Abstract
B7H3, an immune checkpoint molecule, is a highly N-glycosylated membrane protein. However, the key glycosylated asparagine residues that mediate the function of the B7H3 protein are still unclear. Here we identify that N-glycans attached to asparagine residues N91/309 and N104/322 are required for proper B7H3 localization on the cell surface membrane. We demonstrate that mutations in these two pairs of N-glycosylation sites induce ER accumulation of B7H3 by blocking its ER-to-Golgi translocation and subsequently promote its degradation via the endoplasmic reticulum-associated protein degradation pathway. Additional evidence suggests that N-glycosylation at N91/309 and N104/322 of B7H3 is essential for its inhibition of T-cell proliferation and activation. More importantly, a monoclonal antibody, Ab-82, preferentially targeting B7H3 glycosylated at N91/309 and N104/322 is developed, which exhibits the ability to elicit cytotoxic T lymphocyte-mediated antitumor immunity via B7H3 internalization. Together, these findings offer a rationale for targeting glycosylated B7H3 as a potential strategy for immunotherapy.
Similar content being viewed by others
Introduction
N-Glycosylation is an important posttranslational modification in which carbohydrate moieties are attached to proteins1. These glycans are covalently attached to asparagine (Asn) residues in an N-glycosylation consensus sequence (NXT motif, Asn-X-Ser/Thr, where X may be any amino acid except for Pro)2. N-glycosylation plays critical roles in many biochemical and biological events, such as protein folding, degradation, cellular localization, intracellular transport and protein‒protein interactions3,4,5. This type of posttranslational modification is first catalyzed by an oligosaccharyl transferase (OST) complex that transfers a preformed oligosaccharide to an asparagine side chain acceptor in the endoplasmic reticulum (ER). Further processing occurs in the Golgi apparatus via a sequential glycosidase- and glycotransferase-mediated glycoprotein biosynthesis pathway6. The ER is the major site of membrane protein folding, assembly, and maturation. When a protein is misfolded or when an abnormal glycan structure is present, the protein is recognized by the lectin ER-degradation enhancing a-mannosidase-like protein (EDEM), undergoes retrograde translocation from the ER to the cytosol, and is then degraded by endoplasmic reticulum-associated protein degradation (ERAD) via the ubiquitin‒proteasome system for ER quality control7,8.This process requires ER membrane–embedded protein complexes, such as HRD1 and SEL1L, and the ubiquitin-dependent segregase VCP (valosin-containing protein, also known as p97)9,10,11. Many membrane proteins and receptors are N-glycosylated, and this modification plays an essential role in membrane protein function12. For instance, the glycan on N89, among the three glycans present on inducible co-stimulator (ICOS), is required for proper ICOS protein folding in the ER and its intracellular trafficking and ligand binding activity13. Therefore, it is important to determine the specific N-glycans of membrane proteins that are essential for proper ER quality control, membrane trafficking and protein activities.
B7 homolog 3 protein (B7H3), also known as CD276, is a type I transmembrane protein belonging to the B7 immunoglobin superfamily. 4IgB7H3 (encoding 534 amino acids) with two identical pairs of extracellular IgV-like and IgC-like domains is the predominant isoform in humans. B7H3 in mouse is expressed only as the 2IgB7H3 isoform (encoding 316 amino acids) with one pair of extracellular IgV-like and IgC-like domains14. Human B7H3 is highly glycosylated, and widely overexpressed in many solid cancers but exhibit very low expression in matched normal tissues. Previous studies have shown that B7H3 plays important roles in tumor cell migration, proliferation, invasion, and angiogenesis and plays key roles in cancer cell escape from immune surveillance15,16,17. It has been verified that there are four pairs of Asn N-glycosylation sites (N91/N309, N104/N322, N189/N407, and N215/N433) in B7H3 in oral squamous cells and breast cancer cells. And this modification plays an important role in the poor prognosis of patients and is necessary for the inhibitory effect of B7H3 on the activity of cytotoxic T cells18,19. However, it remains largely unknown whether all glycosylation sites of B7H3 contribute to glycan-mediated functions or whether specific positions have separate roles.
In this work, we clarify the biological roles of N-glycans on B7H3 using a series of glycosylation-defective B7H3 mutant proteins. We reveal that the N-glycans attached to N91/309 and N104/322 are essential for B7H3 surface expression and immune evasion, with their absence triggering ER retention and ERAD-dependent elimination. Notably, we develop an optimized monoclonal antibody, Ab-82, that selectively targets these glycosylation sites, inducing B7H3 internalization and degradation while enhancing antitumor immunity through cytotoxic T cell activation. This study provides a substantial contribution to the current mechanistic understanding of the necessity and potential functions of a specific N-glycan among the multiple glycans of B7H3 and a potential strategy for eliciting an antitumor effect.
Results
Identification of the vital N-glycosylation sites that maintain B7H3 protein stability
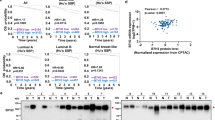
N-linked glycosylation occurs specifically at the NXT motif (Asn-X-Ser/Thr sequon). In the consensus sequence, N represents asparagine (Asn) residue with forming an N-glycosidic bond, X represents any amino acid except proline (Pro), and T represents either serine (Ser) or threonine (Thr). Human B7H3 usually contains four pairs of Asn N-glycosylation sites at N91/N309, N104/N322, N189/N407, and N215/N433 in the nearly exact tandem duplication of IgV-IgC ___domain18,19. N-glycosylation of the NXT motif sites is essential for the expression of B7H3 on the cell surface18. To pinpoint the exact NXT motif on B7H3 that is responsible for its cell surface localization, we first depleted B7H3 using specific single-guide RNAs (sgRNAs) in human HEK-293T to construct B7H3-deficient cell model (Supplementary Fig. 1a). Then, a series of B7H3 mutants were generated in which one or more of the four pairs of asparagine (N) sites were mutated to glutamine (Q), and these B7H3 mutants and wild-type (WT) B7H3 were transiently expressed in HEK293T-B7H3KO cells individually by introduction of the same amount of plasmid (Fig. 1a). Compared with that in B7H3-WT cells, B7H3 glycosylation was completely ablated in B7H3-8NQ mutant cells, as indicated by the significant reduction in the baseline molecular weight of the B7H3 protein (~110 kDa) to ~70 kDa, and B7H3 expression on the cell surface was largely blocked (Fig. 1b, Supplementary Fig. 1a), as we previously reported18. Surprisingly, we found that when compared to that in WT and N91/104/309/322 mutant (in which only the N91/N309 and N104/N322 sites could be N-glycosylated), the N91/104/309/322Q mutant (in which only the N91/N309 and N104/N322 site pairs could not be N-glycosylated) exhibited greatly reduced B7H3 cell surface localization, similar to that of the B7H3-8NQ mutant (Fig. 1b). Furthermore, restoring any single Asn residue in N91/104/ 309/322Q mutant partially recovered the membrane expression of B7H3 (Supplementary Fig. 1b, c).
a Schematic diagram of human B7H3-NQ mutant constructs. WT B7H3 contains four pairs of Asn N-glycosylation sites at amino-acid positions 91 (N91) / 309 (N309), 104 (N104) / 322 (N322), 189 (N189) / 407 (N407) and 215 (N215) / 433 (N433) in the nearly exact tandem duplication of IgV-IgC ___domain. Potential asparagine N-glycosylation sites (N) were mutated to glutamine (Q). b HEK293T-B7H3KO cells were transiently re-expressed with the same amount of plasmids as indicated. Flow cytometry measuring B7H3 protein on the cell membrane in the indicated cell lines. Left, representative images of membrane B7H3. Right, median Fluorescence Intensity (MFI) of B7H3 (n = 3 biological independent samples). c MDA-MB-231-B7H3KO cells and A549-B7H3KO were stably rescued with human B7H3-WT and B7H3-NQ mutant cDNA individually. Flow cytometry measuring B7H3 protein on the cell membrane in the indicated cells. d Schematic diagram of mouse B7H3-NQ mutant constructs. WT B7H3 contains four Asn N-glycosylation sites at amino-acid positions 91 (N91), 104 (N104), 189 (N189) and 215 (N215) in the single IgV-IgC ___domain. Potential asparagine N-glycosylation sites (N) were mutated to glutamine (Q). e 4T1-B7H3KO cells, B16-B7H3KO cells and E0771-B7H3KO cells were stably rescued with mouse B7H3-WT and B7H3-NQ mutant cDNA individually. Flow cytometry measuring B7H3 protein on the cell membrane in the indicated cell lines. f The indicated cell lines were treated with MG132 in the presence of CHX at indicated intervals. The intensity of B7H3 protein was quantified using ImageJ software. g The indicated cell lines were treated with MG132 for 6 h. Immunoprecipitation analysis of Flag-tagged B7H3 ubiquitination with the indicated antibodies. The p-value in (b, f) was determined by one-way ANOVA with Tukey’s multiple comparisons test. Error bars represent mean ± SD. Data in (b–g) are representative of three independent experiments.
To further confirm the involvement of the key site pairs N91/N309 and N104/N322 in B7H3 cell surface localization, the N91/104/309/322Q mutant and N91/104/309/322 mutant constructs were used for stable re-expression of B7H3 in human MDA-MB-231-B7H3KO and A549-B7H3KO tumor cell lines (Supplementary Fig. 1d). As expected, flow cytometric analysis indicated that the level of membrane-bound B7H3 was significantly decreased when N91/N309 and N104/N322 sites could not be N-glycosylated, with the level in these cells similar to that in cells reconstituted with the B7H3-8NQ mutant (Fig. 1c). There were no significant differences in the level of B7H3 mRNA among the groups reconstituted with the various mutant forms (fold change was not more than two times) (Supplementary Fig. 1e). As mouse B7H3 contains four Asn N-glycosylation sites at N91, N104, N189 and N215 in the singular IgV-IgC ___domain, the N91/104Q mutant (in which only the N91 and N104 sites could not be N-glycosylated) and N91/104 mutant (in which only the N91 and N104 sites could be N-glycosylated) constructs were then used for stable re-expression of B7H3 in mouse 4T1-B7H3KO, E0771-B7H3KO and B16F10-B7H3KO tumor cell lines (Fig. 1d, Supplementary Fig. 1f). Also, the B7H3 cell surface localization of N91/104 mutant was greater than that of N91/104Q mutant, and the level of membrane-bound in B7H3 N91/104Q mutant was similar to that in cells reconstituted with the B7H3-4NQ mutant, (Fig. 1e).
Canonical K48-linked polyUb chains are believed to be the principal signal for targeting substrates for degradation by the 26S proteasome20. We previously reported that the 26S proteasome facilitated the degradation of the K48-ubiquitinated B7H3-8NQ mutant18.Indeed, we also observed that the degradation rate of B7H3 protein in N91/104/309/322Q mutant was faster than that in N91/104/309/322 mutant in the presence of the protein synthesis inhibitor cycloheximide (CHX), and this degradation was reversed by treatment with the proteasome inhibitor MG132 (Fig. 1f). In the presence of MG132, the B7H3 in N91/104/309/322Q mutant exhibited a higher level of K48-linked ubiquitination than that in N91/104/309/322 mutant (Fig. 1g, Supplementary Fig. 1g, h). Together, these results suggest that the N-glycosylation of B7H3 at N91/N309 and N104/N322 sites is the major contributor to the cell surface localization and the stabilization of B7H3 protein.
The N-glycans attached to N91/302 and N104/322 sites of B7H3 trigger its ER-to-Golgi translocation
The stepwise process of N-linked glycosylation involves reactions in both the ER and Golgi apparatus. Newly synthesized proteins translocate from the ER to the ER-Golgi intermediate compartment (ERGIC), then to the Golgi, and finally to their destinations. During the intracellular transport of membrane glycoproteins, specific glycosylation in one compartment is closely related to the transfer of protein cargo to the next compartment21.To explore the molecular mechanism by which N-glycosylation at specific sites affects the stability of the B7H3 protein and its localization on the cell membrane, we performed immunofluorescence staining in MDA-MB-231-B7H3KO and A549-B7H3KO cell lines with stably re-expression of WT, 8NQ, N91/104/309/322Q or N91/104/309/322 B7H3 to determine the subcellular ___location of B7H3. We found that the subcellular localization of B7H3 in N91/104/309/322 mutant was located primarily on the cell membrane and within the perinuclear cisternae, which was similar to that in WT-B7H3 (Fig. 2a). In contrast, the subcellular localization of B7H3 in N91/104/309/322Q and 8NQ mutants were detected throughout the cytosol, with reduced signals on the membrane (Fig. 2a), implying that severe abnormalities in the collective glycan structure stemming from the N91/309 and N104/322 mutations lead to the intracellular accumulation of B7H3. KDEL is an ER localization signal that retains tagged proteins in the ER9. Thus, we co-transfected the B7H3 plasmid with fused mNeogreen at the N terminus and the KDEL plasmid with fused mRuby at the C-terminus. We found that more B7H3 proteins were colocalized with mRuby in N91/104/309/322Q and 8NQ mutants than that in WT and N91/104/309/322 mutant, indicating that the B7H3 proteins in N91/104/309/322Q and 8NQ mutants majorly were accumulated in the ER (Fig. 2b). In contrast, co-staining of B7H3 with ERGIC and a Golgi marker (TGN38) revealed that B7H3 was colocalized with the ERGIC and Golgi in the WT and N91/104/309/322 mutant cells, but not in the N91/104/309/322Q or 8NQ mutant cells (Fig. 2c, d). These data suggest that mutation of B7H3 at the N-glycosylation sites N91/302 and N104/322 induces ER accumulation of B7H3 by blocking its ER-to-Golgi translocation.
a Confocal microscopy of MDA-MB-231-B7H3KO and A549-B7H3KO cells reconstituted with human B7H3-WT and B7H3-NQ mutant cDNA. Green, Actin; Red, B7H3. Scar bar, 50 µm. b Confocal microscopy of MDA-MB-231-B7H3KO and A549-B7H3KO cells co-expressed with human mNegoGreen-B7H3-WT cDNA or mNegoGreen-B7H3-NQ mutant cDNA, and KDEL-mRuby cDNA. BF, bright field; Green, B7H3; Red, ER marker (KDEL). Scale bars, 20 μm. c Immuno- fluorescence staining with antibodies against B7H3(red) and ERGIC (green). Scale bars,100 μm. d Immunofluorescence staining with antibodies against B7H3 (red) and TGN38 (green) in the indicated cell lines. Scale bars,100μm. DAPI and Hoechst: nuclear counterstaining. Data are representative of three independent experiments.
The N-glycans attached to N91/302 and N104/322 sites of B7H3 block its ERAD
The aforementioned findings indicated that a lack of N-glycosylation of N91/302 and N104/322 induced B7H3 accumulation in the ER (Fig. 2), which is a common characteristic of ERAD substrates22. We then compared the binding partners of B7H3 in MDA-MB-231-B7H3KO cells with stably re-expression of WT, 8NQ, N91/104/ 309/322Q or N91/104/309/322 B7H3 by immunoprecipitation mass spectrometry (IP-MS) analysis (Supplementary Fig. 2a). As expected, pathway enrichment analysis based on these mass data showed that the binding of B7H3 with components of the ERAD pathway was increased in N91/104/309/322Q and 8NQ mutants (Fig. 3a). p97/VCP is a type II AAA+ adenosine triphosphatase essential for the retro-translocation of substrates from the ER to the cytosolic proteasome system, which is a critical step in ERAD23. The ATP-competitive inhibitor CB-5083 and allosteric inhibitor NMS-873 are the most advanced p97 inhibitors described24. Compared to that in WT control, NMS-873 and CB-5083 attenuated the degradation of B7H3 protein in N91/104/309/322Q and 8NQ mutants, as did the proteasomal degradation inhibitor MG132 (Fig. 3b). ERAD is accompanied by polyubiquitination. Indeed, the N91/104/309/322Q and 8NQ mutations led to marked increases in the total and K48-linked ubiquitination levels of B7H3 after p97/VCP inhibitor treatment (Fig. 3c). These results suggest that the reduction in the B7H3 level upon mutation of the N-glycosylation sites N91/302 and N104/322 is associated with the ERAD pathway.
a IP-MS analysis showing candidates with increased binding to 8NQ or N91/104/309/322Q B7H3 compared to WT or N91/104/309/322Q B7H3. b The indicated cell lines were treated with MG132, NMS-873 (2 μM) or CB-5083 (5 μM) in the presence of CHX at indicated intervals. c The indicated cell lines were treated with NMS-873 or CB-5083. Immunoprecipitation analysis of Flag-tagged B7H3 ubiquitination with the indicated antibodies. d The indicated cell lines were treated with MG132 for 6 h. Flag-tagged B7H3 WT or its NQ mutants were pulled down by anti-Flag beads in the indicated cell lines, followed by western blotting to detect HRD1 and SEL1L. e MDA-MB-231-B7H3KO-N91/104/309/322Q cells were transiently transfected with HRD1 siRNA or SEL1L siRNA for 48 h. Then the cells were treated with CHX at indicated intervals. The intensity of B7H3 protein was quantified using ImageJ software. f MDA-MB-231-B7H3KO-N91/104/309/322Q cells expressing sgRNAs targeting HRD1 or SEL1L were treated with CHX at indicated intervals. The intensity of B7H3 protein was quantified using ImageJ software. g MDA-MB-231-B7H3KO-N91/104/309/322Q cells expressing sgRNAs targeting HRD1 or SEL1L were treated with MG132 for 6 h. Immunoprecipitation analysis of Flag-tagged B7H3 ubiquitination with the indicated antibodies. The p-value in (e, f) was determined by a two-tailed unpaired Student’s t-test. Error bars represent mean ± SD. Data in (b–g) are representative of three independent experiments.
N-linked glycoproteins with abnormal glycan structures are recognized by ERAD substrate recognition factors, which are then recruited to ERAD E3 ligases, such as HRD1, through the adapter protein SEL1L22,25.We thus conducted co-immunoprecipitation (co-IP) to examine the interaction of candidates with B7H3. The co-IP results showed that HRD1 and SEL1L strongly bound to N91/104/309/322Q and 8NQ B7H3 (Fig. 3d, Supplementary Fig. 2b). We further confirmed the effect of HRD1 and SEL1L on the stability of B7H3 protein. HRD1 and SEL1L were knockdown by RNAi or sgRNAs in MDA-MB-231-B7H3KO and A549-B7H3KO cells with re-expression of N91/104/309/322Q B7H3. The CHX chase assay revealed substantial degradation of the N91/104/309/322Q B7H3 protein over a 6-h period, and this effect was largely reversed by knockdown of HRD1 or SEL1L (Fig. 3e, f, Supplementary Fig. 2c). Furthermore, knockdown of HRD1 or SEL1L clearly reduced the K48-linked ubiquitination of the N91/104/309/322Q B7H3 protein (Fig. 3g, Supplementary Fig. 2d), suggesting that the important roles of HRD1 and SEL1L during the ERAD of N91/104/309/322Q B7H3. These data suggest that mutation of B7H3 at the N91/302 and N104/322 N-glycosylation sites promotes its degradation via the ERAD pathway.
The N-glycans attached at N91/302 and N104/322 sites of B7H3 are important for its suppressive function of T cells in vitro
We then determined whether N-glycosylation of B7H3 at the key Asn sites regulates T cell function in vitro. First, carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeled human T cells were co-cultured with irradiated tumor cells in the presence of stimulation with anti-CD3. Flow cytometric analysis revealed that more CD4+ T and CD8+ T cells underwent growth arrest in re-expression of N91/104/309/322 or WT B7H3 than those re-expression of N91/104/309/322Q or 8NQ B7H3 (Fig. 4a). Then we evaluated the activation of T cells. Reconstituted N91/104/309/322 or WT B7H3 had less frequency of IFN-γ+CD8+ T cells than those reconstituted N91/104/309/322 B7H3 (Fig. 4b). To extend these findings, we loaded OVA peptide onto the irradiated mouse tumor cells, and co-cultured these tumor cells with OT-1 T cells. Also reconstituted mouse N91/104 or WT B7H3 had less frequency of IFN-γ+CD8+ T, perforin+CD8+ T and TNF+CD8+ T than those reconstituted N91/104Q or 4NQ B7H3 (Fig. 4c, d). Finally, we evaluated the T-cell response with a T-lymphocyte cytotoxicity assay. Compared to MDA-MB-231-B7H3KO cells reconstituted with N91/104/309/322Q or 8NQ B7H3, cells with re-expression of N91/104/309/322 or WT B7H3 were more resistant to killing by CD3/CD28-activated human T cells, as confirmed by the decreased percentage of cleaved caspase-3+ tumor cells (Fig. 4e). In addition, E0771-B7H3KO and B16F10-B7H3KO cells reconstituted with N91/104 or WT B7H3 were also more resistant to antigen-specific T-cell killing, as evidenced by the reduced percentage of cleaved caspase-3+ tumor cells and the increased proportion of surviving tumor cells by crystal violet assay (Fig. 4f, g, Supplementary Fig. 3a). In addition, we detected the non-immune regulatory function of B7H3 at the key N-glycosylation sites. The result showed that the mutation of key glycosylation sites in B7H3 had no effect on cell proliferation in vitro, as evidenced by growth curve analysis and colony formation assay (Supplementary Fig. 3b-3c). Moreover, no obvious differences were observed in the analysis of proliferation-related signaling pathways, such as PI3K/AKT/mTOR pathway and MAPK pathway (Supplementary Fig. 3d). Together, these data confirm that glycosylation of B7H3 at the N91/302 and N104/322 sites interferes with the proliferation and activation of T cells.
a Human T cells were labeled with CFSE, then co-cultured with the irradiated MDA-MB-231-B7H3KO cells as indicated in the presence of stimulation with anti-CD3. Anti-CD3-activated human T cells alone was used as positive control. Left, representative dot plots of in vitro proliferation of CD4+ T cells and CD8+ T cells measured by fluorescence-activated cell sorting (FACS) as CFSE dilution. Right, percentage of proliferating CD4+ T and proliferating CD8+ T (n = 3 biological independent samples). b Anti-CD3-activated human T cells were co-cultured with the irradiated MDA-MB-231-B7H3KO cells as indicated. Left, representative dot plots of in vitro activation of T measured by FACS. Right, percentage of IFN-γ+CD8+ T (n = 3 biological independent samples). c, d The irradiated E0771-B7H3KO and B16F10-B7H3KO cells as indicated were co-cultured with OT-1 T cells in vitro. Tumor cells were pulsed with OVA peptide. Left, representative dot plots of in vitro activation of T measured by FACS. Right, percentage of IFN-γ+CD8+ T, perforin+CD8+ T or TNF+CD8+ T (n = 3 biological independent samples). e The indicated MDA-MB-231-B7H3KO cell lines were co-cultured with CD3/CD28-activated human T cells. Left, representative dot plots of the cleavage of caspase-3 in tumor cells measured by flow cytometry. Right, percentage of cleaved caspase-3+ tumor cells (n = 3 biological independent samples). f, g The indicated E0771-B7H3KO and B16F10-B7H3KO cell lines were co-cultured with OVA peptide-activated OT-1 T cells. Tumor cells were pulsed with OVA peptide. Left, representative dot plots of the cleavage of caspase-3 in tumor cells measured by flow cytometry. Right, percentage of cleaved caspase-3+ tumor cells (n = 3 biological independent samples). The p-value in (a, c–e) was determined by one-way ANOVA with Tukey’s multiple comparisons test. The p-value in (b, f–g) was determined by one-way ANOVA with Dunnett’s multiple comparisons test. Error bars represent mean ± SD. Data are representative of three independent experiments.
N-glycosylation of B7H3 at the key asparagine residues inhibits antitumor T-cell responses in vivo
Next, we further evaluated the effect of the vital N-glycosylation sites of B7H3 on T cell-mediated anti-tumor activity in vivo. Mouse 4T1-B7H3KO cells reconstituted with N91/104Q B7H3 or N91/104 B7H3 were implanted in immunocompetent BALB/c mice. At the same time, we depleted CD8+ T or CD4+ T cells using systemic administration of an anti-CD8 antibody or anti-CD4 antibody, which was confirmed by FACS analysis of peripheral blood (Supplementary Fig. 4a). We observed that reconstituted N91/104 B7H3 tumors grew faster than the reconstituted N91/104Q B7H3 tumors, as confirmed by the xenograft volume curve and the tumor weights (Fig. 5a). Importantly, T cell depletion, especially CD8+ T cell depletion, reduced the differences in tumor growth rate between these two groups in vivo, suggesting a T cell dependent mechanism (Fig. 5a). Indeed, the tumors induced by the re-expression of N91/104 B7H3 not only had decreased total CD8+ T population, but also had fewer activated cytotoxic CD8+ T and CD4+ T cells (IFN-γ+CD8+ T, TNF+CD8+ T; IFN-γ+CD4+ T, TNF+CD4+ T) in their tumor-infiltrating lymphocyte (TIL) populations than those tumors with re-expression of N91/104Q B7H3 (Fig. 5b–d). And there were no significant differences in the infiltration levels of FOXP3+Treg cells (Supplementary Fig. 4b). However, no significant change in tumor burden was observed in the severe combined immunodeficient (SCID) mice between 4T1-B7H3KO-N91/104 group and 4T1-B7H3KO-N91/104Q group (Supplementary Fig. 4c). In addition, we obtained similar results when mouse E0771-B7H3KO cells reconstituted with N91/104Q B7H3 or N91/104 B7H3 were implanted in syngeneic C57BL/6 mice. Similarly, reconstituted N91/104 B7H3 tumors grew faster than the reconstituted N91/104Q B7H3 tumors in syngeneic C57BL/6 mice (Fig. 5e). Also the tumors derived from the re-expression of N91/104 B7H3 had fewer activated cytotoxic CD8+ T cells (IFN-γ+CD8+ T and TNF+CD8+ T) in their TIL populations than those tumors with re-expression of N91/104Q B7H3 (Fig. 5f, g).
a Tumor growth of the indicated 4T1-B7H3KO cells in female 8 week-old BALB/c mice (n = 7 mice per group). Tumor volumes (left) and tumor weights upon autopsy on day 24 (right) were calculated. Mice were injected with CD8 or CD4 depletion antibody on days -7, -3, 0, +3, +7, and +10 relative to tumor challenge on day 0. b–d FACS analysis of CD4+ T and CD8+ T cells (b), IFN-γ+ and TNF+ in CD8+ T cells (c), IFN-γ+ and TNF+ in CD4+ T cells (d) from the isolated TILs in (a) (right, n = 7 mice per group). Representative dot plots from a representative mouse for each group (left). e Tumor growth of the indicated E0771-B7H3KO cells in female 8 week-old C57BL/6 mice (n = 5 mice per group). Tumor volumes (left) and tumor weights upon autopsy on day 42 (right) were calculated. f-g FACS analysis of IFN-γ+ and TNF+ in CD8+ T cells from the isolated TILs in (e) (right, n = 5 mice per group). Representative dot plots from a representative mouse for each group (left). h The correlation between the expression of B7H3 and the normalized tumor infiltrated CD8+ T cells for TCGA cancer patients was analyzed using the R package “ESTIMATE”. NS, not significance. The p-value in (a–g) was determined by a two-tailed unpaired Student’s t-test. Error bars represent mean ± SD. The p-value in (h) was assessed using the two-sided Pearson correlation test. Data in (a–g) are representative of two independent experiments.
To further assess the clinical significance of B7H3 in tumors, we performed pan-cancer patients analysis including 33 human cancers from TCGA datasets based on RNA-seq data. High B7H3 mRNA expression was significantly associated with poor overall survival in tumor patients (Supplementary Fig. 4d). Consistent with the suppressing T-cell activation of B7H3 in vitro and in vivo, we observed negative correlations between B7H3 expression and normalized CD8+ T-cell infiltration in tumor patients (Fig. 5h).Taken together, these results suggest that N-glycosylation at the key sites is crucial for the ability of B7H3 to suppress T-cell activity in the tumor microenvironment.
Generation of a glycosylation-specific B7H3 antibody Ab-82
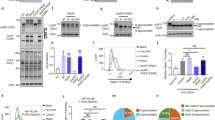
Glycosylated antigen-specific antibodies are valuable in cancer therapy26. Given the importance of glycosylation at the N91/302 and N104/322 glycosylation sites to the localization and function of B7H3, we generated monoclonal antibodies that preferentially recognize glycosylated B7H3. These antibodies against B7H3 were generated by immunizing rabbits with a recombinant human 4Ig-B7H3-His fusion protein with an extracellular ___domain (Met1-Thr461) to allow heavy glycosylation of B7H3. Using a single B cell antibody generation platform, we obtained >200 monoclonal antibodies secreted from the corresponding B cells, whose binding activities to B7H3 were validated by ELISA. To screen the monoclonal antibodies that preferentially bind to N91/302/104/322 B7H3 but not N91/302/104/322Q B7H3 by flow cytometry, we first selected monoclonal antibodies that effectively recognized wild-type B7H3 on the surface of live tumor cells. Then, the antibodies likely to recognize N91/302/104/322 B7H3 were screened in the MDA-MB-231-B7H3KO-N91/104/ 309/322 cell line. Finally, the antibodies fail to recognize N91/302/104/322Q B7H3 were screened in the MDA-MB-231-B7H3KO-N91/104/309/322Q cells with HRD1 deletion, for the ERAD pathway was blocked in these cells (Fig. 6a). Among these monoclonal antibodies tested, Ab-82 preferentially bound to N91/302/104/322 B7H3. Subsequently, we verified the specificity and sensitivity of Ab-82. Actually, Ab-82 significantly recognized WT B7H3 and N91/302/104/322 B7H3 on live tumor cells (Fig. 6b, c). Moreover, compared with that of a commercial anti-B7H3 antibody (Cat. 17-2769-42, eBioscience), the ability of Ab-82 to recognize N91/302/104/322Q B7H3 was not increased by HRD1 deletion (Fig. 6d). Moreover, the affinity of Ab-82 was determined by surface plasmon resonance analysis, and its dissociation constant (KD) was 3.00 × 10-9 M, indicating that Ab-82 has good affinity for the B7H3 antigen (Fig. 6e). When the human 4IgB7H3-His fusion protein was deglycosylated by PNGase F, Ab-82 was unable to recognize the completely deglycosylated B7H3 protein (Fig. 6f). We next enriched WT, 8NQ, N91/302/N104/322 and N91/302/N104/322Q B7H3 proteins through immunoprecipitation. The result showed that Ab-82 failed to recognize the 8NQ B7H3 protein, (Fig. 6g) and Ab-82 preferentially bound to N91/302/N104/322 B7H3 rather than N91/302/N104/322Q B7H3 (Fig. 6h). In addition, compared with the commercial anti-B7H3 antibody (Cat. 17-2769-42, eBioscience), Ab-82 preferentially recognized WT and N91/302/104/322 B7H3 rather than 8NQ and N91/302/104/322Q B7H3 (Fig. 6i). Taken together, these data confirm that Ab-82 preferentially recognize N91/302/N104/322 B7H3.
a Working flow chart for generation of glycosylated B7H3 antibody. Created in BioRender. Wq, Z. (2025) https://BioRender.com/p93p347. b Flow cytometry analysis by the different concentrations of Ab-82 to detect the B7H3 expression on the cell membrane in the indicated tumor cell lines (n = 3 biological independent samples). c Flow cytometry analysis by the different concentrations of Ab-82 to detect the B7H3 expression on the cell membrane in the N91/104/309/322 B7H3 reconstituted tumor cells (n = 3 biological independent samples). d Flow cytometry analysis by Ab-82 to detect the B7H3 expression on the cell membrane in the N91/104/309/322Q B7H3 reconstituted tumor cells with or without HRD1 deletion (n = 3 biological independent samples). e Binding affinity (KD) analysis of Ab-82 by Biacore T200. f Human B7H3-His fusion protein was de-glycosylated by PNGase F and used in Dot blot analysis by Ab-82. g, h Flag-tagged B7H3 WT or its NQ mutants were pulled down by anti-Flag beads, followed by western blotting to detect the binding ability of Ab-82 to them. i Flow cytometry analysis by anti-B7H3 and Ab-82 at the same concentration to detect the B7H3 expression on the cell membrane in the indicated cell lines (n = 3 biological independent samples). NS, not significance. The p-value in (b, c) was determined by one-way ANOVA with Dunnett’s multiple comparisons test. The p-value in (d, i) was determined by one-way ANOVA with Tukey’s multiple comparisons test. Error bars represent mean ± SD. Data in b-i are representative of three independent experiments.
Ab-82 exerts potent antitumor immunity by inducing B7H3 internalization and degradation
Subsequently, we evaluated the therapeutic efficacy of Ab-82 in a syngeneic animal model. We first generated mouse 4T1 cells expressing human (h) B7H3 (4T1-hB7H3) in 4T1-B7H3KO cell line reconstituted with human WT B7H3 (Supplementary Fig. 5a). In 4T1-hB7H3 cell-inoculated immunocompetent BALB/c mice, treatment with Ab-82 significantly reduced tumor growth in a dose-dependent manner compared with that in the vehicle control group, as confirmed by the xenograft volume curves and the tumor weight (Fig. 7a). No obvious toxicity was observed in the mice receiving the treatment. Ab-82 showed good safety profiles, as the levels of enzymes indicative of liver and kidney functions did not change significantly (Supplementary Fig. 5b). Additionally, Ab-82 treatment not only increased the total CD4+ T and CD8+ T populations, but also substantially increased granzyme B (GZMB) release in a dose-dependent manner (Fig. 7b, Supplementary Fig. 5c). And B7H3 expression in tumors decreased with increasing concentrations of Ab-82 (Fig. 7c, Supplementary Fig. 5d). To avoid the immunogenicity reactions caused by the introduction of human antigen and rabbit antibody in an immunocompetent mouse model, which may interfere the antitumor activity evaluation of Ab-82, we humanized Ab-82 by genetic engineering technology and successfully obtained a humanized antibody Hu-Ab-82, whose sequence was >95% homology with human antibody (Fig. 7d). The affinity of Hu-Ab-82 was measured by surface plasmon resonance analysis, and its dissociation constant (KD) was 3.20×10-9 M, suggesting Hu-Ab-82 also has good affinity for the B7H3 antigen like Ab-82 (Fig. 7e). We further examined the antitumor effect of Hu-Ab-82 in a human peripheral blood mononuclear cell (PBMC) engrafted humanized xenograft model (Supplementary Fig. 5e). In A549 cell-inoculated huPBMC-NOG-MHC I/II-2 KO mice, treatment with 5 mg/kg Hu-Ab-82 significantly inhibited tumor growth compared with that in the isotype control group, as confirmed by the xenograft volume curves and the tumor weight (Fig. 7f). Hu-Ab-82 still showed good safety profiles, as the levels of enzymes indicative of liver and kidney functions did not change significantly (Supplementary Fig. 5f). Moreover, Hu-Ab-82 treatment both increased the total human CD8+ T population and GZMB release in the tumor tissue (Fig. 7g, Supplementary Fig. 5g). The B7H3 expression in tumors also decreased after Hu-Ab-82 treatment (Fig. 7h, Supplementary Fig. 5h). These data indicate that Ab-82 can exert its effective anti-tumor activity by eliciting a local immune response characterized by the recruitment of cytotoxic T cells.
a Tumor growth of 4T1-hB7H3 cells in female 8 week-old BALB/c mice following treatment with Ab-82 antibody (n = 9 mice per group). The treatment protocol is summarized by the arrows. Tumor volume (left) and tumor weight upon autopsy on day 23 (right) were calculated. b, c Quantitative IHC analysis of mouse CD4, mouse CD8, mouse GZMB expression (b), and human B7H3 expression (c) (n = 9 mice per group). d Working flow chart for humanization of Ab-82 by using genetic engineering technology. e Binding affinity (KD) analysis of Hu-Ab-82 by Biacore T200. f Tumor growth of A549 cells in female 7 week-old huPBMC-NOG-MHC I/II-2 KO Mice following treatment with Hu-Ab-82 antibody (n = 8 tumors per group, 4 Mice bearing established tumors on both flanks). The treatment protocol is summarized by the arrows. Tumor volume (left) and tumor weight upon autopsy on day 26 (right) were calculated. g, h Quantitative IHC analysis of human CD8, human GZMB expression (g), and human B7H3 expression (h) (n = 8 tumors per group, 4 Mice bearing established tumors on both flanks). i The indicated cells were treated by Ab-82 at different concentrations for 48 h, followed by western blotting to detect the total B7H3 protein level with anti-human B7H3. j Cell surface B7H3 was bound by Ab-82 for 30 min or not, followed by flow cytometry with commercial anti-B7H3 (Cat. 17-2769-42, eBioscience) (n = 3 biological independent samples). k A549 cells were treated by Ab-82 at different concentrations for 24 h, followed by flow cytometry with commercial anti-B7H3 (Cat. 17-2769-42, eBioscience) (n = 3 biological independent samples). l Cell surface B7H3 was labeled by Ab-82 and internalized for 5 min, followed by flow cytometry to measure B7H3 level (n = 3 biological independent samples). m Cell surface B7H3 was labeled by Ab-82 and internalized at different time points in the presence of 50uM primaquine, followed by flow cytometry to measure B7H3 level (n = 3 biological independent samples). n Cells were treated by 20 μg/ml pHrodo red-labeled Ab-82 for 24 h, followed by immunofluorescence analysis with LSM880.BF, bright field. o Cells were treated by 20 μg/ml pHrodo red-labeled Ab-82 for different time, followed by flow cytometry to measure Ab-82 internalization level (n = 3 biological independent samples). p The indicated cells were treated by 20 μg/ml pHrodo red-labeled Ab-82 for different time, followed by flow cytometry to measure Ab-82 internalization level (n = 3 biological independent samples). NS, not significance. The p-value in (a–c) and (f–h) and (o) was determined by one-way ANOVA with Dunnett’s multiple comparisons test. The p-value in (p) was determined by one-way ANOVA with Tukey’s multiple comparisons test. Error bars represent mean ± SD. The data in (a–h) are representative of two independent experiments. The data in (i–p) are representative of three independent experiments.
Then the potential antitumor mechanisms of Ab-82 were explored. The aforementioned findings indicated that the B7H3 expression in tumors decreased after Ab-82 treatment in vivo. To confirm this, we treated tumor cells with Ab-82 and observed changes in B7H3 expression by immunoblotting and flow cytometry in vitro. Actually, the levels of total B7H3 protein were reduced in a dose-depended manner (Fig. 7i). Meanwhile, commercial anti-B7H3 (Cat. 17-2769-42, eBioscience), whose recognition ability would not be interfered by Ab-82 (Fig. 7j), was used to detect cell membrane B7H3 after Ab-82 treatment. The results showed that B7H3 level on cell membrane also reduced after Ab-82 treatment (Fig. 7k, Supplementary Fig. 6a). As a few antibodies will induce antigen internalization, we speculated that Ab-82 could mediate B7H3 internalization through antigen-antibody interaction and might facilitate the degradation of B7H3 after entering cells. Therefore, we examined whether Ab-82 could promote B7H3 internalization using a classic internalization assay based on a temperature change (Supplementary Fig. 6b). Predictably, the amount of Ab-82-bound B7H3 on the cell membrane decreased after the temperature change (Fig. 7l, Supplementary Fig. 6c), suggesting that Ab-82 could mediate B7H3 internalization at the cell culture temperature. Moreover, incubation with primaquine simultaneously, an inhibitor of endocytic recycling, did not increase loss of B7H3 from the cell surface mediated by Ab-82, suggesting that B7H3 would not recycle to cell surface after internalization for a certain time (Fig. 7m, Supplementary Fig. 6d). To visualize the internalization process, we treated cancer cells with pHrodo red-labeled Ab-82 for 24 h and observed B7H3 internalization by immunofluorescence staining. Clearly, pHrodo red-labeled Ab-82 successfully entered tumor cells and exhibited bright fluorescence (Fig. 7n). We also measured fluorescence quantitively at different time points by flow cytometry and found the amount of internalized pHrodo red-labeled Ab-82 increased in a time-dependent manner (Fig. 7o, Supplementary Fig. 6e). In addition, Ab-82 preferentially mediated WT and N91/309/N104/322 B7H3 internalization rather than 8NQ or N91/309/N104/322Q B7H3 internalization (Fig. 7p, Supplementary Fig. 6f). Together, these data suggest that Ab-82, particularly targeting B7H3 at N91/302 and N104/322 sites, induces potent antitumor immunity by the internalization and degradation of B7H3 on the tumor cell membrane.
Discussion
Glycosylation is important for the protein function and stability, and blocking or targeting the protein glycosylation may be an important supplement for tumor immunotherapy27,28. In this study, we demonstrate the essential biological functions and physiological role of N-glycans attached to the well-established immune checkpoint molecule B7H3. Among the four pairs of Asn N-glycosylation sites on human B7H3 in tumor cells, N91/309 and N104/322 sites are essential for proper B7H3 protein trafficking to the cell surface membrane and structural stabilization, which prevents B7H3 from being degraded via the ERAD pathway. We also provide evidence that the glycosylation of N91/309 and N104/322 is essential for the ability of B7H3 to inhibit T-cell proliferation and activation. More importantly, we develop the glycosylation-specific B7H3 antibody Ab-82, which preferentially targets N-glycosylated B7H3 at N91/309 and N104/322, and demonstrate that Ab-82 exerts favorable effects on antitumor immunity by inducing B7H3 internalization and degradation.
Over the past decade, most studies have indicated that B7H3 plays an inhibitory role in the tumor microenvironment. It is reported that blocking B7H3 increased CD8+ T cell infiltration and function, ultimately leading to subsequent tumor inhibition16,29,30. In addition, no significant correlation was observed between B7H3 expression and Foxp3+ Treg cells in the breast cancer and prostate cancer model31,32. B7H3 also promotes M2 macrophage polarization via the B7H3-CCL2-CCR2 axis and JAK2-STAT3 pathway33,34. EBV infection promotes the upregulation of B7H3, which inhibits NK cell-mediated antitumor function in nasopharyngeal carcinoma35. Furthermore, conditional knockout of CD276 in an esophageal squamous model reduces neutrophil extracellular trap (NET) formation via the CXCL1-CXCR2 axis and enhances NK cell function36. The complex interactions between B7H3 and multiple immune cells makes it an appealing target for cancer immunotherapy37,38. B7H3 is a type I transmembrane protein and is heavily glycosylated at Asn sites N91/309, N104/322, N189/407, and N215/433 in human tumor cells18. However, it remains unclear whether all glycans on the B7H3 protein contribute to its function and proper trafficking to the cell surface membrane. N-glycosylation is a highly conserved glycan modification essential for proper protein folding and transport to cell membrane39. It also plays a critical role in regulating the function of ERAD40. AMPK phosphorylates the S195 residue of PD-L1, which results in abnormal glycosylation of PD-L1, blocking its ER-to-Golgi translocation and subsequently leading to its ER accumulation and ER-associated degradation41. In this study, we demonstrated that N-glycosylation of the key Asn residues N91/309 and N104/322 of B7H3 played a decisive role in inhibiting T cell activation by affecting the stability and localization of the B7H3 protein on the cell membrane surface. We also identified glycosylation at N91/309 and N104/322 of B7H3 as a critical event that affected the correct folding of B7H3 protein, and determined whether it was transported from the ER to the Golgi to the cell membrane and degraded by ERAD. According to these results, the N91/309 and N104/322 glycosylation site pairs in B7H3 are closely connected with both its stability and function. Although B7H3 is an important immune checkpoint molecule, its lack of known receptors makes it difficult to target pharmacologically14.Therefore, the identification of the key N-glycosylation sites of B7H3 may provide further guidance for the development of blocking antibodies targeting B7H3 as well as for the search for its receptors.
Abnormally altered glycoproteins can serve as antigen targets for antibodies that specifically recognize tumor cells. Glycosylation-specific antibodies targeting glycosylated antigens are promising potential therapeutic agents for cancer. Researchers have successfully developed MUC1 TR glycopeptides with cancer-associated O-glycosylated forms, such as the Tn and STn glycoforms, and these glycopeptides is found to elicit the strongest antibody response to MUC1 expressed in breast cancer cell lines in mice42. Recently, anti-Tn-MUC1 CAR-T cells that recognized the cancer-associated Tn glycoform of MUC1 successfully control tumor growth in xenograft models43. The neutralizing monoclonal antibody STM108, which can specifically recognize the PD-L1 glycosylated at N192 and N200, blocks the PD-L1/PD-1 interaction and promotes PD-L1 internalization and degradation, and STM108-ADC has shown potent activity against various solid tumors44. The monoclonal antibody STM418 specifically targets N58-glycosylated PD-1, exhibiting greater binding affinity for PD-1 than do FDA-approved PD-1 antibodies, potently inhibiting PD-L1/PD-1 binding and increasing antitumor immunity45,46. In this study, we focus on the development of a specific antibody targeting glycosylated B7H3 to further explore the therapeutic potential of our findings. Here, we developed the monoclonal antibody Ab-82 by single B cell antibody generation technology, and this antibody preferentially recognized the B7H3 glycosylated at N91/309 and N104/322. More importantly, Ab-82 treatment, including humanized Ab-82, inhibited tumor growth in vivo by eliciting a local immune response characterized by the recruitment of cytotoxic T cells. Moreover, because Ab-82 preferentially recognizes the glycosylated N91/309 and N104/322 sites of B7H3, it exhibits specificity and affinity without significant cytotoxicity in normal cells, suggesting that its clinical application would be relatively safe. Interestingly, we found that Ab-82 promoted B7H3 internalization and degradation, and exhibited greater binding affinity for B7H3 than did a commercial anti-B7H3 monoclonal antibody. These data indicate that Ab-82-induced B7H3 internalization occurs in an N91/309- and N104/322-glycosylation-dependent manner.
Over the years, drug research and clinical trials targeting B7H3 have been carried out continuously. Chimeric antigen receptor (CAR) T-cells targeting B7H3 is deemed safe and tolerated47,48. Considering the difficulty of generating blocking antibodies and the failure of ADCC and double-target antibodies in clinical trials, B7H3 ADCs have shown great clinical value as ADC technology has matured49. At present, the most advanced B7H3 targeted drug in clinical trials is a B7H3 ADC, DS-7300, which successfully entered phase III clinical trials in January of 202450. The ideal target antigen is effectively internalized after binding of the corresponding antibody, allowing the ADC-antigen complex to enter the target cell and release the payload51,52. Although the exact mechanism of B7H3 internalization by Ab-82 needs to be further explored, Ab-82 is a new monoclonal antibody that effectively recognizes glycosylated B7H3 and efficiently mediates its internalization. This study is expected to provide a new drug-loading framework for research on ADCs targeting B7H3.
Overall, our findings shed light on the functional significance of a posttranslational modification, specifically N-linked glycosylation, of B7H3 in tumor cells and highlight the rationale of targeting glycosylated B7H3 as a potential approach to improve the efficacy of immunotherapy.
Methods
Cell culture and compounds
Human HEK-293T (Cat. CRL-3216, ATCC), MDA-MB-231(Cat. HTB-26, ATCC), A549 (Cat. CCL-185, ATCC), and mouse B16F10 (Cat. CRL-6475, ATCC), 4T1(Cat. CRL-2539, ATCC) and E0771(Cat. CRL-3461, ATCC) cells, were maintained in Dulbecco’s modified Eagle’s medium (DMEM, Cat. 12491015, Gibco) supplemented with 10% fetal bovine serum (FBS, Cat. A5670701, Gibco) and 1% penicillin/streptomycin at 37 °C under 5% CO2. Compounds MG132 (Cat. S2619), NM-S873(Cat. S7285) and CB-5083(Cat. S8101) were obtained from Selleck Chemical. Cycloheximide (CHX, Cat. BS168A, Biosharp) was obtained commercially. PNGaseF (Cat. P0704S) was obtained from New England Biolab. Primaquine (Cat. T0850-500) was obtained from TargetMol. pHrodo Red (Cat. P36011) was obtained from Thermo Fisher Scientific. HEPES (Cat. V900477) was obtained from Sigma.
Plasmids
Human full-length B7H3 and mouse B7H3 cDNA (with fused C-terminal FLAG tag) was subcloned into pCDH-CMV-MCS-EF1 vector (Cat. CD510B-1, System Biosciences) lentiviral expression vectors to establish stably transfected cells or for transient transfection. Using the human pCDH-B7H3-WT-Flag plasmid as a template, a series of human B7H3-Flag mutant constructs were generated (Supplementary Table 1). Using the mouse pCDH-B7H3-WT-Flag plasmid as a template, a series of mouse B7H3 mutant constructs were developed (Supplementary Table 2). For immunofluorescence experiments, human B7H3-WT-Flag and B7H3-Flag mutant constructs with fused mNeogreen at the N terminus were developed. In addition, the ER marker KDEL plasmid with fused mRuby tag at the C-terminus was developed. All plasmids were generated by using the ClonExpress II One Step Cloning Kit (Cat. C112-01) from Vazyme, and all mutations were verified by DNA sequencing.
CRISPR–Cas9-mediated gene disruption
To establish B7H3-deficient cell models, human B7H3 CRISPR/Cas9 KO Plasmid (Cat. sc-402032) and mouse B7H3 CRISPR/Cas9 KO Plasmid (Cat. sc-430440) were purchased from Santa Cruz Co. All of these plasmids were transfection-ready purified DNA plasmids. According to the manufacturer’s instruction, these CRISPR/Cas9 KO plasmids were transiently transfected into HEK-293T, MDA-MB-231, A549, 4T1, E0771 and B16F10 cell lines using Lipofectamine 2000 (Cat. 11668030, Invitrogen). After 48 h, puromycin positive cells were dissociated and seeded at subcloning density. B7H3-knockout clones were isolated by single-cell dilution cloning from the positive polyclonal sgRNA-transduced populations. All knockout clones were identified by immunoblot. Control CRISPR/Cas9 Plasmid (Cat. sc-sc-418922) from Santa Cruz Co. was used as a negative control. The knockout cells were identified by immunoblot.
For CRISPR-Cas9-mediated HRD1 and SEL1L knockout, the specific 4 sgRNA sequences targeting human HRD1 gene (Supplementary Table 3) were cloned to LentiCRISPR V2 plasmid (Cat. 52961, Addgene), the specific 4 sgRNA sequences targeting human SEL1L gene (Supplementary Table 3) were cloned to LentiCRISPR V2 plasmid. The packaging plasmids were co-transfected with LentiCRISPR V2-HRD1 sgRNAs or LentiCRISPR V2-SEL1L sgRNAs into HEK293T cells, and viral particles were harvested at 48 h post-transfection. The indicated tumor cells were infected with viruses for 24 h in the presence of polybrene (8 μg/ml), and stable cells were subsequently selected by puromycin for 3 days. LentiCRISPR V2 plasmid was used as a negative control53. The knockout cells were identified by immunoblot.
Quantitative Real-Time PCR
Total RNA was isolated by TRIzol according to the manufacturer’s instructions. And reverse transcription was performed using a PrimeScript RT Reagent Kit with gDNA Eraser (Cat. RR047D, Takara). Quantitative Real-Time PCR (qRT-PCR) was conducted using ChamQ SYBR qPCR Green Master Mix (Cat. Q311-03, Vazyme Biotech Co.), and run with a Light Cycler 480 instrument. The relative amount of target gene mRNA was normalized to GAPDH. These primers for B7H3 were listed in Supplementary Table 3.
Generation of stable cells using lentiviral infection
For reconstituted with B7H3 mutants in human B7H3-deficient cell lines, packaging plasmids were co-transfected with human B7H3 mutant plasmids into HEK293T cells. For reconstituted with B7H3 mutants in mouse B7H3-deficient cell lines, packaging plasmids were co-transfected with mouse B7H3 mutant plasmids into HEK293T cells. The viral particles were harvested at 48 h post-transfection. The indicated cells were infected with viral particles for 24 h in the presence of polybrene (8 μg/ml), and stable cell lines were subsequently selected by puromycin for 3 days54,55.
siRNA transfection
The cells were seeded into six-well plate the day before transfection. Transfection of siRNA was performed with lipofectamine RNAimax (Cat. 13778030, Invitrogen) according to the manufacturer’s instruction. Oligonucleotide sequence of siRNAs of SEL1L and HRD1 were listed in Supplementary Table 3.
Animal treatment protocol
Female C57BL/6 mice (Cat. GDMLAC-07) and BALB/c mice (Cat. GDMLAC-03) were obtained from Guangdong Medical Laboratory Animal Center and were 8 week-old. Female 4 week-old BALB/c nude mice (Cat. GDMLAC-01) were obtained from Guangdong Medical Laboratory Animal Center. To evaluate the effect of N-glycosylation of B7H3 in vivo, tumor xenografts were established by 1 × 105 indicated 4T1 in BALB/c and BALB/c SCID mice, and 5 × 105 indicated E0771 cells in C57BL/6 mice. For T cell depletion, BALB/c mice were respectively treated with anti-mouse CD4 monoclonal antibody GK1.5 (Cat. A2101, Selleck), anti-mouse CD8α monoclonal antibody 2.43 (Cat. A2102, Selleck), and Rat IgG2b isotype control (Cat. A2116, Selleck) on days -7, -3, 0, +3, +7, and +10 relative to tumor challenge on day 0. Each depleting antibody was administrated i.p. at 100 μg per mouse, and depletion effect was confirmed by flow cytometry. To evaluate the antitumor effect of Ab-82 in vivo, tumor xenografts were established by 5 × 105 4T1-hB7H3 cells in BALB/c mice. After tumor implantation, the mice were randomly divided into four treatment groups. Ab-82 was intravenously injected on the indicated day. To evaluate the antitumor effect of Hu-Ab-82 in vivo, Female 7 week-old huPBMC-NOG-MHC I/II-2 KO mice (Cat. 801-1063) were obtained from Beijing Vital River Laboratory Animal Technology Co. Five days after intravenous inoculation with human PBMCs in NOG-MHC I/II-2 KO mice, tumor xenografts were established by 5 × 106 A549 cells in both flanks of each mouse. After tumor implantation, the animals were randomly divided into three treatment groups. Hu-Ab-82 was intravenously injected on the indicated day. All laboratory mice are housed in abnormally hygienic specific-pathogen-free (SPF) barrier facilities under a 12 h light-dark cycle, with ad libitum access to food and water in accordance with animal care regulations, while experimental and control animals are bred separately. All mice were monitored for tumor formation, with tumor length and width measured twice per week (for mice implanted with 4T1 and A549 tumor cells) or once per week (for E0771 tumor cells) using calipers. Tumor volume and body weight were recorded. Volumes were calculated using the formula: 0.5 × a × b² (in millimeters), where a represents the length and b the width. Mice were euthanized at the following humane end points: a total tumor volume exceeding 1.6 cm3, tumor ulceration, necrotic tissue or a weight loss >10% of the initial weight. Euthanasia was performed via cervical dislocation following isoflurane anesthesia. After mice were sacrificed, the tumor tissues were excised and weighed, and the peripheral blood was collected to detect liver and kidney function. All procedures involving mice and experimental protocols were approved by Institutional Animal Care and Use Committee (IACUC) of Sun Yat-sen University Cancer Center.
T lymphocytes preparations
T lymphocytes were isolated from human peripheral blood lymphocytes of healthy donors by depletion of non-T lymphocytes using a Pan T Cell Isolation Kit (Cat. 130-096-535, Miltenyi Biotec). Isolated human T lymphocytes cells were then maintained in T cell culture medium (RPMI 1640, 10% FBS, 2% PSG, 1% MEM Nonessential Amino Acids, 1% Sodium Pyruvate) and Interleukin-2 (Cat. 200-02, Pepro Tech) in the pre-coated plate with anti-CD3(Cat. 300313, BioLegend) and anti-CD28 (Cat. 302913, BioLegend). OT-1 T cells were isolated from spleens of OT-I TCR transgenic mice56. Isolated OT-1 T lymphocytes cells were maintained in OT-1 T cell culture medium (RPMI 1640, 10% FBS,20 mM HEPES, 1 mM Sodium Pyruvate,2 mM L-Glutamine,0.05 mM 2-Mercaptoethanol) with murine Interleukin-2(Cat. 2121220, Pepro Tech) and ovalbumin peptide. All procedures were approved by the medical ethics committee of Sun Yat-sen University Cancer Center.
T-cell killing assay
For human T cell killing assay, the prepared tumor cells and CD3/CD28-activated T cells were seeded into U-shaped 96-well microtiter plates at a ratio of 1:10 ~ 1:20. For activated caspase-3 assay, FITC active caspase-3 apoptosis kit (Cat. 550480) was purchased from BD Biosciences. After incubation for 6-10 h, the cells were fixed and permeabilized, then stained with FITC-conjugated activated caspase-3 antibody. Anti-APC-CD3 was used to label CD3- tumor cells at the same time57. For mouse T cell killing assay, the prepared tumor cells were pulsed with OVA(SIINFEKL) peptide for 4 h at 37 °C. Then the tumor cells were washed and plated into 24-well plates along with OVA peptide -activated OT-1 T cells at a ratio of 1:1 ~ 1:2. After incubation for 6–8 h, the cells were fixed and permeabilized, then stained with FITC-conjugated activated caspase-3 antibody. For cell survival assay, the cells were washed and fixed with 4% paraformaldehyde for 15 min, 0.005% crystal violet solution is used to visualize the living cancer cells. Data analysis was performed using ImageJ software53,58.
Flow cytometry analysis
For cell surface staining, cell suspensions were washed and stained with indicated fluorescent labeled antibodies for 30 min on ice and washed with PBS. For TILs isolation, tumors were cut, minced, followed by incubation with 0.05 mg/ml Type IV Collagenase (Sigma) and 0.05 mg/ml Type I Deoxyribonuclease (Sigma) for 60 min at 37 °C with gentle shaking. After passing through a 70 μm filter, lymphocytes were purified from the interface of mouse Ficoll gradient centrifugation (Multi Sciences). For intracellular staining, the cells were sorted for fixation and permeabilization using the Cytofix/CytoPerm Fixation/ Permeabilization kit (Cat. 554714, BD Bioscience). For detecting the proliferation T cell, human T lymphocytes were labeling with CFSE and activated in vitro with anti-CD3 pre-coated U-96-plates in present of 100 U/ml IL2. When needed, irradiated (100 Gy) tumor cells were added in coculture system at the indicated ratio in triples. All flow cytometry analyses were conducted on CytoFLEX (Beckman), and the data were analysed using FlowJo software (FlowJo Vx.10.0.7), Kaluza Analysis software (Kaluza Analysis Version 2.1), or CytExpert software (CytExpert 2.4) according to manufacturers’ instructions. All antibodies used for flow cytometry analysis were listed in Supplementary Table 4.
Immunoblot and immunoprecipitation
For immunoblot cells were harvested and lysed in 1xSDS sample buffer or RIPA buffer (Cat. 9806 s, Cell Signaling Technology) adding 1 mM phenylmethanesulfonyl fluoride immediately before use. A volume of 25–50 μg of total proteins was separated by SDS-PAGE transferred to PVDF membrane. Quantification of Western Blots was performed using ImageJ software. For immunoprecipitation, cells were collected and lysed in Pierce IP Lysis Buffer (Thermo Fisher Scientific) supplemented with Complete Protease Inhibitor Cocktail (Roche). Lysates were incubated with Anti-Flag Affinity Gel (Cat. B23102, Selleck) at 4 °C on the rotator for about 6 h. The beads bound with target proteins were washed with same lysis buffer and eluted with 3X SDS sample buffer. Antibodies used in immunoblot and immunoprecipitation were listed Supplementary Table 5.
Immunohistochemical staining
The tumor mass was isolated from mice and immersed with formalin and embedded into paraffin block. Sections were submerged into sodium citrate buffer or EDTA citrate buffer and subjected to antigen retrieval. Then the slides were incubated with the primary antibody at 4 °C overnight. Normal mouse/rabbit IgG as negative controls were used to ensure specificity. Then the slides were treated by HRP polymer conjugated secondary antibody for 30 min and developed with diamino-benzidine solution (ZSGB-Bio). Nuclei were counterstained with hematoxylin59. Image acquisition was performed using a KFBIO Digital Pathology Slide Scanner. The percentage of CD8 +, CD4+ and GZMB + cells within the tumor cell nest was quantified using the pathology image analysis software HALO (Indica Labs)60. The number of positive cells were calculated and expressed as percentage (percentage of positive cells among total cells). For evaluation of B7H3 staining, we adopted a staining index by multiplying the score for the percentage of positive tumor cells by the intensity score, which obtained as the intensity staining (0, no staining; 1, weak; 2, moderate; 3, strong) and the percentage of positive cells (0, <10%; 1, 10–40%; 2, 41–80%; 3, >80%)18. IHC staining results were reviewed independently by two pathologists in a blinded fashion. The detailed antigen retrieval solutions for different antibodies were listed in Supplementary Table 6.
Liquid chromatography-mass spectrometry (LC-MS)
Protein sample preparation for LC-MS follows the immunoprecipitation experimental methods. The beads bound with target proteins were stored at -80 °C until further proteomic analysis. The samples were extracted and evaporated for LC-MS analysis at the Beijing Omics Biotechnology Co., Ltd. We manually collected an ERAD gene set for mass data analysis22, as listed in Supplementary Table 7.
Immunofluorescence
For Immunofluorescence staining, the samples were stained with primary antibodies against ERGIC-3 (Cat. sc-514611, Santa Cruz,1:100), TGN38 (Cat. sc-166594, Santa Cruz,1:100) and B7H3 (Cat. ab312274, abcam,1:100) diluted in 4% BSA at 4 °C overnight. The cells were washed, and followed by a fluorescently labeled secondary antibody Alexa Fluor 488-conjugated antibodies against mouse IgG (Cat. A-11001, Thermo Scientific, 1:2000), and developed with DAPI (Cat. D9542, Sigma, 1:10000). The confocal microscope (LSM880; Carl Zeiss) was used for image analysis.
Generation of Ab-82 and Hu-Ab-82 antibody
Japanese white rabbit was immunized by human 4Ig-B7H3-His fusion protein with an extracellular ___domain (Met1-Thr461) (Cat. 11188-H08H, Sino Biological) for five times, then its spleen was isolated to obtain B cells. Monoclonal B7H3 antibodies were gained by single B cell antibody generation technology according to ELISA assay (ABclonal, China). Ab-82 was selected from them according to the working flow chart for generation of glycosylated B7H3 antibody. Hu-Ab-82 was obtained after the humanization of Ab-82 using genetic engineering technology (HOEKBIO, China).
Characteristic identification of Ab-82 by flow cytometry
For detecting the recognition ability of Ab-82, tumor cells were labeled by Ab-82 at 4 °C for 30 min, then stained by Alexa Fluor-647-conjugated anti-rabbit secondary antibody (Cat. A21244, Invitrogen) at 4 °C for 30 min. To explore whether Ab-82 influenced B7H3 binding of commercial anti-B7H3(Cat. 17-2769-42, eBioscience), tumor cells were incubated with Ab-82 at 4 °C for 30 min, then were stained with commercial anti-B7H3 at 4 °C for 30 min. To explore whether Ab-82 influenced B7H3 level on tumor cell surface, tumor cells were incubated with Ab-82 at 37 °C for 24 h, then were stained with commercial anti-B7H3 at 4 °C for 30 min.
Antibody affinity detection by surface plasmon resonance
The binding kinetics for Ab-82 and Hu-Ab-82 were determined by Surface Plasmon Resonance (SPR) using a Biacore T200 (GE Healthcare Life Sciences) equipped with a Series S NTA sensor chip. The human B7H3-His protein was used as the ligand and Ab-82/Hu-Ab-82 at different concentrations were used as the samples. The dissociation constant (KD) was calculated by Biacore T200 analysis software.
Dot blot assay
Human 4Ig-B7H3-His fusion protein (Cat. 11188-H08H, Sino Biological) with or without deglycosylation were diluted by PBS and spotted to PVDF membrane. The PVDF membrane was dried for 1 h and soaked in TBST with 5% Skimmed milk powder at room temperature for 1 h. Then the PVDF membrane was incubated by anti-B7H3 or Ab-82 at 4 °C overnight and subsequently incubated with HRP-conjugated secondary antibody. Finally, the PVDF membrane was incubated with ECL reagent and exposed to X-ray film in a dark room45.
Internalization assay based on temperature change
Cells were collected with TrypLE Express (Cat. 12604013, GIBCO), and RPMI/5% FCS/30 mM HEPES (Cat. V900477, Sigma) was used for all staining and wash steps. Cell surface B7H3 was labeled with unconjugated Ab-82 for 1 h on ice and then washed to remove unbound antibody. Cells were resuspended in RPMI medium and divided to a baseline part and an internalization part. The baseline part was kept on ice, while the internalization part was incubated at 37 °C in a water bath for 5 min. Then the internalization part was immediately diluted in ice-cold PBS to stop further internalization. The remaining surface-bound Ab-82 were stained with Alexa Fluor-647-conjugated anti-rabbit secondary antibody (Cat. A21244, Invitrogen) for 30 min on ice. Samples were conducted on cytoFLEX flow cytometer and analysed by FlowJo software61.
Internalization assay based on pHrodo Red
Antibodies were labeled with pHrodo Red (Cat. P36011, Invitrogen) as described by the manufacturer. To validate the label effect, tumor cells were treated by pHrodo Red-labeled antibodies for 24 h, then the B7H3 internalization was observed by immunofluorescence. To monitor a dynamic internalization of antibodies on the live cell surface, tumor cells were incubated with the pHrodo Red-labeled antibodies for different time. Then the level of pHrodo Red-labeled antibodies inside the cells was conducted on cytoFLEX flow cytometer and analysed by FlowJo software44.
Recycling assay based on temperature change
Cell surface B7H3 was labeled with unconjugated Ab-82 for 1 h on ice as described. Cells were treated with primaquine (Cat. T0850, TargetMol) incubated at 37 °C to allow internalization of antibody-labeled B7H3. The remaining surface-bound Ab-82 were stained with Alexa Fluor-647-conjugated anti-rabbit secondary antibody (Cat. A21244, Invitrogen) for 30 min on ice. Samples were conducted on cytoFLEX flow cytometer and analysed by FlowJo software61.
Cell proliferation
For MTT assay, MTT was added to indicate cells and incubated for 4 h at 37 °C. After removing the solution, 150 μl DMSO was added, and absorbance was measured at 570 nm (reference: 650 nm) using a SpectraMax Plus 384 (MD). For the colony formation assay, cells were seeded in six-well plates and cultured in complete DMEM for 1 week. Colonies were fixed, stained with crystal violet, and those with over 50 cells were counted.
Analyses of TCGA data
Patients for 33 human cancers from TCGA datasets has corresponding B7H3 expression levels were used for analysis. The TCGA Pan-Cancer cohort TPM format RNA-seq data and aligned survival information were downloaded using the R package “TCGABiolinks”. Statistical analysis was performed using R software v4.2.1 and visualization was performed using the R package “ggplot2”. To assess the clinical outcomes delineated by expression of B7H3, median of expression values were calculated, overall survival curves were performed using Kaplan-Meier method. The association between the expression of B7H3 and the infiltration of CD8+ T cells was explored using the R package “ESTIMATE”, which infers the immune and stromal components of the tumor microenvironment based on gene expression profiles. To facilitate comparison across samples, the raw immune scores generated by ESTIMATE were normalized to a range of 0–100. This normalization step ensured that immune scores were dimensionless and rescaled, enabling direct comparisons between samples. To estimate the infiltration of CD8+ T cells, we calculated a proxy by dividing the expression of the CD8A gene by the normalized immune score. This ratio reflects the relative infiltration of CD8+ T cells while accounting for the overall immune activity within the tumor microenvironment. This approach was employed to investigate the potential negative association between B7H3 expression and CD8+ T cell infiltration.
Statistical analysis
Statistical analyses were conducted using GraphPad Prism 9.5.1 (GraphPad, La Jolla, CA, USA). The intensity of B7H3 protein was quantified using ImageJ software. The p-values of Kaplan-Meier overall survival curves for TCGA cancer patients were assessed using the two-sided log-rank test. The relationship between the expression of B7H3 and the normalized tumor infiltrated CD8+ T cells was assessed using the two-sided Pearson correlation test. The results presented as the mean ± SD were analysed by an unpaired Student’s t-test, or one-way ANOVA with Dunnett’s multiple comparisons test, or one-way ANOVA with Tukey’s multiple comparisons test using GraphPad Prism. All the statistical tests were two-sided, p < 0.05 was considered statistically significant.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The Pan-Cancer Atlas was downloaded from The Cancer Genome Atlas (TCGA) using the R package “TCGABiolinks”.The source data underlying Fig. 1, Fig. 3–7, Supplementary Fig. 1–5 are provided as a Source Data file. Specific data p-values are also included within the Source Data file. The LC-MS/MS raw data of the identification of the binding partners of B7H3 in MDA-MB-231-B7H3KO cells with stably re-expression of WT, 8NQ, N91/104/309/322Q or N91/104/309/322 B7H3 have been deposited in the PRIDE database under accession code PXD059437. All data are included in the Supplementary Information or available from the authors, as are unique reagents used in this Article. The raw numbers for charts and graphs are available in the Source Data file whenever possible. Source data are provided with this paper.
References
Moremen, K. W., Tiemeyer, M. & Nairn, A. V. Vertebrate protein glycosylation: diversity, synthesis and function. Nat. Rev. Mol. Cell Biol. 13, 448–462 (2012).
Pinho, S. S. & Reis, C. A. Glycosylation in cancer: mechanisms and clinical implications. Nat. Rev. Cancer 15, 540–555 (2015).
Lopez-Sambrooks, C. et al. Oligosaccharyltransferase inhibition induces senescence in RTK-driven tumor cells. Nat. Chem. Biol. 12, 1023–1030 (2016).
de Caestecker, C. & Macara, I. G. A size filter at the Golgi regulates apical membrane protein sorting. Nat. cell Biol. 26, 1678–1690 (2024).
Fang, J. et al. Glucose-mediated N-glycosylation of RPTPalpha affects its subcellular localization and Src activation. Oncogene 42, 1058–1071 (2023).
Schwarz, F. & Aebi, M. Mechanisms and principles of N-linked protein glycosylation. Curr. Opin. Struct. Biol. 21, 576–582 (2011).
Molinari, M. N-glycan structure dictates extension of protein folding or onset of disposal. Nat. Chem. Biol. 3, 313–320 (2007).
Ferris, S. P., Kodali, V. K. & Kaufman, R. J. Glycoprotein folding and quality-control mechanisms in protein-folding diseases. Dis. Models Mech. 7, 331–341 (2014).
Griffiths, G. et al. Localization of the Lys, Asp, Glu, Leu tetrapeptide receptor to the Golgi complex and the intermediate compartment in mammalian cells. J. Cell Biol. 127, 1557–1574 (1994).
Wu, X. & Rapoport, T. A. Mechanistic insights into ER-associated protein degradation. Curr. Opin. Cell Biol. 53, 22–28 (2018).
Sun, Z. & Brodsky, J. L. Protein quality control in the secretory pathway. J. Cell Biol. 218, 3171–3187 (2019).
Chandler, K. B. & Costello, C. E. Glycomics and glycoproteomics of membrane proteins and cell-surface receptors: present trends and future opportunities. Electrophoresis 37, 1407–1419 (2016).
Kamei, N. et al. Definitive evidence that a single N-glycan among three glycans on inducible costimulator is required for proper protein trafficking and ligand binding. Biochem. Biophys. Res. Commun. 391, 557–563 (2010).
Getu, A. A. et al. New frontiers in immune checkpoint B7-H3 (CD276) research and drug development. Mol. Cancer 22, 43 (2023).
Shi, W. et al. Immune checkpoint B7-H3 is a therapeutic vulnerability in prostate cancer harboring PTEN and TP53 deficiencies. Sci. Transl. Med. 15, eadf6724 (2023).
Liu, H. J. et al. mTORC1 upregulates B7-H3/CD276 to inhibit antitumor T cells and drive tumor immune evasion. Nat. Commun. 14, 1214 (2023).
Sutton, M. N. et al. Dimerization of the 4Ig isoform of B7-H3 in tumor cells mediates enhanced proliferation and tumorigenic signaling. Commun. Biol. 7, 21 (2024).
Huang, Y. et al. FUT8-mediated aberrant N-glycosylation of B7H3 suppresses the immune response in triple-negative breast cancer. Nat. Commun. 12, 2672 (2021).
Chen, J. T. et al. Glycoprotein B7-H3 overexpression and aberrant glycosylation in oral cancer and immune response. Proc. Natl Acad. Sci. USA 112, 13057–13062 (2015).
Xu, P. et al. Quantitative proteomics reveals the function of unconventional ubiquitin chains in proteasomal degradation. Cell 137, 133–145 (2009).
Vagin, O., Kraut, J. A. & Sachs, G. Role of N-glycosylation in trafficking of apical membrane proteins in epithelia. Am. J. Physiol. Ren. Physiol. 296, F459–F469 (2009).
Vembar, S. S. & Brodsky, J. L. One step at a time: endoplasmic reticulum-associated degradation. Nat. Rev. Mol. Cell Biol. 9, 944–957 (2008).
van den Boom, J. & Meyer, H. VCP/p97-mediated unfolding as a principle in protein homeostasis and signaling. Mol. Cell 69, 182–194 (2018).
Wei, Y., Toth, J. I., Blanco, G. A., Bobkov, A. A. & Petroski, M. D. Adapted ATPase ___domain communication overcomes the cytotoxicity of p97 inhibitors. J. Biol. Chem. 293, 20169–20180 (2018).
Xu, C. & Ng, D. T. Glycosylation-directed quality control of protein folding. Nat. Rev. Mol. Cell Biol. 16, 742–752 (2015).
Xiao, H., Woods, E. C., Vukojicic, P. & Bertozzi, C. R. Precision glycocalyx editing as a strategy for cancer immunotherapy. Proc. Natl Acad. Sci. USA 113, 10304–10309 (2016).
Xiao, L. et al. B7 family protein glycosylation: promising novel targets in tumor treatment. Front. Immunol. 13, 1088560 (2022).
Shi, J. et al. NEU4-mediated desialylation enhances the activation of the oncogenic receptors for the dissemination of ovarian carcinoma. Oncogene 43, 3556–3569 (2024).
Wang, C. et al. CD276 expression enables squamous cell carcinoma stem cells to evade immune surveillance. Cell Stem Cell 28, 1597–1613 e1597 (2021).
Cai, D. et al. Tumor-expressed B7-H3 mediates the inhibition of antitumor T-cell functions in ovarian cancer insensitive to PD-1 blockade therapy. Cell. Mol. Immunol. 17, 227–236 (2020).
Maeda, N. et al. Expression of B7-H3, a potential factor of tumor immune evasion in combination with the number of regulatory T cells, affects against recurrence-free survival in breast cancer patients. Ann. Surg. Oncol. 21, S546–S554 (2014).
Zhou, Q. et al. B7 score and T cell infiltration stratify immune status in prostate cancer. J. Immunother. Cancer 9, e002455 (2021).
Miyamoto, T. et al. B7-H3 suppresses antitumor immunity via thesuppresses antitumor immunity via the CCL2-CCR2-M2 macrophage axis and contributes to ovarian cancer progression. Cancer Immunol. Res. 10, 56–69 (2022).
Gao, Y. et al. LncRNA NEAT1 sponges miR-214 to regulate M2 macrophage polarization by regulation of B7-H3 in multiple myeloma. Mol. Immunol. 117, 20–28 (2020).
Chen, H. et al. EBV-upregulated B7-H3 inhibits nk cell-mediated antitumor function and contributes to nasopharyngeal carcinoma progression. Cancer Immunol. Res. 11, 830–846 (2023).
Xiong, G. et al. CD276 regulates the immune escape of esophageal squamous cell carcinoma through CXCL1-CXCR2 induced NETs. J. Immunother. Cancer 12, e008662 (2024).
Zhao, B. et al. Immune checkpoint of B7-H3 in cancer: from immunology to clinical immunotherapy. J. Hematol. Oncol. 15, 153 (2022).
Cheng, M. et al. CD276-dependent efferocytosis by tumor-associated macrophages promotes immune evasion in bladder cancer. Nat. Commun. 15, 2818 (2024).
Sitia, R. & Braakman, I. Quality control in the endoplasmic reticulum protein factory. Nature 426, 891–894 (2003).
Feng, D. et al. Regulation of Wnt/PCP signaling through p97/VCP-KBTBD7-mediated Vangl ubiquitination and endoplasmic reticulum-associated degradation. Sci. Adv. 7, eabg2099 (2021).
Cha, J. H. et al. Metformin promotes antitumor immunity via endoplasmic-reticulum-associated degradation of PD-L1. Mol. cell 71, 606–620 e607 (2018).
Sorensen, A. L. et al. Chemoenzymatically synthesized multimeric Tn/STn MUC1 glycopeptides elicit cancer-specific anti-MUC1 antibody responses and override tolerance. Glycobiology 16, 96–107 (2006).
Posey, A. D. Jr. et al. Engineered CAR T cells targeting the cancer-associated tn-glycoform of the membrane Mucin MUC1 control adenocarcinoma. Immunity 44, 1444–1454 (2016).
Li, C. W. et al. Eradication of triple-negative breast cancer cells by targeting glycosylated PD-L1. Cancer Cell 33, 187–201 e110 (2018).
Sun, L. et al. Targeting glycosylated PD-1 induces potent antitumor immunity. Cancer Res. 80, 2298–2310 (2020).
Lee, T. A. et al. Post-translational modification of PD-1: potential targets for cancer immunotherapy. Cancer Res 84, 800–807 (2024).
Zhang, Y. et al. Safety and efficacy of B7-H3 targeting CAR-T cell therapy for patients with recurrent GBM. J. Clin. Oncol. 42, 2062–2062 (2024).
Tian, M. et al. CAR T-cells targeting FGFR4 and CD276 simultaneously show potent antitumor effect against childhood rhabdomyosarcoma. Nat. Commun. 15, 6222 (2024).
Seaman, S. et al. Eradication of tumors through simultaneous ablation of CD276/B7-H3-positive tumor cells and tumor vasculature. Cancer Cell 31, 501–515 e508 (2017).
Yamato, M. et al. DS-7300a, a DNA topoisomerase I inhibitor, DXd-based antibody-drug conjugate targeting B7-H3, exerts potent antitumor activities in preclinical models. Mol. Cancer Ther. 21, 635–646 (2022).
Colombo, R., Tarantino, P., Rich, J. R., LoRusso, P. M. & de Vries, E. G. E. The journey of antibody-drug conjugates: lessons learned from 40 years of development. Cancer Discov. 14, 2089–2108 (2024).
McPherson, M. J. et al. An anti-TNF-glucocorticoid receptor modulator antibody-drug conjugate is efficacious against immune-mediated inflammatory diseases. Sci. Transl. Med. 16, eadd8936 (2024).
Zhang, K. M. et al. Inactivated cGAS-STING signaling facilitates endocrine resistance by forming a positive feedback loop with AKT kinase in ER+HER2- breast cancer. Adv. Sci. 11, e2403592 (2024).
Zhang, H. L. et al. TRPML1 triggers ferroptosis defense and is a potential therapeutic target in AKT-hyperactivated cancer. Sci. Transl. Med. 16, eadk0330 (2024).
Zhang, H. L. et al. PKCbetaII phosphorylates ACSL4 to amplify lipid peroxidation to induce ferroptosis. Nat. Cell Biol. 24, 88–98 (2022).
Hogquist, K. A. et al. T cell receptor antagonist peptides induce positive selection. Cell 76, 17–27 (1994).
Li, Z. L. et al. Autophagy deficiency promotes triple-negative breast cancer resistance to T cell-mediated cytotoxicity by blocking tenascin-C degradation. Nat. Commun. 11, 3806 (2020).
Cai, R. et al. A high immune-related index with the suppression of cGAS-STING pathway is a key determinant to herceptin resistance in HER2+ breast cancer. Int. J. Biol. Sci. 20, 3497–3514 (2024).
Mai, J. et al. AKT-mediated regulation of chromatin ubiquitylation and tumorigenesis through Mel18 phosphorylation. Oncogene 40, 2422–2436 (2021).
Subudhi, S. K. et al. Neoantigen responses, immune correlates, and favorable outcomes after ipilimumab treatment of patients with prostate cancer. Sci. Transl. Med. 12, eaaz3577 (2020).
Burr, M. L. et al. CMTM6 maintains the expression of PD-L1 and regulates anti-tumour immunity. Nature 549, 101–105 (2017).
Acknowledgements
This study was supported by the Natural Science Foundation of China (project number 82173092 and 82372808, to R.D.; project number 82341029 and 82130079, to X.F.Z.; project number 82303041, to Z.L.L.; project number 82404008, to Y.H.), the Guangdong Basic and Applied Basic Research Foundation (project number 2023A1515110974, to Y.H.), and the Science and Technology Project in Guangzhou (project number 2024A04J4844, to T.D). We thank Beijing Vital River Laboratory Animal Technology Co., Ltd, for its support with huPBMC-NOG-MHC I/II-2 KO mice. In addition, we acknowledge the use of BioRender (https://biorender.com) for the creation of Fig. 6a, Supplementary Fig. 2a, Supplementary Fig. 6b and Supplementary Fig. 6d.
Author information
Authors and Affiliations
Contributions
Y.H, W.Q.Z., X.Y.Y., Z.L.L., J.L.S., Y.Q.G. and H.L.Z. performed the experiments and analyzed the data. R.D., D.Y., T.D. and K.M.Z. performed bioinformatics analyses. Y.H., L.Z., D.Y., B.X.H., Y.H.C. and G.K.F. provided experimental materials. R.D., Y.H., W.Q.Z. and X.Y.Y. wrote the manuscript. R.D., X.F.Z. and J.T. designed and supervised this project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Peer review
Peer review information
Nature Communications thanks Charles Dimitroff and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Huang, Y., Zhong, WQ., Yang, XY. et al. Targeting site-specific N-glycosylated B7H3 induces potent antitumor immunity. Nat Commun 16, 3546 (2025). https://doi.org/10.1038/s41467-025-58740-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-58740-3