Abstract
Intervertebral disc degeneration (IDD) remains a global healthcare challenge. Here, by analyzing clinical samples, we discover the loss of milk fat globule-EGF factor 8 (MFG-E8) from the nucleus pulposus (NP) tissue—along with increased enzymatic breakdown of glycosaminoglycans (GAGs)—as an overlooked factor for IDD development. Repairing the degraded NP extracellular matrix (ECM) with a structurally-mimicking glycan glue to enrich MFG-E8 may counter the degeneration. Accordingly, we synthesize a glucomannan octanoate (GMOC) with robust resistance to ECM-cleaving enzymes, which forms assemblies with MFG-E8 to maintain a healthy NP cell phenotype. GMOC injected into the degenerated intervertebral disc enriches MFG-E8 in situ, leading to NP tissue regeneration in a rat and a rabbit model, which represent two clinical scenarios of pre-surgical intervention and post-surgical regeneration of IDD, respectively. In summary, we report enriching MFG-E8 in ECM with a glycan glue as a mechanism to promote NP regeneration for IDD treatment.
Similar content being viewed by others
Introduction
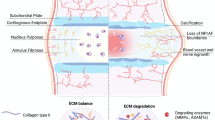
Intervertebral disc degeneration (IDD) accounts for nearly half of the cases of low back pain (LBP), a leading cause of disability worldwide1. The progression of IDD is characterized by decreased intervertebral disc height and water content in the nucleus pulposus (NP) tissue2, which lies in the center of the intervertebral disc and is surrounded by annulus fibrosus (AF)2. Current clinical interventions involve endoscopic excision of the protruding NP to relieve nerve root compression, which can effectively reduce the symptoms3; however, such practices cannot reverse the damage to the degenerated tissue, leading to common recurrence of the disease4,5. A highly expected, fundamental solution for IDD treatment is regenerating the NP tissue, which remains an unmet challenge6.
A key change to the NP tissue along IDD development is the increasing loss of glycosaminoglycan (GAG) polysaccharides. GAGs are a main component of the gel-like extracellular matrix (ECM) that maintains the morphology and phenotypes of NP cells (NPCs)7. Although replenishing GAGs has emerged as a promising strategy8, its efficacy remains unclear, with hardly any clinical success achieved. Recent findings have raised questions that the NP tissue under degeneration is maintained as a catabolic microenvironment by the elevated presence of enzymes that can degrade native GAGs9—such as abundant hyaluronidases that cleave hyaluronic acid (HA)10. Thus, GAGs supplemented to the degenerated site may suffer premature degradation. An alternative approach is to implant a biomaterial substitute of GAGs—serving as a glue to the damaged ECM of NP. This glue material should avoid recognition by the enzymes in the pathological niche, and meanwhile, mimic native GAGs in exerting specific bioactivities to support NPC functionality. However, the biological functions that GAGs provide in maintaining NPC phenotypes—which are lost under IDD—remain elusive. Existing knowledge is limited to their physical role in holding water among hydrophilic chains to maintain osmotic pressure11, while the essential bioactivities of GAGs that this glue should possess are unclear.
A signature function of GAGs in various other mammalian tissues is to bind and enrich soluble biological signals in the ECM, e.g., growth factors (GFs), forming assemblies12. Such glycan-protein assemblies are crucial: they control the local concentration gradient of the GFs and stabilize the GFs’ interactions with corresponding cell-membrane receptors13, thereby modulating the behavior of chondrocytes14, osteoblasts15, neurons16, and endothelial cells17. However, much less is known about which protein(s) GAGs bind in regulating NPCs’ phenotype (and what assemblies are lost during IDD). In addition, the physicochemical status of GAGs, including their degree of polymerization and thus molecular weight, greatly influences their binding with soluble factors18,19. As mentioned above, it remains unknown whether the catabolic environment during IDD could break the glycan-protein assembly, consequently affecting the NPC phenotype. Therefore, to design a biomaterial to replenish the lost GAGs for NP regeneration, the first gap to fill is finding the key protein(s)—its assembly with GAGs is essential for maintaining NPC functions, followed by the preparation of a GAG-mimicking, enzyme-resisting substitute to restore this assembly in situ to activate the regenerative process.
To realize this goal, in this study, we start with clinical evidence by investigating patient specimens and the Human Musculoskeletal System database (MSdb)20, in search of the glycan-binding factor pivotal in maintaining NPCs’ phenotype but undergoing abnormal changes during IDD. Intriguingly, we notice the significant loss of the milk fat globule-EGF factor 8 (MFG-E8)—an integrin-binding bridging molecule in ECM21—from the tissue of higher-Pfirrmann grade patients, in correlation with increased GAG-cleaving enzymes, suggesting an unexpectedly vital role of MFG-E8 in maintaining NP homeostasis. A previous inspirational finding that MFG-E8 activates the Nrf2/TXNIP/NLRP3 axis to protect NPCs and resist IDD progression22 supports our hypothesis that restoring MFG-E8 in NP has therapeutic potential. Accordingly, we synthesize and screen a glucomannan octanoate (GMOC) polysaccharide with enzyme-resisting glycosidic structures and an optimal binding affinity for MFG-E8. We inject GMOC in the form of injectable particles into IDD models in rats and rabbits to validate its efficacy to glue the damaged ECM and regenerate the NP tissue (Fig. 1).
Inspired by our clinical evidence that the loss of the MFG-E8 protein along with GAGs in the ECM of NP is correlated with IDD development (upper row), we synthesized a GAG-mimicking glycan biomaterial (GMOC) with an optimal binding affinity for MFG-E8 and enzyme-resisting activity in the pathological tissue matrix (lower row). GMOC injected into the degenerated NP tissue functions as a glue for ECM to retain MFG-E8 and promote NP regeneration.
Result
Decreased MFG-E8 content and increased HA degradation in higher-grade IDD patients
We first employed the Human MSdb (www.msdb.org.cn) to screen genes related to GAGs and glycan-interacting proteins (ECM components) during IDD development (Fig. 2A, B). The analysis revealed that genes encoding the ECM-degrading enzymes, including hyaluronidases and matrix metalloproteases, were significantly higher in IDD patients than in healthy individuals, as reflected by the Integrated Expression Atlas of Fig. 2C. Notably, among the genes encoding the glycan-interacting proteins that mostly had minor changes, MFGE8 was increased at the transcriptional level in IDD patients than in healthy individuals with statistic differences (Fig. 2D). As a bridging protein in the ECM of many tissues, MFG-E8 regulates the functions of various cells in wound healing, containing an N-terminal epidermal growth factor (EGF)-like ___domain and two C-terminal domains homologous to the C1 and C2 domains of the coagulation factors V and VIII (F5/8-C1 and -C2)21,23. MFG-E8 can bind carbohydrates (including GAGs), as mediated by the interaction between its basic amino acids, e.g., arginine, located in F5/8-C1 and -C2, and the carboxylic acid groups of the glycans24, though its role in NP is rarely reported22.
Comparison of the genes of (A) and (B) ECM components, (C) ECM metabolic enzymes, and (D) glycan-interacting proteins between healthy individuals (blue) and IDD patients (red) based on the data from Human MSdb (Data are presented as mean values ± SD, n = 7, biological replicates). E The representative image showing radiological examination of IDD patients at Pfirrmanna’s Grade II and IV. The red arrow indicates surgically removed lesion tissues. F, G Immunohistochemical staining for (F) Type II collagen and (G) Aggrecan in human degenerated NP specimens (scar bar: 150 μm, n = 7, biological replicates and quantified in Supplementary Fig. 1F, G). Immunofluorescent (IF) staining for (H) Hyal-1 and (I) MFG-E8 in human degenerated NP specimens (scar bar: 150 μm, n = 7, biological replicates and quantified in Supplementary Fig. 1H, I). J The representative image showing IF staining for Integrin alpha-V (red), MFG-E8 (green), Type II collagen (magenta), and nucleus (DAPI, blue) in rat NP specimens. The yellow box indicates the area magnified on the right side. AF annulus fibrosus, NP nucleus pulposus (n = 3, left scar bar: 200 μm, right scar bar: 100 μm). K The representative image showing IF staining of Aggrecan (red), MFG-E8 (green), and nucleus (DAPI, blue) (n = 3, scar bar: 100 μm). Data were analyzed using two-sided Mann-Whitney test (D). Source data is provided as a Source Data file.
We next collected specimens of human patients of IDD with varying degrees of degeneration to uncover first-hand evidence of GAG-related changes in NP. The samples were endoscopically extracted from two groups of patients of Pfirrmann’s Grade II and Grade IV25, respectively (Fig. 2E), where a higher grade indicates a more severe degree of degeneration. Histological analysis revealed decreased ECM content, including a 71.4% decrease in Type II collagen (Fig. 2F and Supplementary Fig. 1A; quantification in Supplementary Fig. 1F) and 54.1% in Aggrecan (Fig. 2G and Supplementary Fig. 1B; quantification in Supplementary Fig. 1G), but 59.7% increased expression trend of hyaluronidase 1 (Hyal-1, Fig. 2H and Supplementary Fig. 1C; quantification in Supplementary Fig. 1H) in the Grade IV samples as compared with Grade II samples, in accordance with the database analysis. Nevertheless, the expression of MFG-E8 was significantly lower (59.5%) in Grade IV than in Grade II (Fig. 2I and Supplementary Fig. 1D; quantification in Supplementary Fig. 1I), which is opposite to that in the database. In parallel, a control group applied with only secondary antibodies verified the staining outcome (Supplementary Fig. 1E). This contrast between transcriptional and expressional levels implied that cells probably attempted to express more MFG-E8 to compensate for those lost from the ECM, further suggesting a possible role of MFG-E8 in IDD progression.
To validate the change of MFG-E8 in IDD, we created a needle-puncture model in rats and collected the NP tissue for immunofluorescent (IF) co-staining for MFG-E8, Type II collagen, Integrin alpha-V and Aggrecan, since Arg-Gly-Asp (RGD) sequence of MFG-E8 on ECM can bind with Integrin αv receptor on the cell membrane26. Our results illustrated that healthy NP contained abundant MFG-E8 protein (green) spreading through Type II collagen (magenta), in connection with Integrin alpha-V (red) in magnified images; in comparison, the degenerated tissue contained much lower content of collagens and MFG-E8, with diminished MFG-E8-Integrin alpha-V connections (Fig. 2J). Similarly, the distribution of GAGs represented by Aggrecan in NP is similar to that of Type II collagen; it connects with MFG-E8, yet these connections decreased as the degeneration deteriorated (Fig. 2K). Additionally, at the protein level, MFG-E8 was detected in the Grade IV group (80.46 ± 26.98 pg/mL) only half of that in the Grade II group (156.98 ± 9.85 pg/mL) (Supplementary Fig. 1J). Similarly, in the rat IDD model, the MFG-E8 content in NP decreased with the progression of degeneration and dropped to 35.34 ± 3.95 pg/ml at day 14, which was two-thirds of the control group’s level (52.28 ± 3.06 pg/mL) (Supplementary Fig. 1K). Collectively, these data confirmed the destruction of the ECM structure and reduced MFG-E8 under the IDD circumstance.
Design of MFG-E8-enriching glycan
Based on the clinical findings, we sought to prepare a GAG substitute to enrich MFG-E8 to promote NP tissue regeneration. We selected glucomannan (GM) polysaccharide as the building block to develop an MFG-E8-enriching glycan matrix for NP regeneration based on several reasons. Its monosaccharide components are glucose and mannose, the two most fundamental carbohydrates composing the mammalian tissue matrix. More importantly, its glycosidic linkages are not recognized by the two hyaluronidases, Hyal-1, and Hyal-2, widely present in the pathological NP tissue, hence potentially evading premature degradation. Finally, previous studies demonstrated that GM chemically modified with different aliphatic side groups could exhibit different protein-binding affinities27,28. Given that MFG-E8 can bind polysaccharides, including GAGs24, we expect to obtain GM-based glycans with a relatively high affinity for MFG-E8.
Thus, we prepared a series of GM derivatives via esterification: GM-acetate (GMAC), GM-octanoate (GMOC), GM-decanoate (GMDE), and GM-laurate (GMLA), with -OH groups on the polysaccharide chain replaced with -(CO)-(CH2) n-CH3, where n = 0, 6, 8 and 10, respectively (Fig. 3A). The modification was confirmed by the infrared spectrum (Supplementary Fig. 2A), and the degree of substitution could be controlled between 1.54 and 2.28, as determined by 1H NMR spectrum (Supplementary Fig. 2B). These GM esters could readily be prepared in various forms (Supplementary Fig. 2C), including coating on glass substrates, uniformly sized particles, or electrospun porous scaffolds.
A Schematic illustration of synthesizing GM esters with different aliphatic groups. The representative image showing high-performance liquid chromatography (HPLC) analysis of HA and GM treatment with (B) Hyal-1 or (C) Hyal-2 or without treatment (D). The red arrow indicates degraded fractions. E The binding energy and sites of MFG-E8 for GM esters from molecular docking. F The representative image showing QCM-D assay for the binding between MFG-E8 and GM esters. Dot-blotting assay for the binding between GM esters and (G) recombinant MFG-E8 protein or (H) secreted MFG-E8 from living NPCs (Data are presented as mean values ± SD, n = 4, biological replicates). I The representative image showing morphology of the glycans alone, including GMOC (110 kDa), high molecular-weight HA (150 kDa), and low molecular-weight HA (3 kDa) alone (upper row), and their association with MFG-E8 (lower row), as characterized by the tapping mode AFM (scar bar:600 nm). The cellular thermal shift assay (CETSA) to examine the binding between the glycans and MFG-E8 at a temperature gradient of 55–71 °C, with (J) the blotting images and (K) quantitative analysis demonstrating the thermal stability of different complexes. All samples derive from the same experiment, and blots were processed in parallel. (Data are presented as mean values ± SD, n = 3, biological replicates). Rheological testing assessed the storage modulus of collagen gel (L) and gelation time (M) of a collagen solution following the incorporation of GMOC into the collagen solution (Data are presented as mean values ± SD, n = 5, biological replicates). Data were analyzed using the Kruskal-Wallis test, followed by Dunn’s post hoc test for multiple comparisons (G, H), or two-sided Mann-Whitney test (L, M). Source data is provided as a Source Data file.
To verify the enzyme-proof capability of GM as a key consideration for its in vivo stability, we exposed GM or HA to Hyal-1 (Fig. 3B) and Hyal-2 (Fig. 3C)—the abundantly presented ECM-degrading enzymes in the NP tissue under IDD—for 6 h. As the high-performance liquid chromatography (HPLC) analysis illustrated, GM was completely resistant to these enzymes, with no cleaved fragments detected, while HA—as representative of native GAGs in the ECM—was quickly degraded to fragments within 6 h compared with the control group (Fig. 3D and Supplementary Fig. 2D). Further measurements confirmed that the four GM esters soaked in 0.5% (w/v) Hyal-1 (Supplementary Fig. 2E) or Hyal-2 (Supplementary Fig. 2F) had little loss of mass after 7 days, proving the resistance of GM after esterification to the enzymes. After the addition of hyaluronidase (0.5% w/v, Hyal-2) to the gel-like hyaluronic acid (4% w/v, 150 K), the viscosity of HA decreased progressively with the extent of degradation, resulting in fluidity, which demonstrated rapid degradation of HA. In contrast, for GM esters, as exemplified by GMOC, neither morphological nor volumetric changes were observed through 7 days of enzymatic hydrolysis (Supplementary Fig. 2G).
Next, we evaluated the binding affinity of GM derivatives for MFG-E8. First, with molecular docking, we noticed that GMOC exhibited the highest interaction energy absolute value (7.50 kcal/mol) with MFG-E8, forming tentative hydrogen bonds at Lys180 (Fig.3E). The binding sites of hydrogen bonds were identified on the F5/8-C2 β-barrel core spikes 1 of MFG-E8. Then, to validate this simulation, we measured the binding affinity between MFG-E8 and GM ester samples with quartz crystal microbalance with dissipation (QCM-D). Although all the GM esters could interact with MFG-E8 to varying degrees, GMOC exhibited the strongest binding, with the highest binding mass value (6799.77 ng/cm2) during association. GMDE and GMLA showed similar affinities for MFG-E8, higher than that of GMAC but lower than that of GMOC, consistent with the docking outcomes (Fig. 3F).
Further, we performed the dot-blotting assay28 to validate the ability of GM ester samples to bind MFG-E8 from different sources (recombinant protein and NPC-secreted), by coating different GM esters on the glass substrate. These substrates were incubated with the recombinant MFG-E8 or co-cultured with living NPCs, respectively. After PBS rinsing or removal of NPCs by trypsin-EDTA, the substrates were labeled by horseradish peroxidase (HRP) conjugated antibody to determine the amount of bound MFG-E8. The outcomes confirmed that GMOC exhibited the highest affinity (6.42-fold higher than glass substrate) for recombinant MFG-E8 protein (Fig. 3G) and shared the highest affinity (3.56-fold higher than glass substrate) with GMLA for secretion MFG-E8 (Fig. 3H). Based on these results, we selected GMOC out of the GM derivatives for its superior binding of MFG-E8.
Finally, to validate that GMOC could maintain the assembly with MFG-E8 that was possibly disrupted by GAGs degradation under the pathological conditions, we examined the capability of HA of higher (150 K) and lower (3 K) molecular weight (2%, w/v) or GMOC (110 kDa, 2%, w/v) to assemble with MFG-E8. First, we employed the tapping mode imaging of the atomic force microscope (AFM) to examine the morphology of the glycan-MFG-E8 complex. Indeed, HA with a higher molecular weight formed large, rod-like complexes with MFG-E8 (M@HA-150K), in contrast to the small, dot-like morphology observed in the group of smaller HA bound with MFG-E8 (M@HA-3K). Notably, GMOC, which was proven to be enzyme-proof, formed assemblies with MFG-E8 (M@GMOC) similar to M@HA-150K (Fig. 3I). Then, we performed the cellular thermal shift assay (CETSA) to assess the degradation of MFG-E8 at different temperatures with or without HA or GMOC. The blotting data (Fig. 3J) and quantification outcomes (Fig. 3K) showed that both GMOC and HA (150 K) could protect MFG-E8 from degradation (less than 20%), suggesting these glycans forming relatively tight assemblies with the protein, while HA (3 K) failed to prevent over 75% of the protein from degradation.
To assess the glue effect of GMOC, we tested its interaction with collagen, a major component of the NP tissue, to reflect its long-term stability upon in situ application. In a collagen system where GMOC (1% suspension in PBS; w/v) was incorporated into collagen (0.5% in DMEM/F-12 medium; w/v), a significant increase in the storage modulus (G’) of the collagen after gelation was observed, rising from 358.37 ± 97.02 Pa to 937.75 ± 162.58 Pa (Fig. 3L). Furthermore, GMOC markedly accelerated the gelation of the collagen solution, reducing the transition time from 650.16 ± 71.7 s to 349.31 ± 88.49 s (Fig. 3M). These data confirmed the interaction between GMOC and collagen in agreement with other studies that hydrophobic modifications could promote a more compact aggregation of collagen molecules29,30.
GMOC maintains the morphology and function of NPCs
We next evaluated the behavior of NPCs on GMOC. First, the number of NPCs adhering to the GMOC substrate was comparable to that on the glass surface (as control) in the first 12 h, yet being higher than on GMDE and lower than on GMAC (Supplementary Fig. 3A). After a drop on day 2 in cell numbers, NPCs kept proliferating throughout the rest of the 7-day observation (Supplementary Fig. 3B). Second, the morphology of NPCs on GMOC, GMDE, and GMLA appeared spheroidal, with the highest circularity observed on GMOC (Supplementary Fig. 3C), which is reported to match the morphology of healthy and juvenile NPCs in native ECM31,32,33. Notably, the cells formed tight clusters while those in the center of aggregation maintained viable (Fig. 4A). By defining a gathering of at least 16 cells as a large cluster, we found that 45.22 ± 11.46% of the total NPCs on GMOC form large clusters, a ratio significantly higher than that on GMAC (3.28 ± 2.27%), while hardly any large clusters were observed on glass (Fig. 4B). Third, GMOC most effectively promoted the intracellular expression of Type II collagen (141.39 ± 19.44 AU), a positive sign of healthy phenotype for NPCs34, as illustrated by IF staining (Fig. 4C) and quantification (Supplementary Fig. 3D). Consistent with the IF outcome, transcriptional analysis by real-time qPCR revealed the highest level of Col2a1 in NPCs on GMOC after 7 days, over 3-fold higher than that on the glass surface or on GMAC (Fig. 4D). Meanwhile, Sox9, another essential marker of healthy NPC phenotype35, was nearly 4-fold higher in the GMOC group than in the glass group (Fig. 4E). These results showed that NPCs cultured on GMOC in vitro tend to maintain their native and healthy morphology in the healthy NP tissue. Further, the morphology of human nucleus pulposus cells (h-NPCs) was similar to that of rat NPCs, forming larger and more regular clusters on the surface of GMOC (Supplementary Fig. 3G). H-NPCs significantly upregulated the expression of phenotypic markers, showing transcriptionally a 6.94-fold increase in Col2a1 (Supplementary Fig. 3H) and a 3.62-fold in Sox9 compared to the control group (Supplementary Fig. 3I), indicating that GMOC also regulated h-NPCs functions.
A The representative image showing live and dead staining of NPCs on GM esters. Green: living cell. Red: dead cell (n = 3, scar bar: 200 μm). B The quantitative analysis of NPCs cluster size on GM esters (Data were presented as percentage, n = 3, biological replicates). C NPCs cultured on GM esters and IF for Type II collagen (red), cytoskeleton (phalloidin, green), and nuclei (DAPI, blue) (scar bar: 50 μm, n = 5, biological replicates and quantified in Supplementary Fig. 3D). The real-time qPCR analysis of (D) Col2a1 and (E) Sox9 in NPCs cultured on the surfaces of GM esters, glass as the control group (Data are presented as mean values ± SD, n = 5, biological replicates). MA plot for DEG analysis between GMOC and (F) glass or (G) GMAC. Heatmap of differentially expressed genes—associated with (H) ECM components, (I) glycan-interacting proteins, and (J) juvenile NPCs—among glass, GMAC, and GMOC, as determined using bulk RNA-seq analysis. Data were analyzed using the Kruskal-Wallis test, followed by Dunn’s post hoc test for multiple comparisons (D, E). Source data is provided as a Source Data file.
We comprehensively examined the phenotypes of NPCs on the glycan substrate with bulk RNA sequencing (RNA-seq). The cells on GMOC had 4844 and 2315 genes upregulated in comparison with those on glass and GMAC, respectively (Fig. 4F, G). The transcriptional profile of NPCs on GMOC was almost the opposite of that in control (Supplementary Fig. 3E). For example, NPCs on GMOC significantly expressed genes related to the native NP ECM components (e.g., Col2a1, Col11a2, Has1, and Has2), without increasing the levels of matrix proteins representing NP degeneration (e.g., Col1a2 and Fbln2), which were however highly expressed on GMAC or glass, respectively (Fig. 4H). Meanwhile, on GMOC, NPCs expressed increased levels of critical proteins interacting with ECM—including integrin family members (e.g., Itgav and Itgb1) and cadherin family members (e.g., Cdh4 and Cdh10) (Fig. 4I)—and genes representing the juvenile status of NPCs (e.g., Sox9, Hif1a, Ca12, Glut1, and Acan) (Fig. 4J). Further, differentially expressed genes (DEG) and GO analysis revealed high expression of genes associated with ECM organization, cytoskeleton organization, and cell-matrix adhesion in NPCs on GMOC (Supplementary Fig. 3F). These data further highlighted that NPCs maintained a spheroidal morphology in clusters in tandem with a healthy phenotype on GMOC.
GMOC regulates NPCs phenotypes through MFG-E8 enrichment
We carried out a series of tests to validate the central role of MFG-E8 in the phenotypic regulation of NPCs on GMOC. First, we conducted a 4D label-free quantitative proteomics analysis to identify proteins tightly bound to GMOC. The results revealed that, compared with GMAC and glass, GMOC demonstrated distinct profiles of membrane protein binding (MA-plot, Fig. 5A, B; heatmap, Supplementary Fig. 3J), with MFG-E8 among the most enriched proteins on GMOC (Fig. 5C). IF staining showed significantly more MFG-E8 in cells cultured on GMOC (AU: 206.50 ± 11.71) than that on the glass (91.54 ± 5.77) and GMAC (95.0 ± 11.98) (Fig. 5D; quantified in Fig. 5E). The expression of Integrin alpha-V appeared to have a similar trend, much higher in the GMOC group (AU: 190.67 ± 3.67) than on the glass (81.61 ± 6.24) and GMAC (85.31 ± 3.51), which might favor the enrichment of MFG-E8 around the cells (Fig. 5F; quantified in Fig.5G). This noteworthy phenomenon agreed with the transcriptome data, revealing an elevated expression of Integrin alpha-V.
MA plot for different proteins between GMOC and (A) glass or (B) GMAC. C Heatmap of differentially enriched membrane proteins of NPCs between glass, GMAC, and GMOC, as determined by 4D label-free quantitative proteomic analysis. IF staining and quantification for (D, E) MFG-E8 (red) and (F, G) Integrin alpha-V (green) in NPCs cultured on GM substrates or glass, with nuclei counterstained with DAPI (blue) (scar bar: 200 μm; Data are presented as mean values ± SD, n = 5, biological replicates). H The representative image showing live/dead staining of NPCs on GM esters with or without Mfge8 siRNA treatment. Green: living cell; red: dead cell (scar bar: 400 μm). I The representative image showing IF staining for MFG-E8 in NPCs on GM esters with or without Mfge8 siRNA treatment. MFG-E8 (red) and nuclei (DAPI, blue) (scar bar: 100 μm). J IF staining for Type II collagen in NPCs on GM esters with or without Mfge8 siRNA treatment. Type II collagen (red), Cytoskeleton (phalloidin, green) and nuclei (DAPI, blue) (scar bar: 50 μm, n = 5, biological replicates). K Quantitative analysis of Type II collagen IF staining (Data are presented as mean values ± SD, n = 5, biological replicates). L Dot-blotting analysis of secreted MFG-E8 from living NPCs on GM substrates, with or without Mfge8 siRNA treatment (Data are presented as mean values ± SD, n = 4, biological replicates). M The representative image showing IF staining for cytoskeleton (phalloidin, green) and nuclei (DAPI, blue) in NPCs on GM esters, with or without Integrin αv antagonist MK0429 treatment (scar bar: 200 μm). N Western Blotting analysis of Akt and p-Akt and (O) quantification of gray value in NPCs cultured on the GMOC. All samples derive from the same experiment, and blots were processed in parallel. (Data are presented as mean values ± SD, n = 5, biological replicates). Data were analyzed using the Kruskal-Wallis test, followed by Dunn’s post hoc test for multiple comparisons (E, G), or two-sided Mann-Whitney test (K, L, O). Source data is provided as a Source Data file.
Next, we suppressed Mfge8 expression with siRNA (50 nM) in NPCs for 72 h, as validated by IF staining (Supplementary Fig. 4A) and real-time qPCR (Supplementary Fig. 4B), before culturing these cells on GMOC or control substrates. After using trypsin-EDTA to remove the cultured NPCs off the GMOC surface, we performed IF staining for MFG-E8 adsorbed on the GMOC surface and noted less MFG-E8 on the GMOC surface (Supplementary Fig. 4C), verifying again successful silencing of Mfge8 and its lower adsorption on this substrate. The gene silencing, which did not cause cell death (Fig. 5H), markedly reduced NPCs adhesion and clustering on GMOC (Fig. 5I). Also, Mfge8 silencing substantially changed the morphology of NPCs on GMOC, as IF staining indicated a 49.5% decrease in the type II collagen (Fig. 5J; quantified in Fig. 5K) and real-time qPCR test reported a 54.3% and 71.9% decrease in Col2a1 (Supplementary Fig. 4D) and Sox9 (Supplementary Fig. 4E), in comparison with the non-silenced group. Meanwhile, we performed a dot-blotting assay to show that Mfge8 silencing decreased the amount of MFG-E8 protein enriched on the GMOC surface (ratio value: 3.61 ± 0.82 versus 0.44 ± 0.14; Fig. 5L).
Since integrin αv is the main cell-surface receptor to link MFG-E8, we used an integrin antagonist (MK0429, 10 nM) for αvβ3 and αvβ5 to block their binding with MFG-E8, which abolished the cluster formation of NPCs on GMOC (Fig. 5M). This finding highlighted the involvement of the MFG-E8-integrin αv signaling axis in the cluster formation and maintenance of NPCs on GMOC. Further, adding exogenous MFG-E8 (1 mg/mL) reversed the reduction in cell cluster formation caused by the MFG-E8 siRNA silencing in the GMOC group (Supplementary Fig. 3K). Interestingly, cell adhesion on GMAC or control was less affected by Mfge8 silencing, implying that NPCs adhered to these substrates in other ways less depending on the MFG-E8-mediated mechanism, which explained why NPCs exhibited different morphologies when adhering to different matrices.
Finally, we demonstrated significant activation of Akt in NPCs on GMOC, with the ratio of pAkt and Akt being 1.53-fold higher than in the control group (Fig. 5N, O), since the phosphatidylinositol 3-kinase (PI3k)/Akt pathway plays a crucial role in maintaining NP tissue homeostasis36. This result was consistent with the above transcriptome data that also demonstrated increased expression levels of the downstream targets of the Akt pathway, including Sox9 and Hif1a (Fig. 4J). Collectively, these findings proved that MFG-E8 enrichment played a central role in maintaining the adhesion, cluster formation, and phenotype of NPCs on GMOC.
GMOC controls IDD development in rat and rabbit models
Finally, we tested the therapeutic efficacy of GMOC in two animal models of IDD, each representing a different scenario of clinical application. Although surgery is the ultimate option for pain relief in IDD patients in practice, avoiding surgery—or, if a surgery has been performed, promoting tissue repair post-surgery—is desirable. Hence, in the first model, we mimicked pre-operational intervention in rats, by creating caudal needle punctures and injecting GMOC to counter the degenerative process at an early stage, with implications of possibly avoiding surgical treatment in patients. In the second model, we mimicked post-operational regeneration in rabbits, by creating a lumbar discectomy model and injecting GMOC to fill up the defects, aimed at improving the tissue microenvironment and preventing recurrence after endoscopic resection surgery.
For the first rat model, as illustrated in Fig. 6A, we injected the suspension of GMOC particles (1%, w/v; in PBS) immediately after the operation, followed by radiological examinations and histological staining on weeks 2 and 4. These particles, in spheroidal shape under scanning electron microscopy (Fig. 6B) and within a well-injectable size range (~301 nm) as characterized by dynamic light scattering (Fig. 6C). After incubation with MFG-E8 protein, these particles were specifically bound by MFG-E8 antibody and labeled with fluorescence. The IF staining showed that GMOC particles could effectively absorb MFG-E8 (Fig. 6D). Phagocytosis experiments (Supplementary Fig. 5A) showed that only a small portion of GMOC were phagocytosed by NPCs, indicating abundant GMOC remaining in the ECM to enrich MFG-E8. The low phagocytosis might be due to the agglomeration of GMOC particles, with evidence showing doubled size (Supplementary Fig. 5B) and a sharply increased negative surface charge (from −0.33 ± 0.05 mV to −18.96 ± 1.21 mV) of GMOC in the culture medium (Supplementary Fig. 5C).
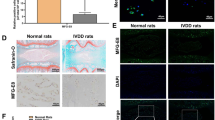
A Schematic diagram of GMOC treatment in a rat IDD model. B The representative image showing scanning electron microscopic image of GMOC particles (scar bar: 500 nm). C The size of GMOC particles was measured by DLS. D The representative image showing IF staining for MFG-E8 (green) enriched on the GMOC particles’ surface (scar bar: 5 μm). E The representative MRI images on week 2 and 4 (n = 8, biological replicates and quantified in (G)). F The representative X-ray images on week 2 and 4 (n = 8, biological replicates and quantified in (H)). G Quantitative analysis of intervertebral disc T2 signal intensity in MRI (Data are presented as mean values ± SD, n = 8, biological replicates). H Quantitative analysis of DHI in X-ray (Data are presented as mean values ± SD, n = 8, biological replicates). I The representative image showing H & E and (J) Safranin O & fast green staining on week 2 and 4 (scar bar: 500 μm). NP tissue representative IF staining for (K) MFG-E8 (green), Type II collagen (red), and (L) Aggrecan (green), Sox9 (enlarged image, red, scar bar: 200 μm), nuclei (DAPI, blue) on week 2 and 4, the expression of MFG-E8 (green) on week 4, highlighted in the orange boxes, were magnified and shown below (scar bar: 100 μm), the white dotted line shows the junction of the AF (annual fibrosus) and NP (nucleus pulposus) (scar bar: 200 μm). M Schematic diagram of mechanical test, including the range of motion (ROM) and compressive stiffness, in rat. N The representative ROM curve of different groups. O The quantified of ROM and (P) compressive stiffness (Data are presented as mean values ± SD, n = 6, biological replicates). Data were analyzed using the Kruskal-Wallis test, followed by Dunn’s post hoc test for multiple comparisons (G, H, O, P). Source data are provided as a Source Data file.
The typical condition of IDD is reduced water content in NP—as reflected by a lowered signal of the NP tissue in MRI-T2—which further leads to decreased height of intervertebral space (DHI) measurable by X-ray. The MRI and X-ray imaging results on week 4 showed that GMOC could effectively maintain the water content of NP (Fig. 6E) and mitigate the decrease in DHI (Fig. 6F). Quantitative results indicated that the T2 signal of the GMOC group was 3.26- and 5.95-fold higher than that of the PBS and IDD group, respectively (Fig. 6G). Also, the DHI of the GMOC group was 1.64- and 1.95-fold higher than that of the PBS and IDD group, respectively (Fig. 6H). Additionally, the treatment with exogenous MFG-E8 alone showed no effect compared with the PBS group at the end of 4-week treatment, according to the MRI data (Supplementary Fig. 6A, B). This finding again highlighted the importance of stabilizing and enriching MFG-E8 by GMOC in the degenerative ECM.
Histological analysis by H & E staining further revealed that, compared to the IDD group, GMOC effectively protected the NP tissue, reduced tissue loss, and attenuated the disorder of the AF caused by the loss of the NP tissue (Fig. 6I). Safranin O & fast green staining illustrated that GMOC efficiently mitigated Type II collagen loss in the degenerative microenvironment, in sharp contrast to the control (Fig. 6J). Despite the presence of irregular arrangement of AF on the edges due to tissue loss and compression, a significant volume of the NP tissue was well maintained in the GMOC group at week 4. Moreover, IF staining showed a clear decrease in MFG-E8, along with the loss of Type II collagen (Fig. 6K) and Aggrecan in the ECM and lowered expression of Sox9 (Fig.6L), in degenerated NP tissue. Particularly, in the IDD group, it was almost impossible to observe a complete ECM signal at week 4, while irregularly arranged AF tissue appeared to have replaced NP. Only GMOC effectively kept a substantial amount of MFG-E8 in the matrix and maintained the expression of the phenotypic marker Sox9.
Pain is one of the most prominent features of IDD clinically. Improving the degenerative microenvironment could effectively alleviate this pain, which can be assessed by the tail-flick test37,38. Here, we observed the effects of GMOC treatment on pain relief in the disc with this method. As illustrated in Supplementary Fig. 6C, 4 weeks after the surgery, the latency time of the IDD group was 4.23 ± 0.42 s, ~62.6% lower than the healthy control group (11.32 ± 0.34 sec). However, the GMOC-treated samples could restore it to 9.29 ± 0.34 s, which was 2.2-fold of that of the non-treated IDD group and closer to (only 18% lower) that of the healthy group. Thus, the GMOC treatment effectively alleviated the pain sensitivity caused by IDD.
Finally, the mechanical loading tests can evaluate the effectiveness of implant treatments, reflecting the mechanical recovery of the disc (Fig. 6M)2,39,40. The total range of motion (ROM) is the mechanical behavior most related to the NP function (Fig. 6N)41. The ROM of the IDD sample (0.78 ± 0.04 mm) significantly increased by over 4-fold compared with that of the healthy disc tissue (0.19 ± 0.08 mm) (Fig. 6O). In comparison, the ROM of GMOC was 0.33 ± 0.08 mm—not statistically different with healthy group—but almost 60% lower than the IDD group. Importantly, neither NP nor implanted GMOC was exuded from the disc during the mechanical testing, which is implication for a positive integration41. Furthermore, the compression stiffness of the intervertebral disc in the GMOC group was 23.98 ± 5.46 N/mm—close to the control group’s value of 20.72 ± 5.44 N/mm, implying that the NP tissue protected by GMOC could effectively absorb and distribute pressure. In contrast, the IDD group lacked enough NP tissue to absorb pressure (evidenced by MRI in Fig. 6E), resulting in a significantly higher compression stiffness of 157.20 ± 82.09 N/mm (Fig. 6P). The above data from mechanical testing, in combination with the collagen-binding data (Fig. 3 L and M), highlighted the capability of GMOC to bind ECM components (e.g., collagen) to offer a desirable strength, which is believed to be an integral part of material-tissue integration42.
For the second model, we excised part of the lumbar intervertebral disc tissues in rabbits to simulate the post-surgical microenvironment5. Then, we filled the defect with 15 μL matrigel mixed with GMOC (vehicle+GMOC group, 1%, w/v) and extended the treatment to 6 weeks (Fig. 7A). Both the MRI (Fig. 7B) and X-ray (Fig. 7C) results showed significant degeneration in the IDD group in week 6 compared with the control group. However, the GMOC treatment managed to maintain the tissue signal throughout the 6-week period, which was significantly higher than the IDD group. Quantitative results on week 6 indicated that the T2 signal of the GMOC treatment was 2.01- and 2.74-fold higher than that of the vehicle and IDD group, respectively (Fig. 7D). The DHI of the GMOC group was 1.4- and 1.5-fold higher than that of the vehicle and IDD group, respectively (Fig. 7E).
A Schematic diagram of GMOC treatment in rabbit IDD model. The vehicle group refers to the injection of Matrigel, a degradable matrix with temperature sensitivity and biocompatibility75,76. B The representative MRI images on week 3 and 6 (n = 5, biological replicates and quantified in (D)). C The Representative X-ray images on week 3 and 6 (n = 5, biological replicates and quantified in (E)). D Quantitative analysis of intervertebral disc T2 signal intensity in MRI (Data are presented as mean values ± SD, n = 5, biological replicates). E Quantitative analysis of DHI in X-ray (Data are presented as mean values ± SD, n = 5, biological replicates). The representative image showing (F) H & E and (G) Safranin O & fast green staining on week 3 and 6. The asterisk (*) shows NP tissue (scar bar: 1000 μm). Data were analyzed using the Kruskal-Wallis test, followed by Dunn’s post hoc test for multiple comparisons (D, E). Source data is provided as a Source Data file.
Histological analyses provided more detailed evidence. First, H & E staining revealed a clear loss of NP tissue and disarray in the AF in the IDD group. In comparison, the GMOC treatment group could alleviate further loss of NP tissue post-excision. Even after 6 weeks, a certain degree of NP tissue was retained (Fig. 7F). Safranin O & fast green staining further showed the morphology of the NP tissue. Notably, the GMOC-treated group exhibited clear annulus fibrosus and retained intact NP, in sharp contrast to the IDD group. (Fig. 7G).
Discussion
Effective treatment of IDD remains an unmet clinical challenge due to an insufficient understanding of key biological events in NP degeneration43, which hampers the design of therapeutic agents or biomedical materials with a clear target and specific mechanism. In this study, by discovering the role of MFG-E8 in maintaining the NP tissue from clinical samples, we devised a glycan biomaterial to function as a glue for the damaged ECM under deteriorated enzymatic degradation, which sequestered MFG-E8 and promoted tissue regeneration in two different IDD models in rats and rabbits.
Our intriguing finding of the MFG-E8 loss from the NP tissue matrix laid the foundation for materials design. From first-hand clinical specimens, we found the loss of this glycan-binding protein in association with the degradation of GAGs in the hypoxic and isolated NP microenvironment as IDD advances8. MFG-E8, a secreted glycoprotein, has its N-terminal EGF-like ___domain containing an RGD sequence recognizing αvβ3 or αvβ5 integrin receptors on the cell membrane21 and C-terminal factor V/VIII-like ___domain containing basic amino acid sequences that bind to carbohydrates in the ECM24. In this process, enriching MFG-E8 from loss during IDD is expected to strengthen the interaction between NPCs and their surrounding pericellular matrix (PCM), which encases and transmits mechanical and biochemical signals to NPCs44, thereby activating essential signaling pathways for phenotype maintenance (e.g., PI3K/AKT, FAK, and MAPK pathways)44,45. Such a bidirectional connection enables MFG-E8 to mediate cell growth in many other tissues21,46. Our finding aligns with previous studies about MFG-E8 in the repair of cartilage47, intestine48, and liver tissues49, such as restoring chondrocyte phenotypes by alleviating senescence and further reprogramming macrophages in an osteoarthritis model47. Their and our studies together, from different aspects and regarding different tissues, highlighted the underestimated function of this protein in mediating cell-matrix interactions, providing fresh insights for designing regenerative approaches for IDD.
However, devising biomaterials to enrich MFG-E8 in ECM must consider its compromised assembly with the GAGs in the ECM under IDD. Previous studies pointed out the possibility of native GAGs prone to degradation due to increased hyaluronidase under pathological conditions8, which we proved in the current study with supporting evidence from enzymatic cleavage experiments and histological examinations. Such a degenerative environment puts supplementing native GAGs at high risk of degradation, posing a challenge for stabilizing MFG-E8 in vivo. Therefore, we selected GM as the backbone structure and eventually screened out GMOC with demonstrated advantages. Compared with HA of similar molecular weight, the GM structure cannot be recognized by hyaluronidases and thus maintains stability to bind and enrich MFG-E8 in the ECM. In addition, like other polysaccharides with a comparable structure that undergo non-enzymatically mediated degradation, such as hydrolysis50, GMOC is expected to lose half of its mass in vivo after approximately 15-20 weeks, which could sustain its role in situ to facilitate neo-tissue growth in comparison with the literature51,52, while balancing the need for eventual degradation of the materials.
Our data showed that GMOC, similar to high molecular weight HA, formed assemblies with MFG-E8, yet the low molecular weight HA failed to do, again reflecting the importance of GAG stability in the matrix. Also, this structure contained only glucose and mannose53, two ubiquitously present monosaccharides in the body, hence minimizing the toxicity concern of potential degradation products. Compared with other saccharide-based GAG mimetics54, such as sulfated GlcNAc backbone with a Mw range between 16.3 kDa and 57.3 kDa55 or sulfated lactose56 with a Mw ranging from 2.1 kDa to 114 kDa, GMOC has a backbone of glucose and mannose, with a polymer Mw of 110 kDa and a certain degree of hydrophobicity owing to the octanyl modification. Also, compared with other minimally invasive treatment strategies, GMOC exerts its role in GAG regeneration by enriching endogenous functional proteins, thereby minimizing the potential risks associated with the introduction of exogenous cytokines or other biological agents57.
GMOC fulfilled the role of a bioactive ECM glue in sticking and enriching MFG-E8, leading to significantly promoted NP tissue repair. Particularly, a series of tests have demonstrated the effective assembly between this glycan and the target protein. Molecular docking predicted Lys as the binding site, confirmed as the polysaccharide-binding site of MFG-E824, followed by quantitative data from QCM-D showing direct evidence of their binding. Further, AFM revealed that GMOC and MFG-E8 assemble into a rod-like complex with a diameter of 150 nm, similar in morphology and size scale to cartilage aggrecan (50 nm)58, the chondroitin sulfate-acryloyl chloride complex (80 nm)59, and the glycan-protein complex formed by pectin and whey protein (300 nm)60. In addition, CETSA demonstrated their binding, resisting degradation at high temperatures, similar to other strong glycan-protein interactions, such as those between oligofructose and the transmembrane protein Rab2161 or the extracellular polysaccharide EPS11 and Type I collagen62. NPCs growing on GMOC exhibited a native-like morphology and healthy phenotype—which nevertheless were disrupted upon MFG-E8 silencing, again underscoring the critical role of restoring GMOC/MFG-E8 assembly in maintaining NP functions.
The regenerative capacity of GMOC was further validated in two different animal models—each representing a different scenario of clinical application. First, we applied a GMOC suspension into the rat intervertebral disc acupuncture degeneration model to simulate the preoperative intervention in IDD. Throughout the subsequent 4-week observation, GMOC effectively reduced NP tissue loss in the disc, relieved pain, and restored mechanical stability, suggesting its capability to not only replenish the lost ECM components but, more importantly, actively counter the degeneration by restoring the glycan/MFG-E8 assembly. This finding may imply a possibility of avoiding surgery for patients. Following this, we simulated discectomy by partially removing intervertebral disc tissue in rabbits. Filling of the defects by GMOC facilitated postoperative healing with a clinical envision for preventing recurrence. The treatment exhibited promising results over an extended 6-week observation, highlighting the translational potential of this glycan for specific clinical applications. The complex pathological mechanisms of IDD have prompted researchers to develop treatment strategies in various aspects63. Intervening in the inflammatory response64, restoring mechanical stability40, and promoting nucleus pulposus regeneration have all yielded positive results65. However, since the initiating factors of IDD are not uniformly defined, single-factor interventions still have certain limitations66. In addition to improving the hydration of NP tissue and the height of the intervertebral space, alleviating pain caused by IDD and restoring disc mechanical stability are also critical for comprehensively assessing the treatment efficacy37,40.
Two limitations of this work provide directions for future research. First, we attempted to seek Mfge8 knockout (KO) animals to study the detailed mechanism of this protein in NP tissue repair. Although Mfge8 KO mice have been reported in immunology research67, the small intervertebral disc structure in mice does not provide sufficient space for biomaterials implantation (hence we used rats and rabbits in this study). However, in rats, the genes encoding Mfge8 and Hapln3 overlap entirely68, and knocking out Mfge8 inadvertently affects the expression of Hapln3, thereby disrupting hyaluronic acid synthesis in the NP. A more delicate transgenic model in larger animals should be created. Second, for higher clinical relevance, the IDD treatment efficacy should be evaluated under the mechanical conditions in an upright setting69. Given the similarity in load-bearing and movement patterns to humans, primates may be considered the preferred IDD model for future biomechanical research. In addition, although the present study focuses on enriching and empowering (endogenous) MFG-E8 in ECM, it is worth exploring the potential of utilizing the affinity between this glycan-protein pair for delivering (exogenous) MFG-E8 with GMOC in future, which may fine-tune the dosage and release kinetics MFG-E8 in a particular tissue ___location in vivo. Addressing the above questions will enhance our understanding of devising more effective treatments for IDD.
Methods
More detailed experimental materials and methods are provided in the Supplementary Information.
Ethical statement
The collection and utilization of human NP specimens were approved by the Ethics Committee of the First Affiliated Hospital of Soochow University (approval no.2024-564). All degenerated NP tissues were obtained through surgical procedures, with written informed consent obtained in advance. The experimental animal procedures were reviewed and approved by the University of Macau Animal Research Ethics Committee (approval no. UMARE‐039‐2021), the Ethics Committee of Soochow University (approval no. SUDA20230522A02), and conformed to the Guidelines for the Care and Use of Laboratory Animals published by the National Institutes of Health, USA.
Chemicals and reagents
Konjac glucomannan (GM, <500 mPa·s) was provided by Shimizu Chemical (Japan). Hyaluronic Acid (HA, molecular weight: 3 kDa and 150 kDa) was purchased from Meilunbio (China). Hyaluronidase type I (Hyal-1) and II (Hyal-2) were purchased from Sigma Aldrich (USA). For primary antibodies, Rabbit anti-Collagen II, Rabbit anti-Aggrecan, and Rabbit anti-Sox9 were purchased from Abcam (UK). Rabbit anti-Hyal-1 and Rabbit anti-MFG-E8 from Thermo Scientific (USA). Rabbit anti-Integrin alpha-V was obtained from Proteintech (China). Rabbit anti-Akt and Rabbit anti-pAkt were obtained from Cell Signaling Technology (USA). The secondary antibodies, Alexa Fluor 488-conjugated goat-anti-rabbit, and Alexa Fluor 594-conjugated goat-anti-rabbit, were purchased from Life Technologies (USA). Phalloidin 488, Calcium AM/PI kit, and DAPI obtained from Beyotime Biotechnology (China). Recombinant rat MFG-E8 protein was purchased from Cusabio (China). Collagen solution was purchased from Advanced Biomatrix (USA). TFAA and other chemicals and reagents were purchased from Sigma Aldrich (USA) unless otherwise stated.
Synthesis of GM esters
The synthesis process followed previous research27,28. TFAA was stirred with different fatty acids at 50 °C for 20 min, and then freeze-dried GM was added to the reaction system, and the reaction continued for 3 h. The reaction solution was added dropwise to absolute ethanol to obtain a precipitate, which was collected and dissolved in chloroform. Then, the solution was added dropwise to absolute ethanol again, and after repeated washing to remove chloroform, the precipitate was collected and freeze-dried. Finally, obtain the corresponding GM ester.
Characterization of GM esters
Fourier transform infrared spectroscopy (FTIR)
A sample of 5 mg was finely ground with 200 mg of pure KBr in an agate mortar. Then, the mixture was placed in a mold and pressed into a transparent sheet in a hydraulic press. This sheet was subsequently used for dry determination in the FTIR spectrometer (Nicolet iS 10, Thermo Scientific, USA).
1H nuclear magnetic resonance (1H NMR) spectroscopy
GM esters were dissolved in CDCl3, and GM was dissolved in D2O. The samples were characterized by 1H NMR spectroscopy (Ascend 600 MHz, Burker, USA). The degree of modification of the acyl group was calculated from the ratio of the integral areas of the methyl proton signals of the acyl group to those of the ring proton signals of glucose and mannose27, as follows: DS = ([CH3]/3)/([ring-H]/7).
Scanning electron microscopy (SEM)
SEM by Hitachi S-4800 (Japan). Different GM esters were dissolved in chloroform, and 2% (w/v) solution was added dropwise onto the glass surface and dried, obtaining the corresponding GM ester coating. After gold sputter coating, images were captured using an accelerating voltage of 2 kV.
Rheological characterization
Rheological testing was carried out on a DHR-2 Rheometer (TA Instruments, USA), using a 20 mm measuring plate. Selected the oscillation time sweep mode and conducted the test at a temperature of 37 °C, applying a strain of 1.0 % and a frequency of 1.0 Hz. Determine the gelation time of the collagen solution by observing the crossover point of G’ (storage modulus) and G” (loss modulus). After gelation, recorded the G’ storage modulus to evaluate the mechanical properties of the collagen gel.
Preparation and characterized of GMOC particles
Preparation of GMOC particles
A GMOC chloroform solution (4 mg.mL−1) was dropped into a 5 mL polyvinyl alcohol (1%, w/v) solution and sonicated for 2 min on ice by a probe sonicator (T-10, IKA, Germany). The solution was collected and subjected to magnetic stirring overnight. To remove residual solvents and unwanted byproducts, the solution was dialyzed against deionized water using dialysis tubes (MW 3500). The dialysis process was carried out for 7 days. Finally, the GMOC particles were obtained through lyophilization and stored for further use.
Characterized of GMOC particles
The sizes and zeta-potential of GMOC particles were measured by Zetasizer Nano ZS dynamic light scattering (DLS, Malvern Instruments, UK). The morphology of the particles was observed by SEM (Hitachi S-4800, Japan).
Phagocytosis of GMOC
The phagocytosis experiment of GMOC was detected using flow cytometry assay. GMOC was labeled with Cy5.5 fluorescence according to the reported method70 and co-cultured with NPCs. At different time points (0, 24, and 72 h), NPCs were harvested and washed with PBS to remove un-phagocytosed GMOC particles. All samples were gated on FSC-H/SSC-H to remove debris. Cy5.5 was used to differentiate between positive and negative populations. Gating strategies were illustrated in Supplementary Fig. 7.
The ability of GMOC to enrich MFG-E8
The characterization of GMOC-enriched MFG-E8 followed a procedure similar to the immunofluorescence (IF) staining protocol. Specifically, 10 mg GMOC particles were incubated with 1 μg.mL−1 MFG-E8 solution at 37 °C overnight. The mixture was then centrifuged at 2000 × g for 5 min and washed three times with PBS. After centrifugation, the particles were incubated with rabbit anti-MFG-E8 antibody at 4 °C overnight. Then, particles were incubated with Alexa Fluor 488-conjugated goat-anti-rabbit antibody at room temperature for 2 h. After further centrifugation and washing, the particles were resuspended in PBS and observed under a DMI8 fluorescence microscope (Leica, Germany).
Molecular docking simulation
The two-dimensional structures of the sugar units serving as ligands were created using ChemDraw 14.0 software (CambridgeSoft Co., UK). Subsequently, molecular docking simulations were carried out and visualized using Molecular Operating Environment (MOE) (Chemical Computing Group Inc., Canada), following established reference protocols. All ligands underwent preparation using the “Prepare” module within MOE, ensuring rigorous validation of the conformational rationality of each ligand. The crystal structure of the Rattus norvegicus MFG-E8 complex (Primary accession code of UniProt: P70490) was selected from the AlphaFold Protein Structure Database (https://alphafold.ebi.ac.uk/entry/P70490) due to its significant similarity to our research subjects and the original ligand. Protein preparation was performed utilizing the “Prepare” modules within MOE, incorporating Amber14: EHT minimization techniques. The binding site was defined based on the original ligand’s positioning. Given the spatial inversion possibilities of the ligands, a total of 30 poses were generated until the most suitable conformation within the protein helix, indicating optimal stability of the resulting complex. Scoring energy values were determined using the London dG scoring function, which underwent two rounds of improvement through the triangle Matcher methods to enhance the ranking of interaction efficiency. The conformations were subsequently ranked based on their energy indices.
Enzymolysis of GM, hyaluronic acid (HA), and GM esters
Enzymolysis of GM and HA
50 mg of GM or HA (150 kDa) was dissolved in 10 mL of ddH2O (PH = 5.3), then 5 mg of Hyal-1 or Hyal-2 was dissolved in 1 mL of ddH2O and added to the sample solution. After 6 h at 37 °C, the reaction solution was collected, passed through 0.45 μm hydrophilic membrane, and detected by High-performance liquid chromatography (HPLC) combined with a refractive index detector (RID) system with a TSK G4000PWXL column (7.8 × 300, 10 μm, from Tosoh Bioscience, Japan).
Enzymolysis of GM ester
For GM esters, 5 mg of Hyal-1 or Hyal-2 was dissolved in 1 mL of ddH2O and mixed with the different series of GM esters (10 mg). Ensure that the GM esters are fully immersed in the reaction solution. The reaction lasted for 7 days at 37 °C. Following the reaction period, the samples were carefully removed and subjected to vacuum drying to remove residual moisture. The weight loss of the samples was measured to assess the extent of reaction.
Fluidity of HA
The 500 µL of a 4% (w/v) hyaluronic acid gel containing 0.5% (w/v) Hyal-2 was prepared and incubated at 37 °C. Samples were recorded at 0, 1, 2, 3, 6, and 12 h, inverted the tubes, and observed the flowability of the HA gel to assess the effectiveness of enzymolysis.
The morphology and volume of GM esters
The GMOC was used as a representative. 5 mg GMOC placed in a solution containing 0.5% (w/v) Hyal-2 and incubated at 37 °C. Photographs were recorded sequentially at 0, 1, 3, 5, and 7 days to monitor changes in appearance.
Validation of the binding between MFG-E8 and GM esters
Quartz crystal microbalance with dissipation (QCM-D)
QCM-D was used to study the interactions between MFG-E8 and GM esters. Different GM esters were coated onto gold QSensor (QSX 301). For the QCM-D procedure, the sensor was equilibrated in buffer (ddH2O) in a Q-sense instrument (Biolin Scientific, Sweden) until a stable baseline. MFG-E8 was dissolved in buffer (1 mg.mL−1) and injected above the sensor at 50 μL.min−1. The binding mass was calculated using the Sauerbrey equation in QTools software (Biolin Scientific, Sweden).
Dot-blotting
Dot-blotting to quantify the enrichment MFG-E8 effect of GM esters. The chloroform solutions of GM esters (2%, w/v) were separately prepared and then carefully dropped onto the surface of glass substrates. After evaporating, the glass coating was washed thrice with PBS and soaked in 75% alcohol overnight to ensure sterilization. For the recombinant MFG-E8 group, the coating glass was immersed in a PBS solution containing 1 μg.mL−1 of MFG-E8 and incubated overnight at 37 °C. Subsequently, the surface of the coating glass was rinsed three times with PBS to remove any unbound MFG-E8. For the NPCs co-culture group, 500 μL of the culture medium containing NPCs was dropped onto the surface of the sterile coating glass. After allowing the cells to adhere for 4 h, an additional 500 μL of culture medium was added, and the cells were cultured for 72 h. Following the culture period, the surface of the coating glass was digested using trypsin-EDTA to remove the adhered NPCs. The substrates were then washed three times with PBS. These substrates were incubated with anti-MFG-E8 antibody overnight at 4 °C. After washed, substrates were incubated with HRP-conjugated anti-rabbit antibody (Cell Signaling Technology, USA) for 1 h at room temperature. The enriched MFG-E8 was visualized with fluorography using an enhanced chemiluminescence system (Biorad, USA).
Atomic force microscopy (AFM) imaging
Briefly, 50 μL of GMOC (2%, w/v), 3 kDa (2%, w/v), or 150 kDa (2%, w/v) hyaluronic acid solutions were separately incubated onto the surface of freshly cleaved mica and dry naturally. Subsequently, a 50 μL droplet of 1 μg.mL−1 solutions of recombinant protein MFG-E8 was incubated on the functionalized mica surface overnight and air-dried to form the binding structure of MFG-E8. AFM images were obtained by tapping mode imaging using nanosized silicon tips in Dimension Icon (Bruker AXS, USA).
Cellular thermal shift assay (CETSA)
The blotting assay was performed based on the CETSA with some modifications71. GMOC (2%, w/v), 3 kDa (2%, w/v), or 150 kDa (2%, w/v) hyaluronic acid was individually mixed with NPCs lysate (200 mg.mL−1) and incubated at 37 °C for 2 h to ensure complete binding to MFG-E8. Single NPCs lysate was used as the control group. Each mixture equally (500 μL) was divided into five small aliquots (100 μL each). A thermal cycler was used to set temperature points at 55, 59, 63, 67, and 71 °C for each aliquot. The samples at each temperature point were heated for 4 min and cooled for 3 min. Then, the samples were centrifuged at 17,000 × g for 30 min at 4 °C. The soluble fractions were collected, and the total protein was separated by 10% SDS-PAGE. The protein bands were labeled using a rabbit-anti-MFG-E8 antibody. Subsequent procedures followed the western blotting protocol, and the intensity of the bands was quantified.
Nucleus pulposus cell (NPCs) isolation and culture
NPCs isolation
NPCs were isolated from rat caudal disks following the previous protocol5. Briefly, NP tissues were collected from caudal disks. Under sterile conditions, NP tissues were washed with PBS to remove blood and digested in 0.5% (w/v) collagenase-II (Sigma, USA) for 20 min at 37 °C. The digested specimen was then centrifuged at 1000 × g for 3 min to collect the NPCs. The NPCs were cultured with the F-12 DMEM (Gibco, USA) with 15% fetal bovine serum (Gibco, USA) and 1% antibiotics (Gibco, USA).
NPCs co-culture with GM esters
For GM esters co-culture with NPCs. Prepared the 2% (w/v) sterile GM esters coating and wetted by F-12 DMEM (Gibco, USA), the suspension containing NPCs was carefully dropped onto the GM esters coating surface.
Live and dead staining
After preparing the sterile GM esters coating as described previously, the NPCs suspension was dropped onto the GM esters coating surface, while the control group was dropped onto the glass surface. All groups were incubated at 37 °C with 5% CO2 for 24 h. After the incubation period, the medium was removed, and the coating surface was covered with the working buffer (AM 1:1000 and PI 1:1000) from the Live-Dead kit (Beyotime, China). The coated surfaces were then incubated at 37 °C in the dark for 2 h and observed under a Dmi8 fluorescence microscope (Leica, Germany).
Number of NPCs adhesion on GM esters
A series of cell quantities was prepared as a gradient in a 24-well plate. The quantities used were 5 × 102, 1 × 103, 2 × 103, 4 × 103, 8 × 103, 16 × 103, and 32 × 103 NPCs per well and incubated for 12 h to allow all NPCs adhere to the well. Following the instructions of Cell Counting Kit-8 (Dojindo Co., Japan), the working buffer was incubated with each sample for 4 h. Optical density (OD) was measured at a wavelength of 450 nm using a Gen5 microplate reader (BioTek Instruments, Inc., USA). A linear regression equation between the number of NPCs and the corresponding OD value was established.
Proliferation curve on GM esters
The number of NPCs adhesion on GM esters coating. Sterile GM esters with a diameter of 14 mm were prepared and placed in 24-well plates. 1×104 NPCs were added to each well, and glass surface as the control group. After 24 h, the culture medium was carefully removed from each well to eliminate non-adherent NPCs. The OD (450 nm) value of each well was detected, and the number of adherent cells was calculated by referencing the standard proliferation curve. For the NPCs proliferation curve, the OD450 nm value was detected by different time points (1, 2, 3, 4, 5, 6, and 7 days).
Circularity of NPCs on GM esters
NPCs were co-cultured with different GM ester coatings for 3 days. Phalloidin at a dilution of 1:200 was used to label the cytoskeleton, while DAPI at 1:1000 was used to label nuclei. Observations were made under a fluorescence microscope. Then, ImageJ (National Institutes of Health, USA) was used for quantification. The circularity72 of NPCs was measured using the formula 4πA/P2 (A: area, P: perimeter) to measure eight cells in each group.
Cluster number of NPCs on GM esters
The clusters of NPCs were quantified based on the labeling of nuclei with DAPI. The number of NPCs forming a cluster was counted manually. NPC clusters were categorized as follows: small clusters (consisting of 2-5 NPCs), middle clusters (6-15 NPCs), and large clusters (more than 16 NPCs).
Immunofluorescence (IF) staining
IF staining of tissue section
Tissue sections were deparaffinized, rehydrated, and subjected to antigen retrieval operations. The sections were blocked with BSA (5%, 30 min) and incubated with primary antibodies at 4 °C overnight. Primary antibodies (diluted 1:200) include anti- MFG-E8, anti-Collagen II, anti-Aggrecan, anti-Sox9 and anti-Hyal-1. Next, the sections were incubated with secondary antibodies for 1 h at room temperature. The next primary antibody staining was performed sequentially.
IF staining of cell sample
For the cell sample, the NPCs were fixed with paraformaldehyde (4 °C, 20 min), treated with Triton X-100 at room temperature for 10 min, and blocked with BSA (5%, 30 min). Follow-up steps are referred to in the section staining protocol. Finally, the cytoskeleton was labeled with phalloidin (diluted 1:200), and the nuclei were stained with DAPI (diluted 1:1000). All fluorescence was captured with the Dmi8 microscope (Leica, Germany).
Fluorescently labeled MFG-E8 distribution
For the spatial distribution of MFG-E8, NPCs were co-cultured with different substrates (glass, GMAC, and GMOC) for 3 days. Living NPCs were removed with trypsin-EDTA and the substrates were washed with PBS three times. These substrates were incubated with anti-MFG-E8 antibody and Fluor 488-conjugated goat-anti-rabbit and observed.
Small interfering (si) RNA silenced Mfge8 expression in NPCs
The siRNA of Mfge8 was synthesized from GenePharma (China). The sequence was 5’-GCCUGAAGUAACAUGUUT-3’ and 3’-AACAUGUUAACAGGCTT-5’. Transient transfection of si-Mfge8 into NPCs by RFectPMsiRNA/miRNA Transfection (Baidai Biotechnology, China) according to instructions. NPCs were transfected with 50 nM siRNA and incubated for 72 h. The transfection efficiency of siRNA was evaluated by observing the entry of Cy3-labeled siRNA into cells using fluorescence microscopy and by measuring the expression levels of Mfge8 using real-time qPCR. The treated NPCs were collected to prepare a suspension and dropped onto the glass, GMAC, or GMOC-coated surface, respectively, for co-culture.
The effect of exogenous MFG-E8 on NPCs
As previously described, NPCs were treated with or without siRNA to reduce MFG-E8 expression. Then, NPCs were cultured on glass or GMOC surfaces, with or without the addition of recombinant MFG-E8 protein (1 mg/mL), and co-cultured for 72 h. The morphology changes of the NPCs were observed by IF.
Inhibition of integrin alpha-V function
The selective and nonpeptide pan-integrin MK0429 was purchased from MedChemExpress (MCE, China). The NPCs were co-cultured with 5 nM MK0429 for 24 h. Subsequently, the treated NPCs were collected and deposited onto the surfaces of glass, GMAC, or GMOC for subsequent experiments, respectively.
Human musculoskeletal system database (MSdb) analysis
The Human Musculoskeletal System Database (MSdb, www.msdb.org.cn) is an integrated expression map for the musculoskeletal system in humans20. On the online platform, we selected the intervertebral disc and Healthy sample data as the control group (n = 7, GSM5098913, GSM5098915, GSM3995365, GSM3995366, GSM3995368, GSM4117765, GSM4117768), while individuals with Degenerative constituted the test group (n = 7, GSM3897507, GSM3897508, GSM4409344, GSM4409346, GSM4663150, GSM4663151, GSM5098919). We utilized the differential expression analysis module available in MSdb to perform a comparative analysis between these two groups. The expression levels of specific genes are depicted using boxplots to visualize the results.
Human NP tissue collection
With informed consent from the patients. The preoperative MRI examination was used to determine the Pfirrmann grade of the degenerated disks25. A total of fourteen degenerated NP tissues (n = 14) were obtained from the patients with lumbar disc herniation. For MRI results, three patients were classified as grade II (n = 7), and three as grade IV (n = 7). During the study, no complications were reported in the patients from whom the NP tissues were collected during surgery.
Enzyme-linked immunosorbent assay (ELISA) assay of MFG-E8
For vivo samples, NP tissue from IDD patients or rat was collected. After weight normalization, the tissue was homogenized in PBS and collected the supernatant for MFG-E8 protein concentration test. The experimental procedures followed the instructions of the ELISA kit (EH0226 human kit from Finetest and 11284R2 rat kit from JINGMEI, China).
Immunohistochemistry (IHC) staining
Human IDD NP tissues were fixed in 10% formalin for 24 h and subsequently embedded in paraffin. The samples were then sectioned at a thickness of 5 μm, then dewaxing, gradient-dehydrated, and antigen retrieval. The sections were immersed in 3% H2O2 buffer for 5 min, then blocked with 5% BSA and labeled Type II collagen (1:200, Abcam) or Aggrecan (1:200, Abcam) solution, respectively. DAB was performed as the chromogen following the DAB Horseradish Peroxidase Color Development Kit (Keygen Biotech, China).
Quantitative real-time qPCR and transcriptome RNA sequencing (RNA-Seq)
Following standard protocols, total RNA was extracted from rat or human NPCs with Trizol (Invitrogen, USA). Subsequently, the extracted RNA was reverse transcribed into cDNA using GoScript™ Reverse Transcriptase kit (Promega, USA) according to the instructions. Quantitative real-time qPCR analysis was performed using a GoTaq® qPCR Master Mix Kit (Promega, USA) in a CFX96 Touch Real-Time PCR Detection System (Bio-Rad, USA). Normalization was performed using GAPDH as an internal control within the same sample to determine the relative mRNA expression levels of the target genes. The primer sequences used for PCR amplification are provided in Supplementary Table 1.
RNA-sequencing libraries were constructed using a Seq-Star™ Rapid RNA-Seq Kit (Illumina, USA), and the cDNA was sequenced on the Illumina Hiseq6000 (Shanghai Meiji Biomedical Technology Inc., China). All data were analyzed on the online Majorbio Cloud Platform (www.majorbio.com).
Western blotting
Total NPCs protein was extracted by RAPI (Beyotime, China), separated by 10% SDS-PAGE, and transferred to a polyvinylidene fluoride membrane. The membrane was blocked with 5% (w/v) skim milk and incubated with primary antibodies AKT or p-AKT (1:1000, Cell Signaling Technology, USA) at 4 °C with gentle shaking overnight. After three times washed with TBST (50 mM Tris. pH 7.4, 150 mM NaCl, 0.05% Tween 20), the blots were incubated with HRP-conjugated anti-rabbit secondary antibody (Cell Signaling Technology, USA) for 1 h at room temperature. After being washed, bands were visualized with fluorography using an enhanced chemiluminescence system.
Animal and IDD model establishment
All animals were kept in an SPF facility and treated with controlled light (12 h light-dark cycles), humidity, and temperature, and provided with a standard chow diet and water.
Rat model
Male Sprague-Dawley (SD) rats (6 weeks old, 200 ± 20 g) were purchased from the Animal Research Core, Faculty of Health Sciences, University of Macau (Macau). For the IDD model, male rats were preferred in the study to exclude the influence of hormones, and the surgical protocol was improved based on the previous procedure73. Briefly, SD rats underwent puncturing at the caudal disc (Co) via a 21 G percutaneous needle, the depth is half of disc, and a 360° rotation within the disc for 30 s. The caudal segments were divided into four groups: group I (Co9-10) as the blank control; group II (Co6-7) receiving GMOC treatment with GMOC PBS solution (1%, w/v) injection 5 μL after punctured; group III (Co7-8) as the PBS treatment, injected 5 μL of PBS solution after punctured, and group IV (Co8-9) as IDD without treatment after punctured. Caudal disks were collected for imaging examinations at 2 and 4 weeks (n = 8) to evaluate the effects of the treatments on IDD. In the exogenous MFG-E8 intervention IDD experiment, the surgical procedures were consistent with the previously described, and the treatment group received a single injection of exogenous MFG-E8 (10 mg/mL, 5 μL) for subsequent radiological examinations.
Rabbit model
The male New Zealand white rabbits (1 year old, 4000 ± 500 g) were purchased from the Zhenhu Experimental Animal Technology Co. Ltd. of Suzhou (China). The rabbits were positioned in the lateral decubitus position, and the lumbar spine structure was exposed using the anterolateral approach. Following the previous IDD surgical procedure5 in a rabbit model, an 18 G needle was utilized to create a partial loss of the annulus fibrosus and nucleus pulposus tissue. The lumbar segments were divided into four groups. Group I (L2-3): blank control; group II (L3-4): Vehicle group with an injection of 15 μL matrigel after puncture; group III (L4-5): IDD, punctured without any treatment, and group IV (L5-6): vehicle+GMOC, injected with 15 μL matrigel mixed with GMOC (1%, w/v) after punctured. Lumbar disks were collected for imaging examinations at 3 and 6 weeks (n = 5) to assess the effects of the treatments on the intervertebral disc degeneration model in rabbits.
Mechanical testing of GMOC implants in rat disc
Consistent with the surgical procedures of the IDD rat model, a single segment disc was used for mechanical testing74. After 4 weeks of implantation, the experimental disc was removed along with the adjacent vertebrae and separated from the surrounding soft tissue. The upper and lower vertebrae were fixed in a mechanical testing device (HY-0580, Shanghai Heng Yi Co., Ltd., China) to ensure that their coronal-sagittal plane was aligned with the testing fixture. The tensile-compression cycle test parameters were set to a tension of 0.5 N, compression of 1.5 N, and frequency of 0.5 Hz. 25 cycles were performed to measure the range of motion and compression stiffness.
Pain assessment assay
The animal surgery was consistent with the previously described surgical procedures for the IDD rat model. A single segment disc was used for the tail flick test. All experimental groups were assessed at 2- and 4-week post-surgery. The method, as shown by Pandit et al. 37, involved focusing a radiation source on the side opposite the surgical wound at 50% of the maximum power. The latency of the rat’s tail flick was recorded, with a maximum exposure time of 20 s to avoid injury. Each rat underwent four measurements at the testing point, with a 5 min interval between each measurement, and the latency was calculated as the average of the four measurements.
Morphological staining
H & E staining
Tissue sections were deparaffinized and rehydrated. Then, sections were stained with hematoxylin (Solarbio, China) for 5 min. The tissue sections were subjected to an acidic differentiation solution to achieve the desired staining intensity under microscopic examination. Following acidic differentiation, the sections were treated with an alkaline solution (Solarbio, China). The sections were then stained with eosin to a suitable degree. Finally, the sections were dehydrated, cleared, and sealed.
Safranin O & fast green staining
Tissue sections were deparaffinized and rehydrated. A Safranin O & Fast Green staining kit (Solarbio, China) was used for staining, following the instructions. Briefly, the sections were stained with Weigert’s staining solution for 5 min, followed by acidic differentiation for 15 s. Then, sections were immersed in Fast Green staining solution for 5 min, washed with the weak acid solution for 10 s, and air dried. The sections were stained to a suitable degree within the Safranin O staining solution, followed by dehydrated cleared, and sealed.
Radiological examination and analysis
According to the time points after IDD surgery, both rats (at 2 and 4 weeks) and rabbits (at 3 and 6 weeks) underwent examination by X-ray and Magnetic Resonance Imaging (MRI). The animals were positioned in a supine position on a molybdenum target radiographic image unit (General Electric, USA) to collect X-ray images. For MRI imaging, a 1.5 T system (General Electric, USA) was used to obtain T2-weighted images. All radiographic images were analyzed in the PACS system (Neusoft, China) by radiologists. In the X-ray image, the disc height index (DHI) was measured following the previous method73. For MRI imaging, the water content of the NP tissue was calculated based on the signal intensity of the NP tissue in the T2-MRI results5.
4D-label free quantification and analysis of protein on GM esters coating surface
Collected 5 × 107 NPCs and followed the instructions of the Membrane and Cytosol Protein Extraction Kit (Beyotime, China) to isolate NPCs’ membrane proteins. The membrane protein solution was incubated with glass and GM esters coating at 37 °C overnight. The samples were washed three times with PBS to remove any unbound or non-specifically adsorbed protein components. The enriched protein was hydrolyzed by protease (V5280, Promega, USA) to obtain peptide fragments, which were desalted and purified by the MonoSpin C18 column (GL Sciences, USA). Subsequently, an equal amount of sample was taken for liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis. The obtained peptide sequences were searched against The Universal Protein Resource (UniProt) database to identify the proteins adsorbed on the sample surface (Shanghai Meiji Biomedical Technology Inc., China). Data were analyzed on the online Majorbio Cloud Platform (www.majorbio.com).
Statistical analysis
The results are presented as fold change, percentage change (%), or mean values ± standard deviation (SD). All data-statistical analysis was conducted using GraphPad Prism 8 (GraphPad Software, USA). Two different groups were analyzed by two-sided Mann-Whitney test, and multiple groups (three or more) were compared using the Kruskal-Wallis test, followed by Dunn’s post hoc test for multiple comparisons. The value of P < 0.05 was considered significant. The numbers of replicates shown in corresponding figure legends, the P-value is shown in each Figure and Source Data file.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The data supporting the findings of this study are available within the Supplementary Information and Source Data file. The mass spectrometry proteomics data have been deposited to the iProX with the project ID IPX0009307000 (https://www.iprox.cn/page/subproject.html?id=IPX0009307001). The RNA-seq data generated in this study have been deposited in NCBI BioProject database under accession code: PRJNA1137599. The RNA-Seq datasets used in Fig. 2A-D are public datasets available in MSdb (IDD sample: GSM3897507, GSM3897508, GSM4409344, GSM4409346, GSM4663150, GSM4663151, GSM5098919. Health sample: GSM5098913, GSM5098915, GSM3995365, GSM3995366, GSM3995368, GSM4117765, GSM4117768) (https://www.msdb.org.cn). Any additional requests for information are available upon request to the corresponding author (C.W.; [email protected]). Source data are provided with this paper.
References
Oichi, T., Taniguchi, Y., Oshima, Y., Tanaka, S. & Saito, T. Pathomechanism of intervertebral disc degeneration. JOR Spine 3, e1076 (2020).
Kirnaz, S. et al. Pathomechanism and biomechanics of degenerative disc disease: features of healthy and degenerated discs. Int. J. Spine Surg. 15, 10 (2021).
Blamoutier, A. Surgical discectomy for lumbar disc herniation: surgical techniques. Orthop. Traumatol. Surg. Res. 99, S187–S196 (2013).
Romaniyanto et al. An update of current therapeutic approach for intervertebral disc degeneration: a review article. Ann. Med. Surg. 77, 103619 (2022).
Liu, Y. et al. Regulation of the inflammatory cycle by a controllable release hydrogel for eliminating postoperative inflammation after discectomy. Bioact. Mater. 6, 146–157 (2021).
Gullbrand, S. E., Smith, L. J., Smith, H. E. & Mauck, R. L. Promise, progress, and problems in whole disc tissue engineering. JOR SPINE 1, e1015 (2018).
Malik, K. & Benzon, H. T. 21 - Low Back Pain. In: Practical Management of Pain (Fifth Edition) (eds Benzon H. T., Rathmell J. P., Wu C. L., Turk D. C., Argoff C. E., Hurley R. W.) (Mosby, 2014).
Silagi, E. S., Shapiro, I. M. & Risbud, M. V. Glycosaminoglycan synthesis in the nucleus pulposus: dysregulation and the pathogenesis of disc degeneration. Matrix Biol. 71-72, 368–379 (2018).
Kazezian, Z., Joyce, K. & Pandit, A. The role of hyaluronic acid in intervertebral disc regeneration. Appl. Sci. 10, 6257 (2020).
Krupkova, O. et al. Expression and activity of hyaluronidases HYAL-1, HYAL-2 and HYAL-3 in the human intervertebral disc. Eur. Spine J. 29, 605–615 (2020).
Sivan, S. S., Wachtel, E. & Roughley, P. Structure, function, aging and turnover of aggrecan in the intervertebral disc. Biochim. Biophys. Acta 1840, 3181–3189 (2014).
Song Y., Zhang F., Linhardt RJ. Glycosaminoglycans. In: The Role of Glycosylation in Health and Disease (ed^(eds Lauc G, Trbojević-Akmačić I). Springer International Publishing (2021).
Hachim, D., Whittaker, T. E., Kim, H. & Stevens, M. M. Glycosaminoglycan-based biomaterials for growth factor and cytokine delivery: Making the right choices. J. Control. Release 313, 131–147 (2019).
van der Kraan, P. M., Buma, P., van Kuppevelt, T. & van den Berg, W. B. Interaction of chondrocytes, extracellular matrix and growth factors: relevance for articular cartilage tissue engineering. Osteoarthr. Cartil. 10, 631–637 (2002).
Yang, S., He, Z., Wu, T., Wang, S. & Dai, H. Glycobiology in osteoclast differentiation and function. Bone Res. 11, 55 (2023).
Kleene, R. & Schachner, M. Glycans and neural cell interactions. Nat. Rev. Neurosci. 5, 195–208 (2004).
Etulain, J. & Schattner, M. Glycobiology of platelet–endothelial cell interactions. Glycobiology 24, 1252–1259 (2014).
Yang, C. et al. The high and low molecular weight forms of hyaluronan have distinct effects on CD44 clustering. J. Biol. Chem. 287, 43094–43107 (2012).
Feng, R., Zhu, L., Heng, X., Chen, G. & Chen, H. Immune effect regulated by the chain length: interaction between immune cell surface receptors and synthetic glycopolymers. ACS Appl. Mater. Interfaces 13, 36859–36867 (2021).
Tian, R. et al. MSdb: An integrated expression atlas of human musculoskeletal system. iScience 26, 106933 (2023).
Oshima, K., Yasueda, T., Nishio, S. & Matsuda, T. MFG-E8: origin, structure, expression, functions and regulation. In: MFG-E8 and Inflammation (eds Wang P.) (Springer Netherlands, 2014).
Ma, H. et al. MFG-E8 alleviates intervertebral disc degeneration by suppressing pyroptosis and extracellular matrix degradation in nucleus pulposus cells via Nrf2/TXNIP/NLRP3 axis. Cell Death Discov. 8, 209 (2022).
Hanayama, R. et al. Identification of a factor that links apoptotic cells to phagocytes. Nature 417, 182–187 (2002).
Kahmann, J. D. et al. Localization and characterization of the hyaluronan-binding site on the Link module from human TSG-6. Structure 8, 763–774 (2000).
Pfirrmann, C. W. A., Metzdorf, A., Zanetti, M., Hodler, J. & Boos, N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine 26, 1873–1878 (2001).
Mai, J., Wang, K., Liu, C., Xiong, S. & Xie, Q. αvβ3-targeted sEVs for efficient intracellular delivery of proteins using MFG-E8. BMC Biotechnol. 22, 15 (2022).
Mu, R. et al. A “Bridge-Building” glycan scaffold mimicking microbial invasion for in situ endothelialization. Adv. Mater. 33, 2103490 (2021).
Feng, Y. et al. A toll-like receptor agonist mimicking microbial signal to generate tumor-suppressive macrophages. Nat. Commun. 10, 2272 (2019).
Li, Y. The mechanism of collagen self-assembly: hydrophobic and electrostatic interactions. University of Florida (2009).
Xie, Q. et al. Beyond temperature: controlling collagen fibrillogenesis under physiological conditions via interaction with cucurbit [7] uril. Chem. Commun. 56, 4946–4949 (2020).
Hwang, P. Y. et al. N-cadherin is key to expression of the nucleus pulposus cell phenotype under selective substrate culture conditions. Sci. Rep. 6, 28038 (2016).
Hwang, P. Y., Chen, J., Jing, L., Hoffman, B. D. & Setton, L. A. The role of extracellular matrix elasticity and composition in regulating the nucleus pulposus cell phenotype in the intervertebral disc: a narrative review. J. Biomech. Eng. 136, 021010 (2014).
Wang, Y. et al. Spheroid formation enhances the regenerative capacity of nucleus pulposus cells via regulating N-CDH and ITGβ1 interaction. Int J. Biol. Sci. 18, 3676–3696 (2022).
Lv, F. et al. In search of nucleus pulposus-specific molecular markers. Rheumatology 53, 600–610 (2014).
Minogue, B. M., Richardson, S. M., Zeef, L. A. H., Freemont, A. J. & Hoyland, J. A. Characterization of the human nucleus pulposus cell phenotype and evaluation of novel marker gene expression to define adult stem cell differentiation. Arthritis Rheumat. 62, 3695–3705 (2010).
Guo, F., Zou, Y. & Zheng, Y. Moracin M inhibits lipopolysaccharide-induced inflammatory responses in nucleus pulposus cells via regulating PI3K/Akt/mTOR phosphorylation. Int. Immunopharmacol. 58, 80–86 (2018).
Mohd Isa, I. L. et al. Implantation of hyaluronic acid hydrogel prevents the pain phenotype in a rat model of intervertebral disc injury. Sci. Adv. 4, eaaq0597 (2018).
Mosley, G. E., Evashwick-Rogler, T. W., Lai, A. & Iatridis, J. C. Looking beyond the intervertebral disc: the need for behavioral assays in models of discogenic pain. Ann. N. Y Acad. Sci. 1409, 51–66 (2017).
Peredo, A. P. et al. Tension-activated nanofiber patches delivering an anti-inflammatory drug improve repair in a goat intervertebral disc herniation model. Sci. Transl. Med. 15, eadf1690 (2023).
Fidai, A. B. et al. Flexible support material maintains disc height and supports the formation of hydrated tissue engineered intervertebral discs in vivo. JOR SPINE 7, e1363 (2024).
Malhotra, N. R. et al. An injectable nucleus pulposus implant restores compressive range of motion in the ovine disc. Spine 37, E1099–E1105 (2012).
Schaefer, D. et al. Tissue-engineered composites for the repair of large osteochondral defects. Arthritis Rheumat. 46, 2524–2534 (2002).
Binch, A. L. A., Fitzgerald, J. C., Growney, E. A. & Barry, F. Cell-based strategies for IVD repair: clinical progress and translational obstacles. Nat. Rev. Rheumatol. 17, 158–175 (2021).
Chery, D. R. et al. Decorin regulates cartilage pericellular matrix micromechanobiology. Matrix Biol. 96, 1–17 (2021).
Berdiaki, A. et al. Extracellular matrix components and mechanosensing pathways in health and disease. Biomolecules. 14, 1186 (2024).
Huang, W. et al. MFG-E8 accelerates wound healing in diabetes by regulating “NLRP3 inflammasome-neutrophil extracellular traps” axis. Cell Death Discov. 6, 84 (2020).
Lu, Y. et al. MFG-E8 regulated by miR-99b-5p protects against osteoarthritis by targeting chondrocyte senescence and macrophage reprogramming via the NF-κB pathway. Cell Death Dis. 12, 533 (2021).
Bu, H.-F. et al. Milk fat globule–EGF factor 8/lactadherin plays a crucial role in maintenance and repair of murine intestinal epithelium. J. Clin. Investig. 117, 3673–3683 (2007).
Matsuda, A. et al. Milk fat globule-EGF factor VIII ameliorates liver injury after hepatic ischemia-reperfusion. J. Surg. Res. 180, e37–e46 (2013).
Soldi V. Stability and degradation of polysaccharides. Polysaccharides: Structural diversity and functional versatility, 395-409 (2004).
Shah, S. S. et al. Optimization of degradation profile for new scaffold in cartilage repair. Cartilage 9, 438–449 (2018).
Tajvar, S., Hadjizadeh, A. & Samandari, S. S. Scaffold degradation in bone tissue engineering: an overview. Int. Biodeterior. Biodegrad. 180, 105599 (2023).
Yin, X. et al. Unmasking chemokine-inducing specificity in oligosaccharide biomaterial to promote hair growth. Adv. Mater. 36, 2304655 (2024).
Morla, S. Glycosaminoglycans and glycosaminoglycan mimetics in cancer and inflammation. Int. J. Mol. Sci. 20, 1963 (2019).
Grande, D., Baskaran, S., Baskaran, C., Gnanou, Y. & Chaikof, E. L. Glycosaminoglycan-mimetic biomaterials. 1. Nonsulfated and sulfated glycopolymers by cyanoxyl-mediated free-radical polymerization. Macromolecules 33, 1123–1125 (2000).
Sun, X.-L., Grande, D., Baskaran, S., Hanson, S. R. & Chaikof, E. L. Glycosaminoglycan mimetic biomaterials. 4. Synthesis of sulfated lactose-based glycopolymers that exhibit anticoagulant activity. Biomacromolecules 3, 1065–1070 (2002).
Wang, M. et al. Articular cartilage repair biomaterials: strategies and applications. Mater. Today Bio 24, 100948 (2024).
Prudnikova, K. et al. Biomimetic proteoglycans mimic macromolecular architecture and water uptake of natural proteoglycans. Biomacromolecules 18, 1713–1723 (2017).
Prudnikova, K. et al. Aggrecan-like biomimetic proteoglycans (BPGs) composed of natural chondroitin sulfate bristles grafted onto a poly(acrylic acid) core for molecular engineering of the extracellular matrix. Acta Biomater. 75, 93–104 (2018).
de Jong, S. & van de Velde, F. Charge density of polysaccharide controls microstructure and large deformation properties of mixed gels. Food Hydrocoll. 21, 1172–1187 (2007).
Liang, J. et al. Fructooligosaccharides and fructans from Platycodon grandiflorum: structural characterization, lung-oriented guidance and targetability. Carbohydr. Polym. 323, 121457 (2024).
Liu, G., Liu, R., Shan, Y. & Sun, C. Marine bacterial exopolysaccharide EPS11 inhibits migration and invasion of liver cancer cells by directly targeting collagen I. J. Biol. Chem. 297, 101133 (2021).
Kehr, P., Härtl, R., Lawrence, J. & Bonassar, Biological approaches to spinal disc repair and regeneration for clinicians. Eur. J. Orthopaed. Surg. Traumatol. 28, 331–331 (2018).
Risbud, M. V. & Shapiro, I. M. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat. Rev. Rheumatol. 10, 44–56 (2014).
Priyadarshani, P., Li, Y. & Yao, L. Advances in biological therapy for nucleus pulposus regeneration. Osteoarthr. Cartil. 24, 206–212 (2016).
Xiang, H. et al. Progress in regulating inflammatory biomaterials for intervertebral disc regeneration. Bioact. Mater. 33, 506–531 (2024).
Peng, Y. & Elkon, K. B. Autoimmunity in MFG-E8–deficient mice is associated with altered trafficking and enhanced cross-presentation of apoptotic cell antigens. J. Clin. Investig. 121, 2221–2241 (2011).
Ensembl. The gene information of Mfge8 (Rat) in Ensemb (eds) (2024).
Lotz, J. C. Animal models of intervertebral disc degeneration: lessons learned. Spine 29, 2742–2750 (2004).
Xie, D. et al. A scaffold vaccine to promote tumor antigen cross-presentation via sustained toll-like receptor-2 (TLR2) activation. Bioact. Mater. 37, 315–330 (2024).
Tu, Y., Tan, L., Tao, H., Li, Y. & Liu, H. CETSA and thermal proteome profiling strategies for target identification and drug discovery of natural products. Phytomedicine 116, 154862 (2023).
Barcellona, M. N. et al. Control of adhesive ligand density for modulation of nucleus pulposus cell phenotype. Biomaterials 250, 120057 (2020).
Wu, X. et al. Prolactin inhibits the progression of intervertebral disc degeneration through inactivation of the NF-κB pathway in rats. Cell Death Dis. 9, 98 (2018).
Di Pauli von Treuheim, T., Torre, O. M., Mosley, G. E., Nasser, P. & Iatridis, J. C. Measuring the neutral zone of spinal motion segments: Comparison of multiple analysis methods to quantify spinal instability. JOR Spine 3, e1088 (2020).
Jung, Y. J. et al. Induction of angiogenesis by matrigel coating of VEGF-loaded PEG/PCL-based hydrogel scaffolds for hBMSC transplantation. Mol. Cells 38, 663–668 (2015).
Aisenbrey, E. A. & Murphy, W. L. Synthetic alternatives to matrigel. Nat. Rev. Mater. 5, 539–551 (2020).
Acknowledgements
The authors thank Dr Yiming Niu, Ms Jiaxi Chen, Ms Feng Guo, Dr Zhencheng Liao, and Dr Haiqing Mao for their technical help. This study was financially supported by the Science and Technology Development Fund, Macao SAR (FDCT, No. 0001/2021/AKP, 0024/2023/AFJ, and 005/2023/SKL; all to C.W.), the National Science Foundation of China (NSFC, No. 32022088, C.W.; 32361163656 and 32230056, L.D.), Jiangsu Provincial Science and Technology Plan Special Fund (BM2023008, L.D.), and the University of Macau (MYRG-GRG2023-00136-ICMS-UMDF and MYRG2022-00100-ICMS, C.W.).
Author information
Authors and Affiliations
Contributions
The conceptualization of the study was initiated by C.M.W. and Y.L. The methodology was developed by Y.L., T.W.C., P.P., and D.C.G., while the investigation was conducted by Y.L., T.W.C., Y.W.L., G.R.G., and D.C.G. The visualization of the data was completed by Y.L., T.W.C., and Y.W.L. The supervision of this study was overseen by L.D. and C.M.W. The original draft was prepared by Y.L., T.W.C., Y.W.L., and C.M.W. Finally, all authors discussed the results, and the writing review and editing were performed by Y.L., L.D., and C.M.W.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, Y., Chao, T., Li, Y. et al. An enzyme-proof glycan glue for extracellular matrix to ameliorate intervertebral disc degeneration. Nat Commun 16, 3629 (2025). https://doi.org/10.1038/s41467-025-58946-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-58946-5