Abstract
Species’ traits and environmental conditions determine the abundance of tree species across the globe. The extent to which traits of dominant and rare tree species differ remains untested across a broad environmental range, limiting our understanding of how species traits and the environment shape forest functional composition. We use a global dataset of tree composition of >22,000 forest plots and 11 traits of 1663 tree species to ask how locally dominant and rare species differ in their trait values, and how these differences are driven by climatic gradients in temperature and water availability in forest biomes across the globe. We find three consistent trait differences between locally dominant and rare species across all biomes; dominant species are taller, have softer wood and higher loading on the multivariate stem strategy axis (related to narrow tracheids and thick bark). The difference between traits of dominant and rare species is more strongly driven by temperature compared to water availability, as temperature might affect a larger number of traits. Therefore, climate change driven global temperature rise may have a strong effect on trait differences between dominant and rare tree species and may lead to changes in species abundances and therefore strong community reassembly.
Similar content being viewed by others
Introduction
Plant communities typically consist of a relatively few dominant and many rare species (MacArthur, 1957; Preston, 1948). Dominant and rare species both contribute to ecosystem function: dominant species provide the majority of ecosystem services, and rare species can increase ecosystem multifunctionality by expanding trait diversity1,2,3,4. Species traits in combination with abiotic and biotic environmental conditions therefore drive the relative abundance of species in local communities5. Macroclimate is an important abiotic trait filter that determines the global distribution of forest biomes6,7 and tree species8,9. Climate change will therefore have a strong effect on the occurrence and distribution of forest biomes, traits, and consequently, forest ecosystem functioning10,11,12. However, the extent to which individual traits of locally dominant and rare tree species differ, and how these differences are affected by climate, remains largely unexplored at a global scale. This lack of knowledge limits our understanding on the processes determining species abundances, functional significance of dominant and rare tree species across the globe and how this is affected by climate3,13.
Community assembly is the process by which species are filtered out from the regional species pool into the local community based on their functional traits, ecological niches or stochastic processes. In this process, climatic factors such as temperature and precipitation, as well as biotic factors such as facilitation, competition, herbivory and pathogens act as filters on species membership in particular assemblages14,15. It is suggested that the strength of different filters depend on the environment, with stronger abiotic filtering at higher latitudes because of harsh environmental conditions and stronger biotic filtering at lower latitudes, because of intense competition under productive conditions16.
After a species’ establishment, its abundance is defined besides habitat suitability by competitive ability related to species’ traits5,17. A trait is defined as any morphological, physiological or phenological feature measurable at the individual plant level that affects plant performance18. In forests across the globe, high wood density and low specific leaf area (SLA) are associated with a stronger competitive ability19. Higher wood density generally increases tissue longevity and shade-tolerance, as it allows species to make persistent deeper and wider crowns that enhance light interception and shade out other species20,21,22. SLA reflects the life-history strategy of plants, with a high SLA associated with a short leaf lifespan and high growth rate, and a lower SLA associated with a long leaf lifespan and efficient nutrient conservation16,23,24.
Plant trait occurrence and abundance is affected by different factors, among which temperature and water availability are of primary importance10,25. Temperature affects the energy balance of leaves, and therefore the balance between photosynthetic rate and respiration26,27. This leads to hump-shaped photosynthesis-temperature response curves which may have profound effects on whole-plant growth rate28. Lower latitudes and altitudes have a higher mean annual temperature and are associated with more productive environments (when water and nutrients are not limiting), taller trees, increased construction costs of stem and leaves (high wood density and low SLA that enhance tissue longevity and shade tolerance), and increased seed mass (provide seedlings with sufficient reserves to establish and survive in the shade)24,25,29,30. Climatic plant water availability, expressed as aridity (i.e., the ratio of mean annual precipitation over mean annual evapotranspiration), is mainly related to traits connected to drought tolerance (high wood density and cavitation resistance), drought avoidance (deciduous leaves with high SLA and deep roots), or efficient water use (wide vessels, high leaf nitrogen concentration and photosynthetic water use efficiency)25,31,32. Extreme temperatures in combination with drought can exacerbate water stress, damage plant tissues, and ultimately lead to plant mortality and species exclusion33,34,35.
In addition to maladaptation to the macroclimate and competition, tree species can be locally rare because of metapopulation processes (e.g., recent invaders or in the process of local extinction)36, extinction of mutualists (e.g., pollinators, dispersers)37, (biogeographical) historical legacies38,39,40, habitat specialization (e.g., specialized for locally rare habitats such as streams, rocky outcrops or treefall gaps)41,42, or adult stature (e.g., attaining a small size, so that only few forest strata can be occupied)19. We do acknowledge the effect of these processes on species abundances, although they are not directly analysed in this study.
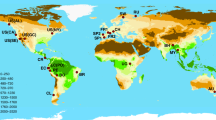
In this study we use global datasets of tree composition of >22,000 forest plots and 11 traits and 2 multivariate trait axes of 1663 tree species (Fig. 1) to ask 1) how do locally dominant and rare tree species differ in their trait values in forest biomes across the globe?, and 2) how are these patterns driven by broadscale climatic gradients in temperature and water availability? We test the hypotheses that (i) dominant species express the more competitive trait values and locally dominant and rare tree species show a larger difference in traits in harsher environments, reflecting larger differences in habitat suitability and competitive ability between species43, and that (ii) differences between trait values of locally dominant and rare tree species are more strongly driven by temperature than water availability because temperature influences a larger number of traits25.
The three most common dominant and randomly chosen rare tree species, according to our dataset, are indicated per forest biome. An overview of the temperature and water availability range per biome can be found in Fig. S5.
Results
Traits of dominant and rare tree species
The first PC axis, which included the traits of the locally dominant and rare tree species together, explained 41% of the variation and reflected a stem strategy spectrum ranging from angiosperms with wide vessels and thin bark to the left, to gymnosperms with narrow tracheids and thick bark to the right. The second PC axis explained 25% of the trait variation and was associated with traits related to photosynthetic carbon gain such as large crown diameter and high specific leaf area (SLA) (Fig. 2). Hence, differences amongst biomes, and more specifically the difference between angiosperms and gymnosperms, were particularly pronounced (Fig. 2).
The dominant and rare species per plot are visualised in this graph, and the circles and triangles represent therefore individual trees. The climatic variables temperature (purple arrow) and water availability (brown arrow), and gymnosperms (green arow) are as well indicated with an arrow. The six different forest biomes are visualized with different colours, see the legend for the colour explanation. The cluster on the left is dominated by angiosperms, while the cluster on the right is dominated by gymnosperms. For the same graph on species level, see Fig. S3A. Source data are provided as a Source Data file.
Additionally, we compared traits of locally dominant and rare species per biome. Across biomes, dominant species had taller stems (Wilcoxon test, 39650337 > W > 475549, 9721 > N > 1015, p < 0.01), softer wood (53798800 > W > 570861, p < 0.01) and higher loadings on the first PC axis compared to rare species (39237289 > W > 463234, p < 0.01) (Fig. 3, Fig. S6 & Table S1). The other trait differences varied with biome, indicating that the environmental context selects for different traits affecting species abundance (Figs. 3 & S6). In temperate and boreal forests rare species had, compared to dominant species, deeper roots (49369516 > W > 1306032, 9721 > N > 1562, p < 0.001) and wider stem conduits (56055893 > W > 1163685, p < 0.001), while in the tropical biomes and temperate conifer forest rare species had a higher SLA (47709647 > W > 1579690, 8813 > N > 1015, p < 0.001) (Table S1). The absolute difference in trait values between locally dominant and rare species increases more than eightfold going from moist tropical forest (mean difference between scaled traits is 0.04 s.d.) to temperate conifer forest (mean difference between scaled traits is 0.3 s.d.), indicating that in harsher environments dominant and rare species differ more in their traits due to gymnosperm dominance (Fig. S6).
Ten traits, percentage gymnosperms and the first two PC axes are evaluated, of which five traits and PC1 are visualized here (see for the other traits and PC2 Fig. S6). The mean and confidence interval of the difference between trait values of dominant and rare species are displayed. If the mean is positive, dominant species have on average a higher trait value, while a negative mean indicates that rare species have a higher trait value. The grey dashed line indicates similar trait values between dominant and rare species. Closed dots indicate a significant difference between trait values of dominant and rare species (Wilcoxon test, N > 1015, p < 0.05). Detailed statistics per biome can be found in Table S1. Source data are provided as a Source Data file.
Traits of dominant and rare tree species correlated with temperature and water availability
Temperature showed the strongest correlation with rooting depth (Pearson correlation, r = 0.73, t = 330.95, N = 95659, p < 0.001), while water availability showed the strongest correlation with bark thickness (Pearson correlation, r = −0.38, t = −127.18, N = 95659, p < 0.001) (Fig. 2). We analysed the absolute difference of trait values between locally dominant and rare species along an environmental gradient of temperature and water availability. Temperature had a stronger effect on trait differences between dominant and rare species (mean variable importance is 34%) than water availability (mean variable importance is 11%) (Fisher’s F-test, F = 2.19, N = 13, p < 0.001) (Figs. 4 & S7). Interactions between temperature and water availability were often non-significant and had an average variable importance <3%. For all traits (except height), the difference between dominant and rare species showed hump-shaped or U-shaped relationships with temperature (Figs. 4 & S7), where the x-axis was crossed (indicating no differences between dominant and rare species) at a temperature between 5–8 °C, a maximum difference was attained around 15 °C, and trait difference became close to zero at high temperatures. For all traits (except height and PC1, PC2) the differences in trait values between dominant and rare species increases linearly with water availability and crosses the x axis at a water availability index of ca. 1.5 (Figs. 4 & S7). This coincides with the dominance of gymnosperms, which is higher at temperatures <4 °C and water availability >1.8.
See for the other traits and PC2 Fig. S7. Positive values indicate that trait values are higher for dominant species, while negative values indicate that trait values are higher for rare species. The graphs on temperature are modelled with a second-order polynomial function, the graphs on availability index are modelled with a linear function as this best fitted the data (See Supplementary Data 1). The standardized regression coefficients of the linear models are displayed of the climatic variables (t = temperature, w = water availability index) and squared climatic variables (t2 = temperature2, w2 = water availability index2). The number of plots included in this analysis is 22,825. The significance of the regression coefficients is indicated with asterisks: *p < 0.05, **p < 0.01, ***p < 0.001. Source data is provided as a Source Data file.
Discussion
In this study, we asked (1) how do locally dominant and rare tree species differ in their trait values in forest biomes across the globe?, and (2) how are these patterns driven by broad-scale climatic gradients in temperature and water availability? We found that in forests globally, dominant tree species grow taller, have softer wood and have higher loadings on the stem strategy axis (traits associated with gymnosperms) compared to rare species (Fig. 2). Locally dominant and rare species show a larger difference in traits in boreal compared to tropical forests (Fig. 3), and the differences in traits are more strongly driven by temperature than by water availability (Fig. 4).
Of the 11 traits and 2 multivariate trait axes evaluated, we found only three consistent trait differences across all biomes, and they are therefore globally important in determining local dominance and rarity. Dominant species are, compared to rare species, taller, have softer wood, and higher loadings on the multivariate stem strategy axis (soft wood, narrow conduit diameter), which are often characteristics of gymnosperm species (Figs. 2 & 3)44,45. Dominant species, therefore, seem to be canopy species and are fast and tall growers to secure light resources, leading to a taller stem height and lower wood density19,46. In contrast, rare species are likely understory or subcanopy species, and are shade-tolerant, slower-growing species with higher wood density22. The consistent trait differences between dominant and rare species might indicate that dominant species are earlier successional, faster growing species and that rare species are later successional slower growing species, a possible indication of human or natural disturbance in the forests evaluated in this study47,48. The difference in tree height is smallest for the tropical forest biomes and we found indeed that difference in height between dominant and rare tree species decreases with higher temperatures and water availability (Fig. 4). This might indicate that in the tropical biomes, dominant and rare species are present in multiple canopy strata, while in the temperate and boreal forests tend to have two canopy strata, where rare species are more restricted to the understory or subcanopy due to their small adult stature49,50. In sum, in forests globally, dominant tree species invest in faster growth and larger tree sizes compared to rare species.
For the other 8 traits and 1 multivariate trait axis, differences in trait values between locally dominant and rare species are dependent on the biome (Fig. 3 & S1), and therefore on the regional biotic and abiotic environmental context. These results are in line with studies across kingdoms of life, concluding that species abundances are related to certain trait values that depend on the environment13,51,52,53. Tropical dry and wet forests showed relatively few (4, 5) significant trait differences between dominant and rare species compared to other biomes, which is probably a reflection of the higher species diversity and, hence, functional redundancy in the tropics54,55,56. A higher functional redundancy may result in less striking trait differences between dominant and rare species, as species have more similar trait values54. Additionally, differences between dominant and rare species may be more difficult to detect because of the long tail of rare species in tropical forests, which may differ widely in their trait values57. Dominant tropical forest species had, next to the three traits mentioned above, also a lower SLA (Fig. S6), which may reflect stronger adaptations to shade for rare species, as moist tropical forests tend to be denser and continuously shaded compared to other forest biomes58,59. Regarding the dry tropical forests, a lower SLA could indicate a drought-tolerance strategy of the dominant, sun-exposed canopy trees60,61,62.
Gymnosperms form a dominant component in temperate conifer and boreal forests, and are subordinates in temperate forests, which may explain why more traits (axes) differences in temperate and boreal forest biomes are significant (10–13) and larger compared to tropical biomes. The trait differences probably reflect a two-layered canopy structure, with a canopy layer occupied by dominants and an understory layer occupied by rare species (King et al.50). Gymnosperm trees have three unique features that set them apart from angiosperms and makes them well adapted to harsh environmental conditions such as low soil fertility, drought63, pathogens64,65 and mainly cold44,45,66,67. First, their water conducting conduits consist of narrow tracheids, which makes them more resistant against freezing- and drought-induced cavitation65,68,69. Second, mechanical strength is delivered by the relatively thick cell walls of their tracheids, and therefore they do not make (heavy) fibres, resulting in a low wood density65. Third, they make structurally enforced needles, with low SLA and leaf nitrogen concentration that increases leaf longevity, and results in an evergreen leaf habit for nearly all gymnosperm species70. As a result, gymnosperm species differ strikingly from angiosperms in their traits and multivariate strategies (Fig. 2).
In general, trait differences are more driven by temperature compared to water availability, and the relationships between climate and trait differences seem to be mostly driven by adaptations to temperature-related productivity and biodiversity gradients, partly by angiosperm-gymnosperm differences, and to a lesser extent by drought adaptations. We found that temperature has a stronger effect on trait differences between locally dominant and rare species compared to water availability and that the interaction between temperature and water availability has a negligible effect (Figs. 4 & S7). These results indicate that it is mainly temperature that drives the range of trait values of dominant and rare species25. Temperature corresponds to the latitudinal gradients in species diversity, biomes, growing season length and productivity, while precipitation is less consistently related to latitude71,72,73,74. Therefore, in global analyses, temperature might influence a wider spectrum of traits, while water availability specifically has a stronger effect on traits related to drought resistance25. Indeed, we observed that water availability only has a stronger correlation with differences in bark thickness compared to temperature75,76 (Fig. 4).
For all traits (except height), the difference between locally dominant and rare species showed hump-or U-shaped relationships with temperature (Figs. 4 & S7). At intermediate temperature conditions (around 15 °C, which coincides with temperate conifer forests) dominants and rare species show the most striking trait differences (Fig. S5). This probably reflects the trait differences between the dominant gymnosperm species and rare angiosperm species (Fig. S7). At colder temperatures (<5 °C, which coincides with boreal forests), the trait differences between dominant and rare species reverse, probably because this reflects a higher gymnosperm dominance (Fig. S7). At high temperatures (ca 25 °C, which coincides with tropical forests) the traits of dominant and rare species tend to converge, probably because of the high diversity and functional redundancy of tropical forests77.
For all traits, the differences in trait values between locally dominant and rare species increased (nearly) linearly with water availability and crossed the x-axis at a water availability index of ca. 1.5 (Figs. 4 & S7). A higher water availability relates to an increase in productivity, forest height, density, complexity and species diversity, and hence, a stronger light competition45,78,79,80. In humid ecosystems, the dominant species show a more acquisitive strategy, as they are larger (larger crown diameter, rooting depth and higher seed mass)46,81, have a higher water transport capacity (larger conduit diameter)65,82, more productive leaves (larger SLA, leaf nitrogen concentration, and leaf N:P ratio)70,83, and higher wood density (angiosperms)65. In arid systems, the dominant species are more conservative, as they are smaller, more drought resistant with narrow conduits or save water with a low SLA. These conservative trait values might indicate a survival strategy in arid systems, with traits adapted to drought to avoid cavitation84.
Global databases of forest inventories are typically under sampled in more speciose tropical regions, potentially leading to an underestimation of trait variation among rare species at biome level. Nevertheless, we found that rare species in these regions (i.e., moist and dry tropical forests) already contain larger trait diversity than the dominant species, indicating that potential under sampling would not lead to a large bias of the overarching patterns (Fig. S8). The trait values used here were estimated based on phylogenetic and environmental information. This allowed for the incorporation of trait plasticity across environmental gradients, but it also introduces model-based uncertainty into the predictions. The imputation uncertainty has been shown to have negligible bias when averaging over many species45. Finally, there are processes shaping tree communities which we did not consider in this study and are subject to future studies, such as metapopulation processes, historical (management) legacies, habitat specialization, and successional stage19,36,38,42,85,86.
Ecosystems consist of dominant and rare species, which have their own unique contribution to ecosystem functioning. Here, we evaluated differences in trait values of locally dominant and rare tree species across global forests and explored how these trait profiles vary along broad environmental gradients. The difference between traits of dominant and rare species is more strongly driven by temperature compared to water availability, as temperature might affect a larger number of traits. Therefore, climate change driven global temperature rise may have a strong effect on the trait differences between dominant and rare tree species and may lead to strong community reassembly.
Methods
Forest inventory data
To identify dominant and rare species at plot level, we initially incorporated H1,2 million forest inventory plots sourced from the Global Forest Biodiversity Initiative (GFBI database). Each forest plot contains information on tree species richness, tree species abundance, year of measurement, plot size and ___location. Tree ferns and palms are not included in the database.
Plot sizes range from 0.0002 to 20 ha in the database and the plots include all trees with stem diameter at breast height ≥ 5 cm. As rare species are likely not captured accurately in very small plots, and trait variation is correlated with plot size as well87, we excluded plot sizes smaller than the first quantile of 0.02 ha and outliers larger than 2 ha (in total 8.3% of the database). Additionally, plots measured before 1990 were filtered out, as these plots likely do not represent current forest composition and do not match with the climatic data we used (filtering out 21% of the remaining database). Also, trait values of the rare and dominant species change with successional forest age85, we therefore excluded early successional plots with a forest age of less than 25 years, which corresponds to 1.3% of the remaining database88. Different forest age thresholds could potentially affect trait values of dominant and rare species. Yet, when comparing trait values using the 25-year threshold with 30- and 35-years thresholds we got very similar results (Fig. S11). Within the filtered database, the correlations between plot size and number of dominant species (r = 0.23, r2 = 0.05, p < 0.01), and plot size and number of rare species was equally weakly related (r = 0.23, r2 = 0.05, p < 0.01) (see for definition of dominant and rare species methods section Identifying dominant and rare tree species). See for an overview of the distribution of plot size within every forest biome Fig. S1 and the relationship between species abundances and plot size for this database78,89. In our filtered dataset, the mean plot size is 0.07 ha, the mean measurement year is 2004 and 53 years is the mean forest age, whereas 1.4% of the dataset is made up of old-growth forest (older than 140 years). Elimination of forest plots based on size, year of measurement, forest age, and incorporating only plots within the forest biomes90, resulted in 660,552 plots in the filtered dataset (Fig. 1). Additionally, we incorporated only plots with six or more species, to clearly separate dominant and rare species, which included 23% of the filtered dataset based on plot size, measurement year and age.
Species names in the GFBI dataset were standardised using The Plant List91. 1.4% of the species names could not be matched using The Plant List, therefore subsequently the Global Biodiversity Information Facility (GBIF) backbone was sourced to standardize these species names to accepted species names92. In every forest plot the dominant and rare species were identified.
Identifying dominant and rare tree species
Dominant and rare species were identified as the top and bottom 10% species according to the rank abundance curve at the plot level93,94,95,96. We defined dominant and rare species at the plot level, as this is the spatial scale at which species interact more directly with each other, and therefore the outcome of both abiotic and biotic interactions affecting species abundances is reflected reflected97,98. There are many different definitions of dominant and rare species, however, we choose the 10% most and least abundant individuals in terms of number of stems as this resembles the outer parts of the species abundance distribution. Also, the number of individuals is not automatically related to trait values, while for example, abundance based on basal area is related to the traits height, crown length and leaf area index99,100,101. As the definition of dominant and rare species could affect the results, a PCA was made where the dominant and rare species were classified as respectively the top and bottom 10% of the number of stems in a plot (Fig. S3B), showing a very similar pattern to Fig. 2. Additionally, dominant and rare species were defined as the top and bottom 5% and 15% of individuals in the plot, showing that the stricter the definition (e.g. lower percentage), the more plots are filtered out and the more pronounced the difference between dominant and rare species trait values are (Fig. S10). As described in Hordijk et al. (2024), both the dominant and rare species in the GFBI database are geographically widespread89.
In forest plots containing between 6 and 19 species, the top two and bottom two species were defined as respectively dominant or rare, whereas for plots with >=20 species the 10% most and least abundant species were identified as dominant or rare, respectively. Additionally, if the rarest species in a plot comprised >10% of the sum of the stems, then this plot was excluded. This assured that the rare species are clearly distinguishable in abundance from the dominant species. Additionally, it also selected for the species-rich plots in the Boreal forest zone. Lastly, we randomly selected 10,000 plots from the temperate forest biome for the plot-level analyses, instead of a total of 135,043 plots, to reduce computational time and have a more balanced dataset representing the different forest biomes. The used subset of the temperate forest biome is a good representation of the traits of dominant and rare straits in this biome, which is verified with a bootstrapping procedure (Fig. S9).
Trait selection
Using the trait imputation models of Maynard et al. (2022), a total of 18 trait values were computed for each tree occurrence in the GFBI dataset, encompassing a variety of leaf, wood and root traits with training data sourced from the TRY database102. In case the tree was identified up to genus level, the species-level average of that trait within that genus was calculated and used as an approximation of the trait value. These models incorporate intraspecific variation and thus provide a unique prediction of each trait for each of the 1663 species in each ___location where the species occurs, based on the combination of phylogenetic and environmental information. From every trait cluster identified by Maynard et al (2022), at least one trait was included for further analysis to guarantee sufficient statistical independence and to cover the range of plant life-history strategies. Ten traits were included reflecting the global Leaf-Height-Seed plant strategy scheme103, and the global spectra of plant form and function44. The traits are related to the size of trees (tree height, crown diameter, rooting depth) and their propagules (seed dry mass), tissue construction costs (wood density, specific leaf area), hydraulics (stem conduit diameter), leaf economics and photosynthesis (leaf nitrogen per mass, leaf nitrogen/phosphorus ratio) and stem defence against disturbances such as fire and insects (bark thickness). For an overview of the traits and their ecological significance see Table 1. The trait values were log transformed (natural logarithm), to diminish the effect of outliers, and trait values were standardized to compare different trait units accurately in the analysis104. For the distribution of the untransformed trait values, see Fig. S2. Additionally, as gymnosperm and angiosperm species have distinct trait values (see also Fig. 2), the dominant and rare species were identified as either gymnosperm or angiosperm, based on their family, and the percentage of dominant and rare species comprising gymnosperms was calculated per plot.
Evaluating the difference between the traits of dominant and rare tree species
To evaluate general trade-offs between traits of the dominant and rare species, a PCA was performed including all dominant and rare species in the six different forest biomes; tropical moist forest, tropical dry forest, tropical conifer forest, temperate forest, temperate conifer forest and boreal forest90 (Fig. 2). The loadings of the dominant and rare species per plot level on the first and second axes of the PCA were incorporated in further analyses. Among a total of 10 traits, the gymnosperm percentage and PC axes loadings, six are presented in the main text, to decrease the information displayed, and seven are presented in the supporting information. The results related to the traits tree height, wood density, leaf nitrogen concentration, seed mass, rooting depth, and the first PC axis are displayed in the main text as they represent a broad spectrum of traits related to different life history strategies44,45,105. To give an insight into the species-level differences, a PCA was made with dominant and rare species as data points in the PCA, rather than the different tree individuals (Fig. S3A). Additionally, to verify if the results are not only caused by chance or the way of calculating the dominant and rare trait values per plot, we randomised the data 100 times within the five main forest biomes, individually keeping the total number of trees per plot and the total number of individuals per tree species constant. Afterwards, we calculated the traits of the dominant and rare species in three different ways: using the trait mean, the median and the interquartile range. The null models indicate that, after randomization of the dataset, the three different ways of calculating trait values show a large to complete overlapping frequency distribution for each of the groups, indicating robust results using the median trait plot level value (Fig. S4).
For the dominant and rare species, the median trait value per plot was calculated, as otherwise, a different number of dominant or rare species could affect the difference in trait values. We calculated the difference in scaled trait value of the dominant and rare species by subtracting the scaled trait value of the rare species from the scaled trait value of the dominant species in the same plot. We included intraspecific variation in the analyses, since across plots trait values of the same species can fluctuate (see methods section Trait selection). With a Wilcoxon signed-rank test, the difference between the mean trait values was evaluated per forest biome. When evaluating the differences between traits for dominant and rare species, the tropical conifer forest was excluded from the analyses due the low number of plots in this biome (N = 182) and therefore high standard error in trait differences between dominant and rare species.
Evaluating the effect of temperature and water availability on trait differences
Climatic water availability was calculated as the ratio of mean annual precipitation over mean annual evapotranspiration at a resolution of 30 arc sec106. A climatic water availability <1 means a water-deficient arid environment, whereas a climatic water availability >1 corresponds to a more humid environment. Other studies also refer to this index as ‘aridity index’, but we prefer to use the term water availability, as a high value indicates a humid environment. Temperature is expressed as mean annual temperature, based on the CHELSA (Climatologies at high resolution for the earth’s land surface areas) data at a resolution of 30 arc sec107. Across our dataset, temperature and water availability are weakly negatively correlated (Spearman correlation, r = −0.09, r2 = 0.008, N = 22,825, p < 0.001), indicating that these two climatic variables represent distinct climatic gradients. An overview of the temperature and water availability range per biome can be found in Fig. S5.
The relationships between trait values and the two climatic variables were evaluated with a Pearson correlation. To explore the effects of temperature, water availability, and their interaction on differences in trait values, a second-order polynomial model was used, in which we corrected for plot size, forest age, elevation and biome. We evaluated the relationships with a second-order polynomial model as we expect that the relationships between trait differences and temperature or water availability can be a concave or convex relationship, as trait differences might be largest in the more extreme climatic conditions43. The independent variables in the model were scaled to a mean of zero and a standard deviation of one to facilitate comparability between the regression coefficients. To quantify the relative importance of temperature, water availability, their interaction, forest age, plot size, elevation and biome on trait differences, we used the scaled calc.relimp function in R108. This function evaluates the contribution of each independent variable to the variation explained by averaging the contribution of each independent variable to the r2 in terms of its sum of squares across all possible fitting sequences. See Supplementary Data 1 for an overview of the regression coefficients and variable importance values. To evaluate the difference between the variable importance of temperature and water availability, including both the non-transformed and squared variables, a two-sample t-test with equal variances was performed (Fisher’s F-test, N = 13, F = 2.19, p < 0.001).
Data management and statistical analyses in this study were performed with the R-Studio interface to R109.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The plot-level data of the difference between trait values of dominant and rare tree species are stored in Zenodo https://doi.org/10.5281/zenodo.15393651. The GFBI database is available upon written request at https://gfbinitiative.net/data/. Source data are provided with this paper.
Code availability
The code used to perform the statistical analyses can be found in the Supplementary Code 1.
References
Fauset, S. et al. Hyperdominance in Amazonian forest carbon cycling. Nat. Commun. 6, 1–9 (2015).
Grime, J. P. Benefits of plant diversity to ecosystems: immediate, filter and founder effects. J. Ecol. 86, 902–910 (1998).
Jain, M. et al. The importance of rare species: A trait-based assessment of rare species contributions to functional diversity and possible ecosystem function in tall-grass prairies. Ecol. Evol. 4, 104–112 (2014).
Mouillot, D. et al. Rare species support vulnerable functions in high-diversity ecosystems. Mace G. M., editor. PLoS Biol. 11, e1001569 (2013).
HilleRisLambers, J., Adler, P. B., Harpole, W. S., Levine, J. M. & Mayfield, M. M. Rethinking community assembly through the lens of coexistence theory. Annu. Rev. Ecol. Evol. Syst. 43, 227–248 (2012).
Kutzbach, J. et al. Climate and Biome simulations for the past 21,000 years. Quat. Sci. Rev. 17, 473–506 (1998).
Woodward, F. I., Lomas, M. R. & Kelly, C. K. Global climate and the distribution of plant biomes. Philos. Trans. R. Soc. B: Biol. Sci. 359, 1465–1476 (2004).
Kirschbaum, M. U. F. Forest growth and species distribution in a changing climate. Tree Physiol. 20, 309–322 (2000).
Woodward, F. I. Climate and plant distribution. Cambridge University Press. (1987).
Joswig, J. S. et al. Climatic and soil factors explain the two-dimensional spectrum of global plant trait variation. Nat. Ecol. Evol. 6, 36–50 (2022).
Kühn, N. et al. Globally important plant functional traits for coping with climate change. Front Biogeogr. 13, 1–18 (2021).
Madani, N. et al. Future global productivity will be affected by plant trait response to climate. Sci. Rep. 8, 2870 (2018).
Avolio, M. L. et al. Demystifying dominant species. N. Phytol. 223, 1106–1126 (2019).
Bruelheide, H. et al. Global trait–environment relationships of plant communities. Nat. Ecol. Evol. 2, 1906–1917 (2018).
Weiher, P., Keddy, E. Ecological assembly rules: perspectives, advances, retreats [Internet]. Cambridge University Press; 2001. Available from: https://books.google.co.in/books?hl=en&lr=&id=RIZDYJ0zKaAC&oi=fnd&pg=PR12&dq=Wilson,+J.+B.++(1999a).+Assembly+rules+in+plant+communities.+Ecological+Assembly+Rules:+Perspectives,+Advances,+Retreats+(eds+E.+Weiher+and+P.+A.+Keddy),+pp.+130%E2%80%93164.+Cambri&redir_esc=y#v=onepage&q&f=false.
Hulshof, C. M. et al. Intra-specific and inter-specific variation in specific leaf area reveal the importance of abiotic and biotic drivers of species diversity across elevation and latitude. de Bello F., editor. J. Veg. Sci. 24, 921–931 (2013).
Whittaker, R. H. Dominance and diversity in land plant communities. Science 147, 250–260 (1965).
Violle, C. et al. Let the concept of trait be functional! Oikos [Internet]. 116, 882–892 (2007).
Kunstler, G. et al. Plant functional traits have globally consistent effects on competition. Nature. 529, 204–207 (2016).
Aiba, M. & Nakashizuka, T. Architectural differences associated with adult stature and wood density in 30 temperate tree species. Funct. Ecol. 23, 265–273 (2009).
Poorter, L., Bongers, L. & Bongers, F. Architecture of 54 moist-forest tree species: traits, trade-offs, and functional groups. Ecol. Soc. Am. 87, 1289–1301 (2016).
Wright, S. J. et al. Functional traits and the growth-mortality trade-off in tropical trees. Ecology. 91, 3664–3474 (2010).
Niinemets, Ü. A review of light interception in plant stands from leaf to canopy in different plant functional types and in species with varying shade tolerance. Ecol. Res. 25, 693–714 (2010).
Poorter, H., Niinemets, Ü., Poorter, L., Wright, I. J. & Villar, R. Causes and consequences of variation in leaf mass per area (LMA): A meta-analysis. N. Phytol. 182, 565–588 (2009).
Moles, A. T. et al. Which is a better predictor of plant traits: Temperature or precipitation? J. Veg. Sci. 25, 1167–1180 (2014).
Harrison, S. P. et al. Ecophysiological and bioclimatic foundations for a global plant functional classification. J. Veg. Sci. 21, 300–317 (2010).
Lambers, H., Chapin, F. S., Pons, T. L. Leaf Energy Budgets: Effects of Radiation and Temperature. In: Plant Physiological Ecology., 210–229. (1998).
Went, F. W. The effect of temperature on plant growth. Annu. Rev. Plant Physiol. 4, 347–362 (1953).
Murray, B. R., Brown, A. H. D., Dickman, C. R. & Crowther, M. S. Geographical gradients in seed mass in relation to climate. J. Biogeogr. 31, 379–388 (2004).
Swenson, N. G., Enquist, B. J. Ecological and evolutionary determinants of a key plant functional trait: wood density and its community-wide variation across latitude and elevation. Am. J. Bot. 94, 451–459 (2007).
O’Brien, M. J. et al. A synthesis of tree functional traits related to drought-induced mortality in forests across climatic zones. J. Appl. Ecol. 54, 1669–1686 (2017).
Wright, I. J., Reich, P. B. & Westoby, M. Least-cost input mixtures of water and nitrogen for photosynthesis. Am. Nat. 161, 98–111 (2003).
Lintunen, A., Hölttä, T. & Kulmala, M. Anatomical regulation of ice nucleation and cavitation helps trees to survive freezing and drought stress. Sci. Rep. 3, 2031 (2013).
Pollastrini, M., Puletti, N., Selvi, F., Iacopetti, G. & Bussotti, F. Widespread crown defoliation after a drought and heat wave in the forests of Tuscany (Central Italy) and their recovery—a case study from summer 2017. Front Glob. Chang. 2, 74 (2019).
Ruehr, N. K., Gast, A., Weber, C., Daub, B. & Arneth, A. Water availability as dominant control of heat stress responses in two contrasting tree species. Tree Physiol. 36, 164–178 (2015).
Magurran, A. E., Henderson, P. A. Explaining the excess of rare species in natural species abundance distributions. Nature. 422, 714–716 (2003).
Dee, L. E. et al. When do ecosystem services depend on rare species? Trends Ecol. Evol. 34, 746–758 (2019).
Hermy, M. & Verheyen, K. Legacies of the past in the present-day forest biodiversity: A review of past land-use effects on forest plant species composition and diversity. Ecol. Res. 22, 361–371 (2007).
Roosevelt, A. C. The Amazon and the Anthropocene: 13,000 years of human influence in a tropical rainforest. Anthropocene 4, 69–87 (2013).
Wiens, J. J. The niche, biogeography and species interactions. Philos. Trans. R. Soc. B: Biol. Sci. 366, 2336–2350 (2011).
Hubbell, S. P., Foster, R. B. Commonness and rarity in a neotropical forest: implications for tropical tree conservation. In: Soulé ME (ed) Conservation biology. Sunderland: Sinauer asociates; 1986. p. 205–231.
Markham, J. Rare species occupy uncommon niches. Sci. 4, 6012 (2014).
Maire, V. et al. Habitat filtering and niche differentiation jointly explain species relative abundance within grassland communities along fertility and disturbance gradients. New Phytol. 196, 497–509. (2012).
Díaz, S. et al. The global spectrum of plant form and function. Nature. 529, 167–171 (2016).
Maynard, D. S. et al. Global relationships in tree functional traits. Nat. Commun. 13, 3185 (2022).
Coomes, D. A. & Allen, R. B. Effects of size, competition and altitude on tree growth. J. Ecol. 95, 1084–1097 (2007).
Li, W. et al. Human fingerprint on structural density of forests globally. Nat. Sustain 6, 368–379 (2023).
Rüger, N. et al. Successional shifts in tree demographic strategies in wet and dry Neotropical forests. Glob. Ecol. Biogeogr. 32, 1002–1014 (2023).
Draper, F. C. et al. Amazon tree dominance across forest strata. Nat. Ecol. Evol. 5, 757–767 (2021).
King, D. A., Wright, S. J. & Connell, J. H. The contribution of interspecific variation in maximum tree height to tropical and temperate diversity. J. Trop. Ecol. 22, 11–24 (2006).
Boyce, A. J., Shakya, S., Sheldon, F. H., Moyle, R. G., Martin, T. E. Biotic interactions are the dominant drivers of phylogenetic and functional structure in bird communities along a tropical elevational gradient. Auk. 136, ukz054 (2019).
Miller, E. T. et al. Fighting over food unites the birds of North America in a continental dominance hierarchy. Stephens D., editor. Behav. Ecol. 28, 1454–1463 (2017).
Delgado-baquerizo, M. et al. A Gobal Atlas of the dominant bacteria found in soil. Science. 325, 320–325 (2018).
Dalerum, F., Cameron, E. Z., Kunkel, K. & Somers, M. J. Interactive effects of species richness and species traits on functional diversity and redundancy. Theor. Ecol. 5, 129–139 (2012).
Fonseca, C. R. & Ganade, G. Species functional redundancy, random extinctions and the stability of ecosystems. J. Ecol. 89, 118–125 (2001).
Monge-González, M. L., Guerrero-Ramírez, N., Krömer, T., Kreft, H. & Craven, D. Functional diversity and redundancy of tropical forests shift with elevation and forest-use intensity. J. Appl. Ecol., Biodivers. Data J. 58, 1827–1837 (2021).
Zhang, S., Zang, R. & Sheil, D. Rare and common species contribute disproportionately to the functional variation within tropical forests. J. Environ. Manag. 304, 114332 (2022).
Reich, P. B. et al. The evolution of plant functional variation: Traits, spectra, and strategies. Int. J. Plant Sci. 164, S143–S164 (2003).
Valladares, F. & Niinemets, Ü. Shade tolerance, a key plant feature of complex nature and consequences. Annu. Rev. Ecol. Evol. Syst. 39, 237–257 (2008).
Greenwood, S. et al. Tree mortality across biomes is promoted by drought intensity, lower wood density and higher specific leaf area. Ecol. Lett. 20, 539–553 (2017).
Legner, N., Fleck, S. & Leuschner, C. Within-canopy variation in photosynthetic capacity, SLA and foliar N in temperate broad-leaved trees with contrasting shade tolerance. Trees - Struct. Funct. 28, 263–280 (2014).
Ramírez-Valiente, J. A. & Cavender-Bares, J. Evolutionary trade-offs between drought resistance mechanisms across a precipitation gradient in a seasonally dry tropical oak (Quercus oleoides). Tree Physiol. 37, 889–901 (2017).
Finér, L. et al. Variation in fine root biomass of three European tree species: Beech (Fagus sylvatica L.), Norway spruce (Picea abies L. Karst.), and Scots pine (Pinus sylvestris L.). Plant Biosyst. 141, 394–405 (2007).
Loram-Lourenço, L. et al. A structure shaped by fire, but also water: ecological consequences of the variability in bark properties across 31 species from the Brazilian Cerrado. Front. Plant Sci. 10, 1718 (2020).
Yang, S. et al. Stem Trait Spectra underpin multiple functions of temperate tree species. Front. Plant Sci. 13, 769551 (2022).
Boulangeat, I., Lavergne, S., Van Es, J., Garraud, L. & Thuiller, W. Niche breadth, rarity and ecological characteristics within a regional flora spanning large environmental gradients. J. Biogeogr. 39, 204–214 (2012).
Cai, H., Li, F. & Jin, G. Forest strata-dependent effects of vegetation attributes and soil nutrients on decadal changes in aboveground net carbon stock in two temperate forests. Catena 194, 104776 (2020).
Crivellaro, A., Piermattei, A., Dolezal, J., Dupree, P. & Büntgen, U. Biogeographic implication of temperature-induced plant cell wall lignification. Commun. Biol. 5, 767 (2022).
Song, Y., Poorter, L., Horsting, A., Delzon, S. & Sterck, F. Pit and tracheid anatomy explain hydraulic safety but not hydraulic efficiency of 28 conifer species. J. Exp. Bot. 73, 1033–1048 (2022).
Wright, I. J. et al. The worldwide leaf economics spectrum. Nature 428, 821–827 (2004).
Adler, R. F., Gu, G., Sapiano, M., Wang, J. J. & Huffman, G. J. Global precipitation: means, variations and trends during the satellite era (1979–2014). Surv. Geophys. 38, 1–21 (2017).
Liang, J. et al. Co-limitation towards lower latitudes shapes global forest diversity gradients. Nat. Ecol. Evol. 6, 1423–1437 (2022).
Rind, D. Latitudinal temperature gradients and climate change. J. Geophys. Res Atmos. 103, 5943–5971 (1998).
Yamahira, K. & Conover, D. O. Intra- vs. interspecific latitudinal variation in growth: Adaptation to temperature or seasonality? Ecology 83, 1252–1262 (2002).
Brunner, I., Herzog, C., Dawes, M. A., Arend, M. & Sperisen, C. How tree roots respond to drought. Front. Plant Sci. 6, 547 (2015).
Poorter, H. et al. Biomass allocation to leaves, stems and roots: Meta-analyses of interspecific variation and environmental control. N. Phytologist 193, 30–50 (2012).
Lamanna, C. et al. Functional trait space and the latitudinal diversity gradient. Proc. Natl Acad. Sci. 111, 13745–13750 (2014).
Hordijk, I. et al. Evenness mediates the global relationship between forest productivity and richness. J. Ecol. 111, 1308–1326 (2023).
Khaine, I. et al. Species diversity, stand structure, and species distribution across a precipitation gradient in tropical forests in Myanmar. Forests 8, 282 (2017).
Liang, J. et al. Positive biodiversity-productivity relationship predominant in global forests. Science 354, aaf8957 (2016).
Weemstra, M. et al. The role of fine-root mass, specific root length and life span in tree performance: A whole-tree exploration. Funct. Ecol. 34, 575–585 (2020).
Olson, M. E., Anfodillo, T., Gleason, S. M. & McCulloh, K. A. Tip-to-base xylem conduit widening as an adaptation: causes, consequences, and empirical priorities. N. Phytol. 229, 1877–1893 (2021).
Goorman, R., Bartual, A., Paula, S. & Ojeda, F. Enhancement of photosynthesis in post-disturbance resprouts of two co-occurring Mediterranean Erica species. Plant Ecol. 212, 2023–2033 (2011).
Bréda, N., Huc, R., Granier, A. & Dreyer, E. Temperate forest trees and stands under severe drought: A review of ecophysiological responses, adaptation processes and long-term consequences. Ann. Sci. 63, 625–644 (2006).
Lohbeck, M., Poorter, L., Mart, M. & Rodriguez-, J. Changing drivers of species dominance during tropical forest succession. Funct. Ecol. 27, 1052–1058 (2014).
van Der Sande, M. T. et al. Tropical forest succession increases tree taxonomic and functional richness but decreases evenness. Glob. Ecol. Biogeogr. (2024).
Karadimou. E. K., Kallimanis, A. S., Tsiripidis, I., Dimopoulos, P. Functional diversity exhibits a diverse relationship with area, even a decreasing one. Sci. Rep. 6, 35420 (2016).
Poulter. B. et al. The global forest age dataset and its uncertainties (GFADv1.1). NASA National Aeronautics and Space Administration, PANGAEA. (2019).
Hordijk, I. et al. Dominance and rarity in tree communities across the globe: Patterns, predictors and threats. Glob. Ecol. Biogeogr. (2024).
Dinerstein, E. et al. An Ecoregion-Based approach to protecting half the terrestrial realm. Bioscience. 67, 534–545 (2017).
The Plant List. The Plant List (2013). Version 1.1. 2013 [cited 2019 Jan 1]. Available from: www.theplantlist.org/
GBIF Backbone Taxonomy. GBIF Secretariat: GBIF Backbone Taxonomy. (2020) [cited 2020 Aug 3]. Available from: https://doi.org/10.15468/39omei
Bracken, M. E. S., Low, N. H. N. Realistic losses of rare species disproportionately impact higher trophic levels. Ecol. Lett. 15, 461–467 (2012).
Gaston, K. Rarity, 13. Chapman & Hall; (1994).
Magurran, A. E. Measuring biological diversity. Blackwell Science Ltd. (2004).
Molina, N. Conservation of rare or little-known species: biological, social, and economic considerations. Island Press; (2013).
Roughgarden, J. J. D. Overview: the role of species interactions in community ecology. In: Diamond J. and Case T. J., eds Community Ecology. New York: Harper & Row Publishers. p. 333–343 (1986).
Stroud, J. T. et al. Is a community still a community? Reviewing definitions of key terms in community ecology. Ecol. Evol. 5, 4757–4765 (2015).
Bartelink, H. Allometric relationships for biomass and leaf area of beech (Fagus sylvatica L). Ann des Sci For [Internet]. 54, 39–50 (1997).
Jonckheere. I., Muys. B., Coppin. P. Allometry and evaluation of in situ optical LAI determination in Scots pine: a case study in Belgium. Tree Physiol. 25, 723–732 (2005).
Sumida, A., Miyaura, T. & Torii, H. Relationships of tree height and diameter at breast height revisited: Analyses of stem growth using 20-year data of an even-aged Chamaecyparis obtusa stand. Tree Physiol. 33, 106–118 (2013).
Kattge, J. et al. TRY plant trait database – enhanced coverage and open access. Glob. Chang. Biol. 26, 119–188 (2020).
Westoby, M. A. Leaf-height-seed (LHS) plant ecology strategy scheme. Plant Soil 199, 213–227 (1998).
Ordonez, A., Wright, I. J., Olff, H. Functional differences between native and alien species: a global-scale comparison. Funct. Ecol. 24, 1353–1361 (2010).
Chave, J. et al. Towards a worldwide wood economics spectrum. Ecol. Lett. 12, 351–366 (2009).
Trabucco, A., Zomer, R. J. Global Aridity Index and Potential Evapo-Transpiration (ET0) Climate Database v2. CGIAR Consortium for Spatial Information(CGIAR-CSI). [Internet]. (2018). Available from: https://cgiarcsi.community
Karger, D. N. et al. Climatologies at high resolution for the Earth’s land surface areas. Sci. Data. 4, 170122 (2017).
Grömping, U. Relative importance for linear regression in R: The Package relaimpo. J. Stat. Softw. 17, 1–27 (2006).
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. (2024).
Westoby, M., Falster, D. S., Moles, A. T., Vesk, P. A., Wright, I. J. Plant ecological strategies: some leading dimensions of variation between species. Annu. Rev. Ecol. Syst. 33, 5–159 (2002).
Muller-Landau, H. C., Wright, S. J., Calderón, O., Condit, R. & Hubbell, S. P. Interspecific variation in primary seed dispersal in a tropical forest. J. Ecol. 96, 653–667 (2008).
Fan, Y., Miguez-Macho, G., Jobbágy, E. G., Jackson, R. B. & Otero-Casal, C. Hydrologic regulation of plant rooting depth. Proc. Natl Acad. Sci. USA 114, 10572–10577 (2017).
Nicoll, B. C., Gardiner, B. A., Rayner, B. & Peace, A. J. Anchorage of coniferous trees in relation to species, soil type, and rooting depth. Can. J. Res 36, 1871–1883 (2006).
Tyree, M. T. & Sperry, J. S. Vulnerability of Xylem to cavitation and embolism. Annu Rev. Plant Physiol. Plant Mol. Biol. 40, 19–36 (1989).
Markesteijn, L., Poorter, L., Paz, H., Sack, L. & Bongers, F. Ecological differentiation in xylem cavitation resistance is associated with stem and leaf structural traits. Plant, Cell Environ. 34, 137–148 (2011).
O’Brien, S. T., Hubbell, S. P., Spiro, P., Condit, R. & Foster, R. B. Diameter, height, crown, and age relationships in eight neotropical tree species. Ecology 76, 1926–1939 (1995).
Rosell, J. A. Bark thickness across the angiosperms: More than just fire. N. Phytol. 211, 90–102 (2016).
Rosell, J. A., Gleason, S., Méndez-Alonzo, R., Chang, Y. & Westoby, M. Bark functional ecology: Evidence for tradeoffs, functional coordination, and environment producing bark diversity. N. Phytol. 201, 299–311 (2014).
Mattson, W. J. Herbivory in Relation to Plant Nitrogen Content. Annu Rev. Ecol. Syst. 11, 199–161 (1980).
Evans, J. R. Improving photosynthesis. Plant Physiol. 162, 1780–1793 (2013).
Güsewell, S. N: P ratios in terrestrial plants: variation and functional significance [Internet]. New Phytologist John Wiley & Sons, Ltd. p. 243–266. (2004). Available from: https://onlinelibrary.wiley.com/doi/10.1111/j.1469-8137.2004.01192.x
Moles, A. T. & Westoby, M. Seedling survival and seed size: A synthesis of the literature. J. Ecol. 92, 372–383 (2004).
Acknowledgements
This research has been funded by a grant from DOB Ecology. Swiss National Science Foundation, Ambizione grant #PZ00P3_193612 to DSM. JCS considers this work a contribution to Centre for Ecological Dynamics in a Novel Biosphere (ECONOVO), funded by Danish National Research Foundation (grant DNRF173), and his VILLUM Investigator project “Biodiversity Dynamics in a Changing World”, funded by VILLUM FONDEN (grant 16549). The GFBI data from New Zealand were drawn from the Natural Forest plot data collected between January 2009 and March 2014 by the LUCAS programme for the New Zealand Ministry for the Environment and sourced from the New Zealand National Vegetation Survey Databank’. Russian Science Foundation Project 21-46-07002 for the plot data collected in the Krasnoyarsk region. Data from National Forest Inventory of Instituto de Conservação da Natureza (ICNF). FCT - Portuguese Foundation for Science and Technology, Project UIDB/04033/2020. GFBi plot data collection in the São Francisco de Paula National Forest, Rio Grande do Sul, Brazil was financed by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq)(project 520053/1998-2). ReVaTene project is funded by the Education and Research Ministry of Côte d’Ivoire, as part of the Debt Reduction-Development Contracts (C2Ds) managed by IRD GFBI data from southern Ethiopia were collected with funding from the International Climate Initiative (IKI) of the German Federal Ministry for the Environment, Nature Conservation, Building and Nuclear Safety (BMU) (IKI-1 project number 09 II 066ETH A Kaffeewälder). GFBI data from Atlantic Forest, Brazil, was funded by the State of São Paulo Research Foundation (FAPESP 03/12595-7) as part of the BIOTA Programme. COTEC/IF 41.065/2005 and IBAMA/CGEN 093/2005 granted permits to establish the permanent plots and collect data. The Exploratory plots of FunDivEUROPE (with sites in Germany, Finland, Poland, Romania, Italy and Spain) received funding from the European Union Seventh Framework Programme (FP7/2007-2013) under grant agreement 265171. Permission to work in the MAWAS region of Indonesia: the BOS Foundation, the Indonesian Institute of Sciences (LIPI), the Direktorat Fasilitasi Organisasi Politik dan Kemasyarakatan, Departamen Dalam Negri, and the BKSDA Palangkaraya. Funding sources: The American Society of Primatologists, the Duke University Graduate School, the L.S.B. Leakey Foundation, the National Science Foundation (Grant no. 0452995), and the Wenner-Gren Foundation for Anthropological Research (Grant No. 7330). This study was supported by the National Natural Science Foundation of China (31800374), Shandong Provincial Natural Science Foundation (ZR2019BC083) The Spanish Agency for International Development Cooperation [Agencia Española de Cooperación Internacional para el Desarrollo (AECID)] and Fundación Biodiversidad, in cooperation with the governments of Syria and Lebanon. Projects D/9170/07, D/018222/08, D/023225/09 and D/032548/10 funded by the Spanish Agency for International Development Cooperation [Agencia Española de Cooperación Internacional para el Desarrollo (AECID)] and Fundación Biodiversidad, in cooperation with the Universidad Mayor de San Simón (UMSS), the FOMABO (Manejo Forestal en las Tierras Tropicales de Bolivia) project and CIMAL (Compañía Industrial Maderera Ltda.). All persons who made the Third Spanish Forest Inventory possible, especially the main coordinator, J. A. Villanueva (IFN3) The German Research Foundation (DFG) Priority Programme 1374 - Biodiversity Exploratories. Research was supported by APVV 20-0168 from the Slovak Research and Development Agency EC acknowledges funding from the project AdAgriF—Advanced methods of greenhouse gases emission reduction and sequestration in agriculture and forest landscape for climate change mitigation (CZ.02.01.01/00/22_008/0004635) We acknowledge collaboration with the International Boreal Forest Research Association (IBFRA, http://ibfra.org). We thank the Ministère des Forêts, de la Faune et des Parcs du Québec for access to their database of permanent sample plots. We thank the Amazon Forest Inventory Network (RAINFOR), the African Tropical Rainforest Observation Network, and the ForestPlots.net initiative for their contributions from Amazonian and African forests. These were supported by many projects, including an ERC Advanced Grant 291585 (“T-FORCES”) and a Royal Society Wolfson Research Merit Award to O.L.P.; RAINFOR plots were additionally supported by the Gordon and Betty Moore Foundation and the UK Natural Environment Research Council (NERC), notably NERC Consortium Grants AMAZONICA (NE/F005806/1), TROBIT (NE/D005590/1), and BIO-RED (NE/N012542/1). This study was supported by GACR project 21-27454S from the Czech Science Foundation. Financial support from DBT, Govt. of India, through the project ‘Mapping and quantitative assessment of geographic distribution and population status of plant resources of Eastern Himalayan region’ is highly acknowledged. (Reference no. BT/PR7928/NDB/52/9/2006 dated 29.09.2006). Financial support from the Monafor network in Mexico was funded by many projects, including the National Forestry Commission (CONAFOR), Council of Science and Technology of the State of Durango (COCYTED), the Natural Environment Research Council, UK (NERC; NE/T011084/1), and local support of Ejidos and Comunidades. The French National Forest Inventory (NFI campaigns, raw data 2005 and following annual surveys) were downloaded by GFBI at https://inventaire-forestier.ign.fr/spip.php?rubrique159 (site accessed on 1 January 2015); the Italian Forest Inventory (2005 and 2015) were downloaded by GFBI at https://inventarioforestale.org/. GA was supported by Italian National Recovery Plan through the National Biodiversity Future Centre. Financial support from the Czech Science Foundation (Project no. 21-26883S). Plots in Mato Grosso, Brazil, were supported by the National Council for Scientific and Technological Development (CNPq), PELD-TRAN 441244/2016-5 and 441572/2020-0, and Mato Grosso State Research Support Foundation (FAPEMAT) – 0346321/2021. C.A.J. and S.V. acknowledge support from the Brazilian National Research Council/CNPq (PELD process 403710/2012–0); NERC and the State of São Paulo Research Foundation/FAPESP as part of the projects Functional Gradient, PELD/BIOTA and ECOFOR (processes 2003/12595-7, 2012/51509-8 and 2012/51872-5, within the BIOTA/FAPESP Programme (www.biota.org.br); COTEC/IF 002.766/2013 and 010.631/2013 permits. H.Y.H.C. acknowledges the support from NSERC (RGPIN-2019–05109 and STPGP428641) and the Canada Foundation for Innovation and Ontario Research Fund (CFI36014). K.S. acknowledges the support of data acqusition from project: “LIFE+ ForBioSensing PL Comprehensive monitoring of stand dynamics in Białowieża Forest supported with remote sensing techniques” which is co-funded by the EU Life Plus programme (contract number LIFE13 ENV/PL/000048) and The National Fund for Environmental Protection and Water Management in Poland (contract number 485/2014/WN10/OP-NM-LF/D) OB was supported by Romania National Council for Higher Education Funding, CNFIS, project number CNFIS-FDI-2024-F-0155. TMF was supported by a grant from the Czech Science Foundation (19-14620S). GFBi plot data collection in Santa Catarina, Brazil (FlorestaSC), was financed by Conselho Nacional de Desenvolvimento Científico e Tecnológico; FAPESC; SEMAE; FAO; SFB. RLC monitoring plots data in Costa Rica was supported by grants from the Andrew W. Mellon Foundation, the US National Science Foundation (NSF DEB-0424767, NSF DEB-0639393 and NSF DEB-1147429), US NASA Terrestrial Ecology Programme, and the University of Connecticut Research Foundation.
Author information
Authors and Affiliations
Contributions
Iris Hordijk, Tom W. Crowther and Daniel S. Maynard conceived of the study. Iris Hordijk extracted and analysed the data, and drafted the manuscript with assistance from Lourens Poorter, Daniel S. Maynard and Tom W. Crowther. Jingjing Liang, Peter B. Reich, Sergio de-Miguel, Gert-Jan Nabuurs, Javier G. P. Gamarra, Han Y. H. Chen, Mo Zhou, Susan K. Wiser, Hans Pretzsch, Alain Paquette, Nicolas Picard, Bruno Hérault, Jean-Francois Bastin, Giorgio Alberti, Meinrad Abegg, Yves C. Adou Yao, Angelica M. Almeyda Zambrano, Braulio V. Alvarado, Esteban Alvarez-Davila, Patricia Alvarez-Loayza, Luciana F. Alves, Iêda Amaral, Christian Ammer, Clara Antón-Fernández, Alejandro Araujo-Murakami, Luzmila Arroyo, Valerio Avitabile, Gerardo A. Aymard C., Timothy Baker, Olaf Banki, Jorcely Barroso, Meredith L. Bastian, Luca Birigazzi, Philippe Birnbaum, Robert Bitariho, Pascal Boeckx, Frans Bongers, Olivier Bouriaud, Pedro H. S. Brancalion, Susanne Brandl, Francis Q. Brearley, Roel Brienen, Eben N. Broadbent, Helge Bruelheide, Roberto Cazzolla Gatti, Ricardo G. Cesar, Goran Cesljar, Robin L. Chazdon, Chelsea Chisholm, Emil Cienciala, Connie J. Clark, David B. Clark, Gabriel Colletta, David Coomes, Fernando Cornejo Valverde, Jose J. Corral-Rivas, Philip Crim, Jonathan Cumming, Selvadurai Dayanandan, André L. de Gasper, Mathieu Decuyper, Géraldine Derroire, Ben DeVries, Ilija Djordjevic, Aurélie Dourdain, Jiri Dolezal, Nestor Laurier Engone Obiang, Brian Enquist, Teresa Eyre, Adandé Belarmain Fandohan, Tom M. Fayle, Leandro V. Ferreira, Ted R. Feldpausch, Leena Finér, Markus Fischer, Christine Fletcher, Lorenzo Frizzera, Damiano Gianelle, Henry B. Glick, David Harris, Andrew Hector, Andreas Hemp, John Herbohn, Annika Hillers, Eurídice N. Honorio Coronado, Cang Hui, Hyunkook Cho, Thomas Ibanez, Ilbin Jung, Nobuo Imai, Andrzej M. Jagodzinski, Bogdan Jaroszewicz, Vivian Johannsen, Carlos A. Joly, Tommaso Jucker, Viktor Karminov, Kuswata Kartawinata, Elizabeth Kearsley, David Kenfack, Deborah Kennard, Sebastian Kepfer-Rojas, Gunnar Keppel, Mohammed Latif Khan, Timothy Killeen, Hyun Seok Kim, Kanehiro Kitayama, Michael Köhl, Henn Korjus, Florian Kraxner, Diana Laarmann, Mait Lang, Simon Lewis, Huicui Lu, Natalia Lukina, Brian Maitner, Yadvinder Malhi, Eric Marcon, Beatriz Schwantes Marimon, Ben Hur Marimon-Junior, Andrew Robert Marshall, Emanuel Martin, Olga Martynenko, Jorge A. Meave, Omar Melo-Cruz, Casimiro Mendoza, Cory Merow, Stanislaw Miscicki, Abel Monteagudo Mendoza, Vanessa Moreno, Sharif A. Mukul, Philip Mundhenk, Maria G. Nava-Miranda, David Neill, Victor Neldner, Radovan Nevenic, Michael Ngugi, Pascal A. Niklaus, Jacek Oleksyn, Petr Ontikov, Edgar Ortiz-Malavasi, Yude Pan, Alexander Parada-Gutierrez, Elena Parfenova, Minjee Park, Marc Parren, Narayanaswamy Parthasarathy, Pablo L. Peri, Sebastian Pfautsch, Oliver L. Phillips, Maria Teresa Piedade, Daniel Piotto, Nigel C. A. Pitman, Martina Pollastrini, Irina Polo, Axel Dalberg Poulsen, John R. Poulsen, Freddy Ramirez Arevalo, Zorayda Restrepo-Correa, Mirco Rodeghiero, Samir Rolim, Anand Roopsind, Francesco Rovero, Ervan Rutishauser, Purabi Saikia, Christian Salas-Eljatib, Peter Schall, Dmitry Schepaschenko, Michael Scherer-Lorenzen, Bernhard Schmid, Jochen Schöngart, Eric B. Searle, Vladimír Seben, Federico Selvi, Josep M. Serra-Diaz, Douglas Sheil, Anatoly Shvidenko, Javier Silva-Espejo, Marcos Silveira, James Singh, Plinio Sist, Ferry Slik, Bonaventure Sonké, Alexandre F. Souza, Hans ter Steege, Krzysztof Stereńczak, Jens-Christian Svenning, Miroslav Svoboda, Ben Swanepoel, Natalia Targhetta, Nadja Tchebakova, Raquel Thomas, Elena Tikhonova, Peter Umunay, Vladimir Usoltsev, Renato Valencia, Fernando Valladares, Fons van der Plas, Tran Van Do, Michael E. Van Nuland, Rodolfo Vasquez Martinez, Hans Verbeeck, Helder Viana, Alexander C. Vibrans, Simone Vieira, Klaus von Gadow, Hua-Feng Wang, James Watson, Gijsbert D. A. Werner, Florian Wittmann, Verginia Wortel, Roderick Zagt, Tomasz Zawila-Niedzwiecki, Chunyu Zhang, Xiuhai Zhao, Zhi-Xin Zhu and Irie Casimir Zo-Bi provided data for the analysis. All authors assisted with revisions and gave final approval for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hordijk, I., Poorter, L., Liang, J. et al. Effect of climate on traits of dominant and rare tree species in the world’s forests. Nat Commun 16, 4773 (2025). https://doi.org/10.1038/s41467-025-59754-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-59754-7