥ 17 cells per repeat.
Five best-performing constructs with bright F0 and large DR values (Fig. 1D) were selected and named as mNeonGreen-based Calcium indicator for ER (NEMOer). These include the medium (NEMOer-m) for general use, high contrast (NEMOer-c) for high contrast applications, fast (NEMOer-f) for rapid signal detection, bright (NEMOer-b) for low-phototoxicity scenarios, and sensitive (NEMOer-s) for detecting subtle fluctuations in ER Ca2+ levels.
We next determined performance of NEMOer variants both in vitro and in situ. The affinities of NEMOer variants were reduced to near milimolar (mM) ranges (Supplementary Fig. 1A; Fig. 1E), either comparable to that of G-CEPIA1er (706 ± 48 μM in situ) or significantly lower. And the Ca2+ dissociation kinetics of NEMOer-f (koff = 156.75 ± 3.11 s−1) is comparable to that of G-CEPIA1er (131.47 ± 7.07 s−1, Supplementary Fig. 1B), whereas the koff values of other NEMOer sensors are markedly slower (ranging from approximately 17 ~ 36 s−1). This highlights NEMOer-f as particularly well-suited for decoding fast acting ER Ca2+ signals in excitable cells, whereas the slower NEMOer variants are restricted to detecting changes occurring with time constants under 60 ms.
More careful in situ characterization revealed that the in cellulo DR of NEMOer sensors are superior to other ER GECIs tested side-by-side. In HEK293 cells, ER-GCaMP6-150 showed a F0 that was close to its Fmax, indicating that it is close to saturation at the basal condition (Fig. 1B, C). We thus only used G-CEPIA1er as a reference as it showed a good combination of affinity, kinetics and DR15 (Fig. 1B, C). In HeLa cells, the DR values of NEMOer-f and NEMOer-b, 68.3 and 139.3, respectively, were 14.3 to 29.9-fold higher than that of G-CEPIA1er (4.5). The DRs of NEMOer-m, NEMOer-s and NEMOer-c were further increased (263.3, 253.8, or 349.3, respectively) to be over 50-fold higher than that of G-CEPIA1er (Fig. 1E). Overall, NEMOer indicators represent a class of bright ER Ca2+ indicators with the in cellulo DRs up to 80-fold larger than G-CEPIA1er.
All NEMOer variants showed in vitro DR values larger than 120-fold (Supplementary Fig. 1A, left), at least 18.8 fold larger than that of G-CEPIA1er (6.4 ± 0.1). Similar to their corresponding template23, the cause of these significantly large DR values is a greater Ca2+-dependent fold-of-increase in the molecular brightness of the anionic fluorophores in NEMOer variants (Supplementary Fig. 1C–G; Supplementary Table 3). In the absence of Ca2+, the anionic fluorophore of NEMOer-c (0.16 ± 0.03 mM–1cm–1) is considerably dimmer, approximately one-fourth that of G-CEPIA1er. And the brightness of Ca2+-saturated anionic NEMOer-c (54.85 ± 2.19 mM–1cm–1), more than ten times that of G-CEPIA1er.
Consistent with the observation that the basal fluorescence (F0) of NEMOer sensors were much brighter than that of G-CEPIA1er (Fig. 1D), over 87% of G-CEPIA1er fluorophores exist in neutral state, the relative dim and less Ca2+-sensitive configuration, while up to 75% of the fluorophore within NEMOer-c could exist in its bright anionic form (Supplementary Table 3). We next more carefully compared F0 of NEMOer indicators with G-CEPIA1er using a P2A-based bicistronic vector to drive the co-expression of GECIs and mKate as an expression marker at a near 1:1 ratio. The normalized basal GECI brightness was shown as the fluorescence ratio of GECI over mKate (Fig. 1F). As expected, all NEMOer sensors showed significantly higher basal brightness than that of G-CEPIA1er, with NEMOer-f the dimmest and NEMOer-b the brightest among all NEMOer variants. Even when examined with settings optimized for G-CEPIA1er (GFP filters), F0 of NEMOer-f and NEMOer-b was about 3 or 8.5 fold of G-CEPIA1er, respectively. When a filter set (YFP) optimized for NEMOer was used, NEMOer-f and NEMOer-b were 7.4 and 17.4 folds brighter than G-CEPIA1er.
NEMOer indicators also showed significantly enhanced photostability than G-CEPIA1er (Supplementary Fig. 2A, B). NEMOer-f exhibited remarkable photostability, enduring illumination over 50 times stronger (0.57 mW) than that applied to G-CEPIA1er (0.01 mW) without apparent photobleaching. The increase in light intensity (from 0.01 mW to 0.57 mW) led to more than a 10-fold rise in the basal fluorescence of NEMOer-f (Supplementary Fig. 2), making it highly suitable for imaging scenarios requiring intense excitation, such as monitoring Ca2+ signals with super-resolution imaging system, or in vivo imaging of subcellular compartments such as dendrites.
Together, these results firmly establish NEMOer indicators as a class of highly bright, photostable GECIs with extraordinarily large Ca2+-dependent changes in fluorescence.
Performance of NEMOer indicators in non-excitable cells
We first examined the ability of NEMOer indicators to report the depletion of ER Ca2+ stores induced by various stimuli. Consistent with their large DR values, in responses to full ER store depletion triggered by ionomycin, NEMOer indicators showed more than 95.8% decreases in fluorescence, significantly larger than those form G-CEPIA1er (71.9 ± 1.3%) (Fig. 2A). In response to sub-maximal activation of muscarinic acetylcholinergic receptors with carbachol (CCh, 10 μM), NEMOer indicators showed stronger response with the mean peak amplitudes 2.1 ~ 2.4 folds higher than G-CEPIA1er (Fig. 2B). Both NEMOer-f and G-CEPIA1er recover to a plateau lower than baseline, with the NEMOer-f signal reaching a smaller plateau (0.65 ± 0.14 vs. 0.93 ± 0.06, respectively) and recovering at a slower rate (half-rise time: 4.47 ± 0.11 vs. 3.51 ± 0.20). Given that G-CEPIA1er and NEMOer-f are kinetically similar (Supplementary Fig. 1B, Supplementary Table 2) and that NEMOer-f is more sensitive, these results suggest that, after the initial Ca2+ release induced by CCh, the subsequent ER Ca2+ refilling occurs more slowly and less efficiently than previously estimated. Similarly, NEMOer sensors also showed superior performance in reporting Ca2+ oscillations over G-CEPIA1er (Fig. 2C). Notably, while the time courses of Ca2+ oscillations detected by NEMOer variants are similar (Fig. 2C), the time-to-nadir of the first CCh-induced transient decay for the other NEMOer variants occur approximately 3~5 s later than those measured by G-CEPIA1er and NEMOer-f. This suggests that the slower NEMOer variants may be less effective at accurately capturing rapid ER Ca2+ decays (Fig. 2B). Consistent with the observed superior performance in NEMOer sensors, simulation results based on the Hill equation and in situ parameters demonstrate that GECIs with larger DRs indeed exhibit significantly enhanced responses (Fig. 2D).
A Typical ER-Ca2+ responses in HEK293 cells induced by ionomycin (iono, 2.5 μM). B, C G-CEPIA1er or NEMOer responses to ER-Ca2+-lowering by submaximal receptor stimulation with carbachol (CCh, 10 μM) in HEK293 cells. Initial ER Ca2+ decreasing recorded in nominally Ca2+ free solution. Left, typical traces; right, statistics. B (**** P < 1E−15, one-way ANOVA). C Typical cells showing ER Ca2+ oscillations in 2 mM Ca2+ imaging solution. D Simulated F/F0 vs. [Ca2+]ER, assuming a 20% [Ca2+]ER drop due to ER Ca2+ release. The simulation was performed using the Hill equation and in situ parameters from Fig. 1E, with Fmin set to 1. E Simultaneous monitoring of 10 μM CCh-induced Ca2+ oscillations in ER and cytosol in HEK293 cells. NEMOer-f or G-CEPIA1er was transiently co-expressed with the cytosolic red indicator, R-GECO1.2. Top left, representative response traces; top right, statistics. (***P = 0.0002, unpaired Student’s t-test, two-tailed.) Bottom, averaged response traces of R-GECO1.2 together with corresponding G-CEPIA1er or NEMOer-f (left two panels), and a direct comparison of the responses between NEMOer-f and G-CEPIA1er (right panel). F–H Ratiometric signals of mTurquoise2 - NEMOer-m (TuNer-m) in COS-7 cells collected with multimodality structured illumination microscopy (Multi-SIM). The TuNer ratio (R) is defined as R = FNEMOer/FsfTq2ox. (F) Typical basal fluorescence and ratiometric images, with enlarged views. Purple arrows show higher fluorescence with a lower ratio, and yellow arrows indicate the opposite. TuNer-m ratios within ER sheets and tubules. (G) Diagram of Region of Interest (ROI) selection in ratio image: ER sheets (white circles), ER sheets edge (yellow circles) or ER tubules (green circles) (H) Statistics of (F), mean size: sheets 0.06 ± 0.01 μm², edge 0.07 ± 0.01 μm², tubules 0.07 ± 0.01 μm². (****P = 1E−7, **P = 0.0019, ns P = 0.0723, one-way ANOVA, n = 70 ROIs from at least 15 cells). 3 independent biological replicates, ≥ 5 cells per repeat. A–C 3 independent biological replicates, ≥ 15 cells per repeat. (A, B, C, E) Error bar denotes SEM.
To better assess whether the fast NEMOer-f sensors could reliably follow cytosolic Ca2+ oscillations with bigger responses, we co-expressed a cytosolic red GECI, R-GECO1.225, with either NEMOer-f or G-CEPIA1er in HEK293 cells. 10 μM CCh induced synchronized changes in R-GECO1.2 and G-CEPIA1er or NEMOer-f fluorescence (Fig. 2E, top left panel). Notably, while G-CEPIA1er only detected synchronized ER Ca2+ oscillations in 50% cells with cytosolic Ca2+ oscillations as indicated by R-GECO1.2 signal, NEMOer-f successfully captured all events, establishing it as a more reliable indicator for monitoring ER Ca2+ oscillations (Fig. 2E, top right panel). The cytosolic Ca2+ increases observed with R-GECO1.2 were comparable between the two groups (Fig. 2E, bottom left two panels), indicating a similar reduction in ER Ca2+ levels. However, in response to the first ER Ca2+ release, NEMOer-f exhibited 2.7-fold larger responses compared to G-CEPIA1er (Fig. 2E, bottom right panel). This superior sensitivity of NEMOer-f in reporting sub-maximal ER Ca2+ releases is likely due to its higher Kd and DR values. These results establish that NEMOer-f indicator is more sensitive than G-CEPIA1er, and exhibits the necessary speed to capture ER Ca2+-releasing events that drive cytosolic Ca2+ oscillations. Together, intensiometric NEMOer response to these investigated Ca2+ signals in non-excitable cells are remarkably larger than those of G-CEPIA1er tested side-by-side.
To enable monitoring of ER Ca2+ homeostasis, we generated ratiometric mTurquoise2- NEMOer (TuNer) indicators by using a mTurquoise2 (mTq2) variant as both an indicator for the sensor expression level and a Föster energy resonance transfer (FRET) donor for NEMOer sensors (Supplementary Fig. 3A). We reasoned that the FRET efficacy between mTq2 and NEMOer indicators may be proportional to NEMOer fluorescence. This would ensure reciprocal change in NEMOer and mTq2 fluorescence, resulting in amplified NEMOer responses (Supplementary Fig. 3B). Since ER Ca2+ level is less conveniently manipulatable as compared with cytosolic Ca2+, we used cytosolic mNeonGreen based indicator NCaMP726 to optimize the linkers and interfaces between the FRET pair (Supplementary Table 4), and then applied the optimized strategy for NEMO and NEMOer sensors to obtain ratiometric mTurquoise2–NEMO (TurN, cytosolic version) or TuNer sensors (Supplementary Table 5, 6). The DRs of all these TurN indicators were 1.4~2 fold higher than the corresponding mono-fluorescent templates (Supplementary Tables 4–6). When stimulated with 10 μM CCh, decreases in ratios of TuNer indicators were more than 1.5-fold of miGer, a ratiometric ER Ca2+ indicator based on G-CEPIA1er (Supplementary Fig. 3C). This finding aligns with the data from monomeric indicators (~1.7-fold, Fig. 2B–D). When measured with TuNer sensors, the basal ER Ca2+ levels within HEK293 and HeLa cells fell in the range of 0.8-1.3 mM (Supplementary Fig. 3D).
We further examined TuNer-m responses with a commercial confocal imaging system, coupled with offline sparse deconvolution analysis27, to obtain super resolution time-lapsed images. Tubular ER structure within COS-7 cells transiently expressing TuNer-m was clearly visible with a good signal-to-noise ratio (Supplementary Fig. 4A, B). Nuclear Ca2+ signaling is crucial for proper cell function28, yet nuclear ER Ca2+ content has been less studied. Here we observed that basal nuclear ER Ca2+ levels are slightly, but significantly higher than the rest portion of ER (indicated by white and yellow circles, respectively, Supplementary Fig. 4A, C). Further analysis showed that the TuNer-m ratio also differed significantly among different subcellular areas at rest, demonstrating uneven distribution of resting ER Ca2+ levels (Supplementary Fig. 4D). When examined with structured illumination microscopy (SIM) super-resolution imaging, the TuNer-m ratios within ER tubules are higher than the adjacent ER sheets in COS-7 cells (Fig. 2F–H), suggesting that Ca2+ levels within ER sheets are significantly lower than those in their corresponding neighboring ER tubules.
Of note, the basal subcellular NEMOer-m fluorescence does not correlate with that of TuNer-m ratio (microdomains indicated by arrows, Fig. 2F and Supplementary Fig. 4B), clearly demonstrating an uneven distribution of GECIs within ER. The mean peak basal Ca2+ concentration in the hot spots are approximately 1.9~2.0 mM, about two-folds higher than in adjacent Ca2+ microdomain (~0.9 mM) (Fig. 2F). Upon stimulation with 5 μM ATP, different subcellular regions of COS-7 cells showed repetitive fluctuations of TuNer-m ratios with varying amplitudes, illustrating slightly unsynchronized Ca2+ oscillations (Supplementary Fig. 4E, Supplementary Video 1). The discrepancies between NEMOer-m signals and TuNer-m ratios within microdomains are more pronounced in enlarged pictures or zoom-in videos (Dots indicated by white arrows, Supplementary Fig. 4B and Supplementary Video 2). These results thus underscore the need for caution when interpreting results obtained with intensiometric GECIs.
During ER Ca2+ oscillations induced by 5 μM ATP (Supplementary Fig. 4E) as well as dynamic re-arrangements of ER tubules, we could clearly see transient punctate microdomains with higher TuNer-m ratios (Dots indicated by white arrows, Supplementary Video 3), highlighting the highly discrete nature of Ca2+ refilling within ER tubules. Transient “Moving dark dots” with decreased TuNer-m ratios were also observed (pointed by yellow arrows, Supplementary Video 3), likely representing the brief reductions of ER Ca2+ levels within microdomains of ER tubules that are responsible for propagating cytosolic Ca2+ waves. To the best of our knowledge, this is the first report to visualize organelle dynamics and concurrent ER Ca2+ signals in microdomains with super-resolution.
Collectively, our mostly proof-of-concept data show that the bright and sensitive TuNer sensors are well suited for super-resolution imaging of dynamic ER Ca2+ signaling in non-excitable cells.
Performance of NEMOer sensors in cultured neurons
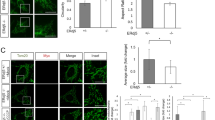
We proceeded to investigate NEMOer responses in cultured primary hippocampal neurons under electrical field stimulation. Since it is established that excited neurons often show ER Ca2+ overload14,24,29, we expressed NEMOer-c variant in cultured primary hippocampal neurons, and examined its ability to report ER Ca2+ signals induced by electrical field stimulation. In transiently transfected neurons, NEMOer-c fluorescence displayed an ER-like reticular network resembling the endoplasmic reticulum in both dendrites and soma. With a train of 50 action potentials (APs) at 25 Hz stimulation, we observed a robust increase in NEMOer-c fluorescence, with dendrites showing a more pronounced response compared to the soma (Fig. 3A, B), indicating more ER Ca2+ overload in dendrites. Next, we examined the responses of other NEMOer variants following stimulations of 10 Hz, 25 Hz or higher frequency, and found that NEMOer sensors had a dramatic increase in the peak signal-to-background ratio (SBR) compared to the G-CEPIA1er sensor. Notably, the NEMOer-c sensors had the highest response amplitude, while NEMOer-c and NEMOer-f were fast enough to pick up individual responses to 10 Hz stimulation (Fig. 3C).
A Typical images showing the fluorescence distribution and responses of NEMOer-c to 25 Hz field stimulation. Pseudocolor cellular images of NEMOer-c before (top left) and after 50 AP (25 Hz) stimulation (bottom left) (scale bar, 10 μm). Enlarged view of the dendrite region (top right), with a white dashed line indicating the position of line scan. Kymograph showing NEMOer-c responses to 25 Hz stimulation within a dendritic region (bottom right) along the indicated line. Scale bar, 2 μm. B Representative tracing of NEMOer-c responses to 25 Hz stimulation, right, statistics (n = 12 cells). Error bar denotes SEM. C Response of NEMOer variants or G-CEPIA1er within dendrites induced by electric stimulation with varies frequencies, with mean response curves shown on the left and statistics shown on the rightmost. Error bars denote SEM. (For stimulation at varied frequencies, G-CEPIA1er, n = 11,11,12,14 cells; NEMOer-f, n = 15, 14, 12, 13 cells; NEMOer-s, n = 11, 13, 12, 13 cells; NEMOer-m, n = 14, 17, 16, 13 cells; NEMOer-c, n = 13, 14, 12, 12 cells). D Typical images showing the individual spine fluorescence and responses of NEMOer-c to 25 Hz field stimulation. Scale bar, 2 μm. Right, statistics. Error bars denote SEM. (G-CEPIA1er, n = 36 spines; NEMOer-c, n = 68 spines).
Subsequently, we detected spines Ca2+ signals elicited by 50 AP at 25 Hz in secondary dendrites of hippocampal neurons. Compared to signals in G-CEPIA1er-expressing neurons, the 50AP-induced spines transient Ca2+ response in NEMOer-c-expressing neurons was significantly enhanced (Fig. 3D), while the other NEMOer variants showed a similar response. These results suggest that NEMOer sensors are capable of effectively responding to synaptic ER Ca2+ dynamics in primary hippocampal neurons.
To further verify if the responses reported by NEMOer sensors represent Ca2+ increase in ER, we examined the effects of SERCA inhibition by incubating cells with cyclopiazonic acid (CPA) for one hour and then stimulating them at a frequency of 100 Hz for 2 s. As expected, the NEMOer-c and NEMOer-s signals were significantly reduced in response to the stimulation in the presence of CPA compared to DMSO (0.33 vs 0.81 (peak response in CPA vs DMSO), P = 0.02; 0.12 vs 0.46 (peak response in CPA vs DMSO), P = 0.004, respectively). These results suggest that NEMOer sensors have a significantly superior ER Ca2+ responses to physiological frequency stimulations in primary neurons compared to G-CEPIA1er sensors.
We also assessed GECIs’ responses to ER Ca2+ lowering induced by 100 μM 3,4-Dihydroxyphenylglycol (DHPG), which activates metabotropic glutamate receptor (mGluR), leading to the generation of IP3 and Ca2+ releases from ER. In the spines of cultured rat cortical neurons, all NEMOer sensors exhibited significantly larger declines compared to G-CEPIA1er (Supplementary Fig. 5A). The amplitudes of lowering in GECI signals were comparable to those observed in HEK293 cells (Fig. 2A).
Performance of NEMOer sensors in cultured cardiac muscle cells
We moved on to further compare the responses of the fast-acting NEMOer-f with G-CEPIA1er in neonatal cardiomyocytes challenged with 30 mM caffeine, an agonist of ryanodine receptors (RyR), the primary Ca2+ releasing channels on SR. Decreases in NEMOer signals were more than 80%, significantly larger than those of G-CEPIA1er or ER-GCaMP6-150 (~66%) (Fig. 4A, Supplementary Fig. 5B–D). Specifically, NEMOer-f demonstrated the fastest kinetics among NEMOer variants, significantly faster than ER-GCaMP6-150 (decay rate constant: 2.47 ± 0.06 s−1 vs 0.81 ± 0.02 s−1, P = 2.37E-6), indicating its capability to detect rapid SR Ca2+ dynamics in cardiac muscle cells.
A SR load measurement by 30 mM caffeine perfusion in neonatal rat cardiomyocytes. Top, pseudocolor image showing a typical cell expressing NEMOer-f; bottom, averaged time curves showing caffeine-induced responses of transiently expressed indicators. The arrow indicates the time of caffeine stimulation. Scale bar, 10 μm. Typical figure captured from 6 cells across 75 one-day-old Sprague-Dawley rats in three independent experiments, showing consistent reproducibility across experiments. B Subcellular distribution of NEMOer-f fluorescence in adult rat cardiomyocyte revealed with confocal imaging. Top left, typical confocal images; top right, enlarged view of boxed area shown in top left image; bottom trace, spatial profile for the boxed region. Typical figure captured from 15 cells across 3 adult rats in three independent experiments, showing consistent reproducibility across experiments. C–E Local and global cytosolic and SR Ca2+ dynamics in adult rat cardiac myocytes revealed by dual-color imaging using NEMOer-f and a cytosolic Ca2+ indicator, Rhod-2. Top, typical line-scan images; bottom, corresponding mean time course plots. Ca2+ responses induced by caffeine (30 mM) (C). Local SR Ca2+ scraps and accompanying cytosolic Ca2+ transient are triggered by electric field stimulation (D) or during a spontaneous release (E), either under control (CT) (left) or when treated with 100 nM norepinephrine (NE) (right). (C, n = 6 cells; D, n = 14 cells; E left trace, n = 11 cells; (E) right trace, n = 20 cells. Data from 3 adult rats). Data is shown as mean ± s.e.m. F Spontaneous elementary cytosolic Ca2+ spark and SR Ca2+ blink events under CT (left) or NE-treated (right) conditions revealed by Rhod-2 and NEMOer-f, respectively. Typical line-scan images are shown on the left; corresponding time-course curves are shown on the right.
We then infected adult rat cardiomyocytes with adenovirus carrying NEMOer-f and imaged them with confocal microscopy 36–48 h later. We found that NEMOer-f fluorescence exhibited a striated sarcomeric pattern, indicating its correct localization in the SR (Fig. 4B). To better assess NEMOer-f performance, we co-loaded cardiomyocytes with a cytosolic chemical Ca2+ indicator Rhod-2, and simultaneously monitored the responses of both indicators. In response to 30 mM caffeine, Rhod-2 signal increased approximately four-fold, indicating a substantial drop in SR Ca2+ levels. Concurrently, NEMOer-f fluorescence rapidly decreased to about 75% of baseline, significantly surpassing values reported with a chemical indicator Fluo-5N (66.7%)30. This result highlights the high sensitivity of NEMOer-f for directly monitoring SR Ca2+ decreases in intact cardiomyocytes (Fig. 4C).
Next, we examined Ca2+ dynamics during excitation-contraction (E-C) coupling with NEMOer-f and Rhod-2. In response to field electrical stimulation (15 V, 1 Hz), cytosolic Ca2+ pulse indicated by Rhod-2 signal (ΔF/F0 = 3.1 ± 0.3) was accompanied by a large transient decrease in NEMOer-f fluorescence (ΔF/F0 = 0.35 ± 0.02) (Fig. 4D, Supplementary Fig. 6A), demonstrating simultaneous lowering of local SR Ca2+ levels, or Ca2+ scraps. Compared with those reported by a commercial Ca2+ dye (Fluo5N, ΔF/F0 = 0.1 ~ 0.2) or R-CEPIA1er31,32, Ca2+ scarps indicated by NEMOer-f demonstrated a similar time-to-nadir (126.8 ± 11.4 ms) and recovery time (414.8 ± 26.9 ms). Nevertheless, peak NEMOer-f responses were larger than R-CEPIA1er or Fluo-5N, showcasing its increased sensitivity over these two sensors.
Using Rhod-2 and NEMOer-f, we subsequently examined the corresponding spontaneous cytosolic and SR Ca2+ activities under resting conditions or upon adrenergic receptor activation (100 nM norepinephrine, NE). We found that both Rhod-2 and NEMOer-f responses were similar to those triggered by field stimulation (Fig. 4E, left). In line with previous reports32, cardiomyocytes under adrenergic activation exhibited significantly larger spontaneous Rhod-2 transients, concomitant with faster responses from NEMOer-f (Fig. 4E, right; Supplementary Fig. 6B, C). The observed phenomenon—larger cytoplasmic Ca2+ transients despite similar relative changes in ER Ca2+—can be attributed to SR Ca2+ overload induced by NE in cardiac muscle cells. Under SR overload conditions, the elevated basal SR Ca2+ level results in a larger absolute Ca2+ release for a given relative change.
We further assessed the ability of NEMOer-f to detect Ca2+ blinks, the elementary decreases in SR Ca2+ level leading to Ca2+ sparks, the fundamental units of excitation-contraction coupling in cardiomyocytes. We successfully observed the Ca2+ spark-blink pairs by Rhod-2 and NEMOer-f, respectively (Fig. 4F). Similar to previous reports22,30, the Rhod-2 signal peaks earlier than the SR Ca2+ indicator. This phenomenon suggests that the cytosolic Ca2+ spark reaches its peak when the decaying SR Ca2+ release rate (due to decaying SR Ca2+ concentration) is balanced by the increasing Ca2+ diffusion rate (due to increasing local cytosolic Ca2+ concentration). Afterward, the corresponding SR Ca2+ blink reaches its nadir when SR Ca2+ release terminates, outlasting the peak of a Ca2+ spark. The peak amplitude of Ca2+ blink indicated by NEMOer-f (ΔF/F0 = 0.17 ± 0.01), much larger than the Fluo-5N signal documented in rabbit muscle cells22,33. The duration of blink response indicated by NEMOer-f was slower than those reported by Fluo-5N. Further researches are needed to determine if the observed effect is due to the slower speed of NEMOer-f or facilitated Ca2+ diffusion by Fluo-5N, a smaller Ca2+ buffer molecule within SR. Nevertheless, to the best of our knowledge, this marks the first measurement of Ca2+ blinks with genetically encoded Ca2+ sensors, underscoring NEMOer-f’s exceptional sensitivity.
In vivo performance of NEMOer indicator in zebrafish
The successful detection of Ca2+ blinks with NEMOer-f in cardiac cells prompted us to test its performance in muscle cells in vivo. We took advantage of larval zebrafish that is optically transparent for live imaging. We constructed Tol2 transposase-based transgenic vectors that express codon-optimized NEMOer-f specifically in the SR of muscle cells using the β-actin promoter34. By injecting the fertilized eggs at their 1-cell stages, we obtained larvae with transient expression of NEMOer-f and imaged sparsely labeled muscle cells on day 5 post fertilization (dpf) (Fig. 5A). As expected, the baseline fluorescence of NEMOer-f indicated the SR localization of the sensor in muscle cells with a typical striated morphology (Fig. 5B). We also performed parallel experiments using G-CEPIA1er following the same procedures. When muscles contracted, NEMOer-f fluorescence exhibited robust signals with a sharp decrease followed by a slow rising phase (Fig. 5C, D, Supplementary Video 4). G-CEPIA1er detected spontaneous Ca2+ releases in fewer than 22% cells, with a median nadir amplitude of 0.15 (Supplementary Video 5). In contrast, NEMOer-f successfully reported these events in 48% cells, with responses 2.2 times larger (Fig. 5D, E). These data demonstrate that NEMOer-f functions in intact animals to detect spontaneous Ca2+ releases in SR and is indeed a superior indicator to G-CEPIA1er.
A A schematic depiction of the NEMOer-f in vivo imaging workflow in zebrafish. Created in BioRender. Wang, Z. (2025) https://BioRender.com/k83b811. B A typical sparsely labeled skeletal muscle cell, expressing NEMOer-f. Scale bar, 10 μm. Typical figure captured from 12 cells and 4 zebrafish larvae, showing consistent reproducibility across experiments. C Image montage of time-lapse imaging at 1 Hz. Black circle indicates the ROI for extracting time series in (D). Pink asterisks indicate single Ca2+ events. Scale bar, 10 μm. D Representative time series showing spontaneous SR Ca2+ release indicated by NEMOer-f and G-CEPIA1er signal. Pink and orange asterisks indicate single NEMOer-f and G-CEPIA1er Ca2+ events. E Cumulative distribution of amplitude of NEMOer-f responses (for NEMOer-f, n = 22 events from 12 cells and 4 zebrafish larvae; for G-CEPIA1er, n = 14 events from 9 cells and 4 zebrafish larvae, ****P = 1.97E−7, Welch’s t-test, two-sided).
Overall, here we reported NEMOer, a highly sensitive green GECIs for ER/SR with large dynamics. In comparison to commonly used GECIs derived from GCaMP-like series, such as G-CEPIA1er, NEMOer indicators show 10-fold higher brightness with the dynamic ranges 50 times larger, enabling the detection of physiological ER/SR Ca2+ dynamics with significantly larger signals. NEMOer sensors also exhibit significantly enhanced photochemical properties, showing minimal sensitivity to physiological pH variations and enduring 50 times more illumination without apparent bleaching. These ideal properties make NEMOer compatible with super-resolution recording of SR/ER Ca2+ homeostasis and dynamics. Remarkably, the fast NEMOer variant, NEMOer-f, stands out as the inaugural GECI that enables the detection of Ca2+ blink, the elementary Ca2+ releasing signal from SR of cardiomyocytes, as well as in vivo detection of spontaneous SR Ca2+ releasing event in a model organism zebrafish. Collectively, the exceptionally dynamic NEMOer sensors emerge as the premier choice for monitoring Ca2+ dynamics and homeostasis in mammalian cells, particularly within muscle cells.
Methods
Ethical statement
Animal experiments involving cultured primary neurons were approved by the Animal Care and Use Committee of the University of Science and Technology of China (Approval No. USTCACUC26120223022). Experiments involving cultured neonatal and adult rat cardiomyocytes were approved by the Institutional Animal Care and Use Committee of Peking University (Permit No. lsc-wangsq-1). All experimental procedures involving zebrafish were approved by the Institutional Animal Care and Use Committee of the Center for Excellence in Brain Science and Intelligence, Chinese Academy of Sciences (Approval No. NA-082-2024). We adhered to all relevant ethical guidelines and regulations.
Plasmids construction and recombinant adenovirus production
ER-GCaMP6-150 and superfolder turquoise2ox (sfTq2ox)35 were synthesized and incorporated into a pCDNA3.1(+) vector by BGI Geneland Scientific Co., Ltd, Jiangsu, China. Zebrafish codon-optimized NEMOer-f and G-CEPIA1er were synthesized by the entrusted company, Beijing Tsingke Biotech Co., Ltd., Beijing, China. The construction of other plasmids followed previously described methods. Briefly, target fragments were PCR amplified from corresponding templates, and the vector backbone was linearized with appropriate restriction enzymes. Corresponding mutations or substitutions were all included in primers. Fragments were then reassembled and inserted into the linearized vector utilizing a Ready-to-Use Seamless Cloning Kit (B632219, Sangon Biotech, Shanghai, China). Plasmids were all confirmed by sequencing. The vector backbones were linearized using the following restriction enzymes: BamHI and EcoRI for pCDNA3.1, EcoRI and XhoI for pTol2-β-actin, and NcoI and XhoI for pET28a. The recombinant plasmids were amplified in the DH5α strain (Vazyme Co., Ltd, Jiangsu, China).
To construct a variant library of NEMOer candidates, we performed PCR amplification of NEMOs23, incorporating known Ca2+-affinity-reducing mutations26 via primers. Subsequently, we added the endoplasmic reticulum localization signal sequence from GCEPIA1er26 and the coding sequence for ER-resident sequence KDEL to the N and C-termini of the constructs, respectively. Then, we reassembled and inserted them into a pCDNA3.1 vector backbone. To construct the TurN (mTurquoise2–NEMO)/TuNer (mTurquoise2–NEMOer) plasmids, we amplified the coding sequence of sfTq2ox-ΔC11 from pCDNA3.1(+)-sfTq2ox and the coding sequences of NEMO/NEMOer-ΔN5 from NEMO/NEMOer, respectively. Subsequently, we reassembled and inserted them into a custom-made pCDNA3.1(+) vector with puromycin resistance. To generate the mKate-P2A-GECI plasmids, we assembled the coding sequence of mKate-P2A, which was amplified from the mKate-P2A-NEMO plasmid23, along with the coding sequences of NEMOer/GCEPIA1er, into the custom-made pCDNA3.1 vector. For expression in Danio rerio, codon-optimized NEMOer-f and G-CEPIA1er were subcloned into the pTol2-β-actin vector, resulting in the generation of pTol2-β-actin: NEMOer-f and pTol2-β-actin: G-CEPIA1er.
The NEMOer-f gene sequences were amplified and inserted into pENTR2B vector (Invitrogen). The adenovirus was produced using the adenoviral expression system (Invitrogen) in HEK293A cells.
The sequences of primers used to generate GECI constructs are provided in Supplementary Table 7.
Bacterial expression and protein purification
Transetta (DE3) bacteria (TransGen Biotech, Beijing, China) were transformed with pET28a plasmids carrying GECI coding sequences and cultured in LB medium until reaching an optical density (OD) of 0.5. Protein expression was then induced overnight at 20 °C by adding 300 mM isopropyl-β-d-thiogalactoside (IPTG). The recombination proteins were purified following a previously described protocol36. Briefly, cells were harvested by centrifugation and re-suspended in 20 mL buffer 1 (mM, 20 Tris, 300 NaCl and 1 imidazole, pH 7.2). Cells were initially lysed and subsequently subjected to ultrasonic disruption, followed by incubation with 1 mL Ni Sepharose (17-5318-01, GE Healthcare, Piscataway, NJ, USA) to enrich recombinant GECI proteins with histidine-tags. The columns were sequentially washed with 20 mL buffer 1 and 10 mL buffer 2 (mM, 20 Tris, 500 NaCl, and 10 imidazole, pH 7.2). Finally, the GECI proteins were eluted using 5 mL buffer 3 (mM, 20 Tris, 100 NaCl, and 300 imidazole, pH 7.2).
In vitro Ca2+ titrations and kinetics measurements
The fluorescence intensity from buffer solutions containing 50 μg/mL GECI proteins, used to calculate Ca2+ dissociation constant (Kd), were recorded by a multi-mode microplate reader (Flexstation 3, Molecular Devices, USA). The buffer solution contained 130 mM KCl, 50 mM MOPs15, and CaCl2 with varying concentrations ranging from 0 mM to 128 mM (pH 7.2). The excitation/emission wavelengths of GCEPIA1er and NEMOer were 485 /510 nm and 490/520 nm with 10 nm bandpass, respectively. Ca2+ titration curves of Ca2+ concentration versus relative fluorescence intensity were then plotted and fit with specific binding with the hill slope function23 in Prism 9.5 software.
The fluorescence traces used to calculate disassociation kinetics (koff) were recorded using a stopped-flow spectrometer (SX20, Applied Photophysics Ltd., United Kingdom) with a 2-mm path length and a 1.1 ms dead time at room temperature. Buffer A contained 50 μg/mL GECI proteins, 1 mM Ca2+, 50 mM MOPS, 100 mM KCl, pH 7.414. Buffer B contained 10 mM EGTA, 50 mM MOPS, 100 mM KCl, pH 7.4. Subsequently, changes in fluorescence were monitored in response to rapidly mixing with buffers A and B. The fluorescence intensity changes of GECIs were recorded using a 515 nm long-pass filter with excitation at 488 nm. The koff value was determined by fitting the fluorescence decay curve with a single exponential function using Prism 9.5 software.
Spectra recording and measurement of quantum yields of GECIs
Purified proteins were diluted to a final concentration of 50~100 μg/mL in a buffer containing 130 mM KCl, 50 mM MOPs, and supplement with or without 128 mM CaCl2, pH 7.2. Fluorescence spectra were measured with a FS5 spectrophotometer (Edinburgh Instrument, Scotland) controlled by Fluoracle 2.15.2 software. Absorption spectra were measured with an ultraviolet and visible spectrophotometer (UV2600, Shimadzu, Japan) controlled by UVProbe 2.42 software.
The emission spectra and optical density(OD) used to calculate Quantum yields (Φ) of GECIs were determined with a FS5 spectrophotometer and a an ultraviolet and visible spectrophotometer23. We used the obtained the emission spectra to calculate the total integrated fluorescence intensity with different concentrations of GECI protein. Linear regression of integrated fluorescence intensity and absorbance values to get the slopes (S). Φ was determined by the equation: Φprotein = Φstandard × (Sprotein/Sstandard)9. The reference standards are fluorescein (φ = 0.925) and TOLLES (φ = 0.79) in 0.1 M NaOH37, excited at 470 nm and 405 nm, respectively38.
Chromophore extinction coefficients
The absorption spectra of buffer solutions containing 200 μg/ml GECI proteins, used to calculate extinction coefficients (ε), were recorded by Flexstation 3. Buffer solutions containing 30 mM trisodium citrate, 30 mM borate with varying pH values were supplemented with or without 128 mM CaCl2. pKa values were then obtained with previously described calculations and curve-fittings performed by Prism 9.5 software employing specific-binding-with-Hill-slope function23. Error bars represent SEM.
Cell isolation and cultures
Culture of non-excitable cell lines
HEK293, HeLa and COS-7 cells (ATCC, cat#: CRL-1573, CL-0101 and CRL-1651, respectively) were grown at 37 °C in a humidified atmosphere containing 5% CO2 in DMEM (HyClone, Chicago, IL, USA) with 10% fetal bovine serum (cat: FBSSA500-S, AusGeneX, Australia), 5% penicillin and streptomycin (Thermo Scientific, Waltham, MA, USA).
Primary dissociated hippocampal and cortical neuronal cultures
The cultured of primary hippocampal and cortical neurons involves a meticulous process starting with the dissection of hippocampi from both sides were dissected from embryonic day 18 CD®(SD) IGS rats of either gender using dissection tools previously sterilized by autoclaving. The dissected tissues are treated with a 0.25% trypsin solution (Sigma-Aldrich) at 37 °C for 15 min, followed by gentle shaking at 5-minute intervals to ensure thorough digestion. Subsequently, the dissected tissues were washed with Hanks’ balanced salt solution (HBSS) without Ca2+ and Mg2+ (Thermo Fisher) to remove debris and blood, followed by pipetting with a Pasteur glass tube (15 mm) in DMEM-F12 (with L-glutamine +10% FBS) complete medium (Thermo Fisher) to obtain a cell suspension. The dissociated hippocampal and cortical neurons were plated on 50k cells/18 mm or 300k cells/25 mm coverslips in 12-well plates or 6-well plates, which were coated overnight with 0.01% poly-L-lysine (Sigma‒Aldrich, Cat# P1274) at 37 °C. On the first day of culture, the neurons were incubated in DMEM-F12 (with L-glutamine +10% FBS) complete medium in a 5% CO2 atmosphere at 37 °C to facilitate initial neuron adhesion and viability in the optimal developmental growth conditions. After 24 h of plating, half of the plating medium was replaced with fresh neurobasal medium without serum (with 2% B27, 0.5 mM L-glutaMAX, 37.5 mM NaCl) and once every 4 days thereafter. Neurons are prepared for experimental use following a development period of 18–20 days in culture.
Neonatal and Adult rat cardiomyocytes isolation and culture
Hearts were excised from 1-day rats and immersed in ice-cold Hanks solution (Sigma) supplemented with NaHCO3 (3.6 mM) and taurine (30 mM). The cardiac tissue was subsequently minced into fragments and subjected to sequential enzymatic digestion with 5 mL pre-warmed collagenase II (Worthington, 0.08%) and trypsin solution (Yeasen, 0.1%) at 37 °C. After each 5-6 min digestion interval, the supernatant was collected and immediately neutralized with FBS. Cell suspensions were centrifuged at 800 g for 5 min, followed by supernatant removal and resuspension in DMEM (Invitrogen) containing 10% FBS (Gibco) and 0.1 mM 5-bromo-2’-deoxyuridine (Sigma). Fresh enzymatic solution was applied to the remaining tissue fragments, with this digestion-neutralization cycle repeated 8–10 times until complete tissue dissociation. Myocytes were plated at 5 × 105 cells/3.5 cm dish.
Adult ventricular cardiomyocytes were enzymatically isolated as previously described39. Briefly, hearts were rapidly removed from anesthetized adult Sprague-Dawley rat and perfused using Ca2+-free Tyrode’s solution (in mM: 120 NaCl, 4 KCl, 1.2 MgCl2, 1.5 NaH2PO4, 20 NaHCO3, 10 glucose, 30 taurine, 95% O2 /5% CO2 saturated) in a Langendorff system. Collagenase Type II (0.7 mg/mL) (Worthington Biochemical) was used to digestion of the heart into single cells. After digestion, the myocardium tissue was cut into small pieces and filtered into Ca2+-free Tyrode’s solution with a sieve, then replaced with gradient Ca2+ solution, resulting in a final solution with 1 mM Ca2+. Freshly isolated cardiomyocytes were plated on laminin-coated (Sigma) culture dishes for 20 min, and then the attached cells were maintained in M199 medium (Gibco) at 37 °C under 5% CO2 until usage.
Gene transfections
Gene transfection animal cell lines
Gene transfection of cells was completed with electroporation (Bio-Rad Gene Pulser Xcell system, Hercules, CA, USA). The electroporation protocol was a voltage step pulse (180 V, 25 ms) using 4 mm cuvettes and 0.4 mL OPTI-MEM medium. Transfected cells were seeded on round coverslips and cultured with DMEM. Imaging experiments were carried out 24 h after transfection.
Transfection for primary dissociated hippocampal and cortical neurons
Cultured neurons were transfected with 0.5-1 µg plasmids G-CEPIA1er or NEMOer -encoding plasmids at approximately 10-11 DIV using a calcium phosphate transfection kit (Takara, Cat# 631312). Specifically, cultured neurons on coverslips were transferred to a new 12-well plates or 6-well plates with pre-warmed DMEM-F12 (with L-glutamine + 25% 1 M HEPES) complete medium. Mix plasmids with 10% of 2 mM CaCl2 in ddH2O. Subsequently, the DNA-containing mixed solution was transferred eight times into 2×HBS solution, vortexing for 3 s after each transfer. The mixture was then incubated for 15–20 min at room temperature and added dropwise to the culture 12-well plates or 6-well plates with medium. When an evenly distributed layer of precipitate particles was observed across the neurons (approximately 2 h later), the DNA-Ca2+-phosphate precipitates were dissolved using 1×HBS solution (pH 6.8). The transfected neurons were then transferred back to their original 12-well plates or 6-well plates containing neurobasal medium.
Gene transfection of rat cardiomyocytes
For adenovirus-mediated gene expression of adult cardiomyocytes. Cells were infected with adenoviruses carrying the NEMOer-f calcium sensor at a multiplicity of infection (m.o.i.) of 30. Confocal imaging was performed 48 h after virus infection. As to neonatal rat cardiomyocytes, 36 to 48 hr after cell culture, 4 μg plasmids was transiently transfected into cells with Lipofectamine 3000 according to the manufacturer’s instructions.
Fluorescence imaging
The fluorescence imaging was performed using a ZEISS observer Z1 imaging system controlled by Slidebook 6.0.23 software (Intelligent Imaging Innovations, Inc.)23. Cells grown on coverslips were immersed in a physiological salt solution (107 mM NaCl, 7.2 mM KCl, 1.2 mM MgCl2, 11.5 mM glucose, 20 mM HEPES-NaOH at pH 7.2), supplemented with 0.1% BSA. The excitation/emission filters used: NEMOer (500 ± 12/542 ± 13 nm), G-CEPIA1er/ER-GCaMP6-150 (470 ± 11 /510 ± 14 nm) and TurN/TuNer (For YFP channel, 500 ± 10/535 ± 15 nm; for CFP channel, 438 ± 12 /470 ± 12 nm). Fluorescence was collected every two seconds, and the mean fluorescent intensity of the corresponding regions was exported for analysis using Matlab 2023b (The MathWorks, Natick, MA, USA), and the results were plotted using Prism 9.5 software. The perfusion rate is 6.7 s/mL, allowing the complete exchange of the solution in the perfusion chamber within 1.5 s. Error bars denote SEM.
Confocal imaging
Confocal imaging of COS-7 cells
Recordings were undertaken with a ZEISS LSM880 microscope equipped with 63x oil objective (NA 1.4) and ZEN 2.1 software. For measurement of TuNer-m fluorescence ratio excited by optimal excitation with and without calcium, excitation was set at 458 and 514 nm, with the emission collected at 460–500 nm and 520–600 nm. The acquired images were analyzed using Image J and Matlab 2023b software. To improve image resolution and contrast, the software MicroscopeX FINER(Computational Super-resolution Biotech Co.,Ltd, Guangzhou, China), which uses sparse deconvolution, was utilized, following the methodology reported previously27. To estimate ER Ca2+ level based on the ratiometric measurement using TuNer-m (Supplementary Fig. 4C–E), ER Ca2+ level was calculated using the following equation: \({[C{a}^{2+}]}_{free}={[\frac{R-{R}_{min}}{{R}_{max}-R}]}^{\frac{1}{n}}\times {K}_{d}\), where R = FNEMOer-m/FsfTq2ox, n = 1.28 and Kd = 958.67 μM.
Confocal line-scan imaging of cardiomyocytes
The imaging was performed on a confocal microscope (LSM-710; Carl Zeis) equipped with an argon laser (488 nm) and 40×, 1.0 N.A. water immersion objective. Myocytes were loaded with 10 μM Rhod2-AM (Invitrogen) under 37 °C for 10 min (Ca2+ transient and SR load measurement) or 20 μM Rhod2-AM (Invitrogen) under 37 °C for 5 min (Ca2+ spark measurement), and the experiments were performed within 1 hr after loading. In pacing Ca2+ transient recording experiments, 15 V, 1 Hz field stimulation to the myocytes. For Ca2+ transient, Ca2+ spark, and SR load measurement, line-scan images were acquired at sampling rates of 3.78 ms/line, the excitation was at 488 and 543 nm, and their fluorescence emission were collected at 490 to 550 and 560 nm to 800 nm. The fluorescence-time curves of image were analyzed by python, the ΔF/F0 ((F-F0)/F0), time to peak (the time of Fmax) and τ90 (decay time of 90% fluorescence change of Fmax) were calculated from curve by matlab R2018a program.
Ca2+ imaging and quantitative analysis of fluorescence intensity in neurons
Cultured hippocampal neurons of DIV 18–20 were imaged using a W1 spinning disc confocal microscope (Ti2-E, NIKON) with a 100 × oil-immersion objective (1.45 NA, NIKON) in extracellular solution (120 mM NaCl, 3 mM KCl, 10 mM glucose, 10 mM HEPES-NaOH, 2 mM MgCl2, and 2 mM CaCl2, pH 7.4). Field stimulations were performed in a stimulation chamber (Warner Instruments, RC-49MFSH) with a programmable stimulator (Master-8, AMPI). The hippocampal neurons were imaged with an exposure time of 200 ms with consecutive and uninterrupted acquisition. For pharmacological experiments, 50 μM CPA (MCE, Cat# HY-N6771) was incubated for 1 h in neurobasal medium at 37 °C. In the control group, neurons were incubated with DMSO (dimethyl sulfoxide, Sigma) for the corresponding time.
Cultured cortical neurons of DIV 16–18 were imaged with a ZEISS LSM880 microscope equipped with 63x oil objective (NA 1.4) and ZEN 2.1 software in extracellular solution (124 mM NaCl, 3 mM KCl, 2 mM CaCl2, 2 mM MgCl2, 10 mM HEPES, 10 mM D-Glucose, pH 7.4). Ca2+ release from cultured cortical neuronal ER was induced by 100 μM DHPG (MCE, Cat# HY-12598A). The cortical neurons were imaged with an exposure time of 2.2 s with consecutive and uninterrupted acquisition.
All the quantifications of fluorescent signals in neuronal cultures were performed on the secondary dendrites and somata of transfected neurons. A region of interest (ROI) was chosen based on the imaging criteria, e.g., the quality of fluorescent signals, the background level. ROI of dendrites, somata, and spines were manually defined in ImageJ for the calculation of mean fluorescent signal Fs. A similar region near the dendrites and somata of interest was selected for the calculation of fluorescent background Fb. The effective signal was calculated as F = Fs – Fb. Since all our quantifications were based on F normalized with the averaged fluorescence intensity within the region during the baseline period, the area of defined polygon would have no impact on the normalized F. We also simultaneously monitor x-y drift to ensure that the identification of the target area in ImageJ does not deviate from the defined ROI.
Multimodality Structured Illumination Microscopy (Multi-SIM) imaging
SIM images were acquired on a Multi-SIM imaging system40 (NanoInsights-Tech Co., Ltd.) equipped with a 100 × 1.49 NA oil objective (Nikon CFI SR HP Apo), and a Photometrics Kinetix camera. SIM images were collected with VSIM 2.2.1.10 software. The SIM images of COS-7 cells expressing TuNer-m were taken using low NA GI-SIM mode with laser power set to 300 mW (445 nm) and an exposure time of 30 ms, enabling authentic simultaneous detection in the 415–475 nm and 500–550 nm range using two detectors. Bleed-through correction was performed to ensure accurate spectral separation. Images were then reconstructed using the SIM Imaging Analyzer software (NanoInsights-Tech). During the acquisition of images, cells were in a humidified chamber maintained at 37 °C in the presence of 5% CO2. The reconstructed images were analyzed using Image J software.
Animals
CD®(SD) IGS rats were obtained from Charles River and used for the culture of primary hippocampal and cortical neurons. The rats were group-housed in cages of three under a 12-hour light/dark cycle (lights on at 7 a.m.) in a stable environment (temperature maintained between 22 °C and 24 °C), with water and food available ad libitum. Female CD®(SD) IGS rats were sacrificed at 18 days of gestation for neuron culture.For cardiomyocyte isolation and culture, adult male SD rats aged 8–12 weeks were used, and neonatal ventricular myocytes were isolated from 1-day-old SD rats. Adult and larval zebrafish (Danio rerio) of the nacre background were maintained at 28.5 °C under a 14-hour/10-hour light/dark cycle. Adult zebrafish were fed twice daily. Zebrafish larvae at 5–6 dpf were used for imaging.
Experiment in zebrafish (Danio rerio)
To generate transient expression larvae for imaging, mating pairs of adult fish of nacre background were set up to avoid dark pigmentation. Injection mix contained 25 ng/uL of pTol2-β-actin: NEMOer-f or pTol2-β-actin: G-CEPIA1er and 25 ng/uL of Tol2 mRNA. Approximately 1 nL of the cocktail was injected into fertilized eggs. Adult and larval zebrafish were maintained at 28 degrees on a 14 h/10 h light/dark cycle. All experiments were conducted in 10% Hank’s solution containing (in mM): 140 NaCl, 5.4 KCl, 0.25 Na2HPO4, 0.44 KH2PO4, 1.3 CaCl2, 1.0 MgSO4, and 4.2 NaHCO3 (pH 7.2). All experimental procedures were approved by Center for Excellence in Brain Science and Intelligence, Chinese Academy of Sciences.
Confocal microscopy was performed using Olympus FV3000 upright microscope with a LUMPlanFL N 40x water immersion objective (N.A. 0.8, W.D. 3.3). Scanning speed was 1 Hz and a single plane was imaged for time-lapse imaging. The frame size was set as 512 × 512 at 16-bit depth, and the actual scan size was slightly cropped to allow 1 Hz speed. The 5 dpf or 6 dpf zebrafish larvae were embedded sideways in 2% low-melting agarose (Invitrogen, 16520100) under system water. Zebrafish larvae were neither anesthetized nor paralyzed. Time-lapse images were registered using StackReg to correct mild drifts and fluorescence values of ROI were measured in Fiji. Baseline fluorescence (F0) was defined as the top fifteen percentile valu,e and normalized fluorescence changes (ΔF/F0) were calculated in MATLAB R2020b.
Statistics and reproducibility
Sample sizes were determined based on well-established studies in the field and the observed animal-to-animal variability during the experiments. For the detection of the performance of transiently expressed NEMOer variants in non-excitable cells (HEK293 cells, HeLa cells, and COS7 cells), the sample size involved at least 3 experimental repeats, with 5 to 20 cells per repeat. For detecting the performance of transiently expressed NEMOer variants in cardiomyocytes, 3 to 32 cells from 3 adult rats and 60 neonatal rats were used, with at least 3 experimental repeats. For detecting the responses of NEMOer variants in dissociated rat hippocampal neurons excited by electric field stimulation, the sample size involved at least 10 neurons from three different primary hippocampal neuron cultures. The sample size for detecting the responses of NEMOer variants in dissociated rat cortical neurons involved at least 7 neurons from 30 fetal rats, with 3 experimental repeats. For detecting the performance of transiently expressed NEMOer-f and G-CEPIA1er sensors in zebrafish, 4 zebrafish larvae per sensor were used. The specific replicates for each experiment are provided in the corresponding figure legends.
No data were excluded from the analyses. Multiple experiments using zebrafish or cells were conducted to assess the reproducibility of our findings. At least three independent repeats were performed for each set of experiments, and all attempts at replication were successful, confirming the reliability of our results.
Samples were allocated randomly, and investigators were blinded to group allocation during data collection and analysis.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All relevant data from this study, including generated and analyzed datasets, are provided in the published article and its supplementary information files. The relevant data generated in this study are provided in the Source Data file. Source Data file is provided with this paper. Source data are provided with this paper.
Code availability
The Matlab code used in this study consists of simple scripts for basic matrix operations and is available at Zenodo (https://doi.org/10.5281/zenodo.15094049).
References
Clapham, D. E. Calcium signaling. Cell 131, 1047–1058 (2007).
Berridge, M. J. The endoplasmic reticulum: a multifunctional signaling organelle. Cell Calcium 32, 235–249 (2002).
Schwarz, D. S. & Blower, M. D. The endoplasmic reticulum: structure, function and response to cellular signaling. Cell Mol. Life Sci. 73, 79–94 (2016).
Zheng, S., Wang, X., Zhao, D., Liu, H. & Hu, Y. Calcium homeostasis and cancer: insights from endoplasmic reticulum-centered organelle communications. Trends Cell Biol. 33, 312–323 (2023).
Parkkinen, I. et al. Pharmacological regulation of endoplasmic reticulum structure and calcium dynamics: importance for neurodegenerative diseases. Pharm. Rev. 75, 959–978 (2023).
Miyawaki, A. et al. Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature 388, 882–887 (1997).
Tallini, Y. N. et al. Imaging cellular signals in the heart in vivo: Cardiac expression of the high-signal Ca2+ indicator GCaMP2. Proc. Natl Acad. Sci. USA 103, 4753–4758 (2006).
Chen, T. W. et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300 (2013).
Zhao, Y. et al. An expanded palette of genetically encoded Ca(2)(+) indicators. Science 333, 1888–1891 (2011).
Llinás, R., Blinks, J. R. & Nicholson, C. Calcium transient in presynaptic terminal of squid giant synapse: detection with Aequorin. Science 176, 1127–1129 (1972).
Tang, S. et al. Design and application of a class of sensors to monitor Ca2+ dynamics in high Ca2+ concentration cellular compartments. Proc. Natl Acad. Sci. USA 108, 16265–16270 (2011).
Deng, X. et al. Tuning protein dynamics to sense rapid endoplasmic‐reticulum calcium dynamics. Angew. Chem. Int. Ed. 60, 23289–23298 (2021).
Reddish, F. N. et al. Rapid subcellular calcium responses and dynamics by calcium sensor G-CatchER+. iScience https://doi.org/10.1016/j.isci.2021.102129 (2021).
Henderson, M. J. et al. A low affinity GCaMP3 Variant (GCaMPer) for imaging the endoplasmic reticulum calcium store. PLoS One 10, e0139273 (2015).
Suzuki, J. et al. Imaging intraorganellar Ca2+ at subcellular resolution using CEPIA. Nat. Commun. 5, 4153 (2014).
Li, J. et al. Visible light excited ratiometric-GECIs for long-term in-cellulo monitoring of calcium signals. Cell Calcium 87, 102165 (2020).
Luo, C., Wang, H., Liu, Q., He, W. & Xu, P. A genetically encoded ratiometric calcium sensor enables quantitative measurement of the local calcium microdomain in the endoplasmic reticulum. Biophys. Rep. 5, 31–42 (2019).
Farhana, I., Hossain, M. N., Suzuki, K., Matsuda, T. & Nagai, T. Genetically encoded fluorescence/bioluminescence bimodal indicators for Ca(2+) Imaging. ACS Sens. https://doi.org/10.1021/acssensors.9b00531 (2019).
Navas-Navarro, P. et al. GFP-Aequorin protein sensor for ex vivo and in vivo imaging of Ca 2+ dynamics in high-Ca 2+ organelles. Cell Chem. Biol. 23, 738–745 (2016).
Suzuki, J., Kanemaru, K. & Iino, M. Genetically encoded fluorescent indicators for organellar calcium imaging. Biophys. J. 111, 1119–1131 (2016).
Reddish, F. N., Miller, C. L., Gorkhali, R. & Yang, J. J. Calcium dynamics mediated by the endoplasmic/sarcoplasmic reticulum and related diseases. Int. J. Mol. Sci. 18, https://doi.org/10.3390/ijms18051024 (2017).
Brochet, D. X. et al. Ca2+ blinks: rapid nanoscopic store calcium signaling. Proc. Natl Acad. Sci. USA 102, 3099–3104 (2005).
Li, J. et al. Engineering of NEMO as calcium indicators with large dynamics and high sensitivity. Nat. Methods 20, 918–924 (2023).
de Juan-Sanz, J. et al. Axonal endoplasmic reticulum Ca2+ content controls release probability in CNS nerve terminals. Neuron 93, 867–881 e866 (2017).
Wu, J. et al. Improved orange and red Ca²± indicators and photophysical considerations for optogenetic applications. ACS Chem. Neurosci. 4, 963–972 (2013).
Subach, O. M. et al. Novel genetically encoded bright positive calcium indicator NCaMP7 based on the mneongreen fluorescent protein. Int. J. Mol. Sci. 21, https://doi.org/10.3390/ijms21051644 (2020).
Zhao, W. et al. Sparse deconvolution improves the resolution of live-cell super-resolution fluorescence microscopy. Nat. Biotechnol. 40, 606–617 (2022).
Bading, H. Nuclear calcium signalling in the regulation of brain function. Nat. Rev. Neurosci. 14, 593–608 (2013).
Deng, X. et al. Tuning protein dynamics to sense rapid endoplasmic-reticulum calcium dynamics. Angew. Chem. Int Ed. Engl. 60, 23289–23298 (2021).
Lu, F. et al. Imaging sarcoplasmic reticulum Ca2+ signaling in intact cardiac myocytes. Circulation 42, 1503–1505 (2020).
Bovo, E., Martin, J. L., Tyryfter, J., de Tombe, P. P. & Zima, A. V. R-CEPIA1er as a new tool to directly measure sarcoplasmic reticulum [Ca] in ventricular myocytes. Am. J. Physiol. Heart Circ. Physiol. 311, H268–275 (2016).
Shannon, T. R., Guo, T. & Bers, D. M. Ca2+ scraps: local depletions of free [Ca2+] in cardiac sarcoplasmic reticulum during contractions leave substantial Ca2+ reserve. Circ. Res. 93, 40–45 (2003).
Brochet, D. X., Xie, W., Yang, D., Cheng, H. & Lederer, W. J. Quarky calcium release in the heart. Circ. Res. 108, 210–218 (2011).
Tao, R. et al. A genetically encoded ratiometric indicator for tryptophan. Cell Discov. 9, 106 (2023).
Meiresonne, N. Y. et al. Superfolder mTurquoise2ox optimized for the bacterial periplasm allows high efficiency in vivo FRET of cell division antibiotic targets. Mol. Microbiol. 111, 1025–1038 (2019).
Dana, H. et al. High-performance calcium sensors for imaging activity in neuronal populations and microcompartments. Nat. Methods 16, 649–657 (2019).
Wilkinson, J. G. Principles of fluorescence spectroscopy - Lakowicz. Jr. Chem. Brit 20, 442–442 (1984).
Katayama, H. et al. Visualizing and modulating mitophagy for therapeutic studies of neurodegeneration. Cell 181, 1176–1187.e16 (2020).
Yang, L. et al. Transcriptional regulation of intermolecular Ca(2+) signaling in hibernating ground squirrel cardiomyocytes: The myocardin-junctophilin axis. Proc. Natl Acad. Sci. USA 118, https://doi.org/10.1073/pnas.2025333118 (2021).
Guo, Y. et al. Visualizing intracellular organelle and cytoskeletal interactions at nanoscale resolution on millisecond timescales. Cell 175, 1430–1442.e1417 (2018).
Wu, S., Skolnick, J. & Zhang, Y. Ab initio modeling of small proteins by iterative TASSER simulations. BMC Biol. 5, 17 (2007).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (92254301 and W2411015 to Y. W., 32171026 to Y. M., 32250003 and 32230048 to S.-Q.W.), STI2030-Major Projects (2021ZD0202503 to A.-H. T., 2021ZD0204500 and 2021ZD0203704 to Y. M.), the National Key Research and Development Program of China (2020YFA0112200 to A.-H. T. and 2024YFA0916804 to S.-Q.W.), China Postdoctoral Science Foundation General Program (2022M723246 to Z. W.), the Natural Science Foundation of Anhui Province (2408085QH256 to J.-H.C.), Scientific Instrument Developing Project of the Chinese Academy of Sciences (YJKYYQ20210029 to Y. M.) and the Ministry of Science and Technology of China (2019YFA0802104 to Y. W.). We would like to thank the Experimental Technology Center for Lifesciences, Beijing Normal University and the National Center for Protein Sciences at Peking University. We thank Dr. Qian Hu and the Optical Imaging Facility from the Institute of Neuroscience for their support. We thank the Core Facility for Biomolecule Preparation and Characterization, Tsinghua University.
Author information
Authors and Affiliations
Contributions
Y. W., A-H. T., S-Q W, and Y. M. supervised and coordinated the study. W. G. and J. L. designed and generated all the plasmid constructs, with help from Z.Z.. J. L., W. G., and H. Z. performed the in vitro assays. W. G. performed all fluorescence imaging in non-excitable cells, with help from J. L. and J. Z.. J. C. isolated and cultured primary cortical neurons with help from W. G. and P. L. J. C. performed confocal imaging of dissociated hippocampal neurons. W. G. performed confocal imaging of dissociated cortical neurons. Y. Z. performed confocal imaging of dissociated cardiac cells, and Z. X. analyzed spark and blink data. W. G., A. J., and H. Z. performed Multi-SIM imaging on COS-7 cells under the guidance of Y. W. and D. L. Z. W. performed in vivo Ca2+ imaging of zebrafish muscle cells with help from S. W. C. X. provided tech support for live cell imaging. W. G., J. C., Y. Z., Z. W., J. L., and H. Z. analyzed data with input from the other authors. D. L. G., S. W., Y. M., T. H., and J. L. provided intellectual input to the manuscript. Y. M., A-H. T. and Y. W. wrote the manuscript with inputs from all the other authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Yi Shen, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Gu, W., Chen, JH., Zhang, Y. et al. Highly dynamic and sensitive NEMOer calcium indicators for imaging ER calcium signals in excitable cells. Nat Commun 16, 3472 (2025). https://doi.org/10.1038/s41467-025-58705-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-58705-6