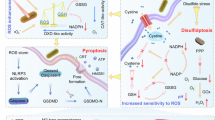
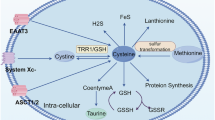
Abstract
Disulfidptosis, a recently identified form of programmed cell death, is initiated by depletion of endogenous nicotinamide adenine dinucleotide phosphate (NADPH) under glucose starvation. Tumor cells, owing to their heightened requirements of energy and nutrients, are more susceptible to disulfidptosis than normal cells. Here, we introduced an effective strategy to induce tumor disulfidptosis via interrupting cellular energy supply and reducing power by integrating a copper single-atom nanozyme (CuSAE) and glucose oxidase (GOx). GOx induces glucose starvation, impeding generation of NADPH through pentose phosphate pathway (PPP). CuSAE mimics NADPH oxidase, depleting existing NADPH, which intensifies the blockade of disulfide reduction and efficiently triggers disulfidptosis of tumor cells. Furthermore, CuSAE exhibits peroxidase- and glutathione oxidase-mimicking activities, catalyzing generation of •OH radical and depletion cellular GSH, which enhances oxidative stress and exacerbates cell damage. Disulfidptosis is confirmed as the predominant type of cell death induced by GOx/CuSAE. In vivo assays demonstrated the high antitumor potency of GOx/CuSAE in treating with female tumor-bearing mice, with minimal systemic toxicity observed. This work introduces a promising strategy for designing antitumor agents by inducing disulfidptosis. The enzyme hybrids that combine nanozymes and natural enzymes offer a feasible approach to achieve this multifaceted therapeutic goal.
Similar content being viewed by others
Introduction
Disulfide bonds play a pivotal role in intracellular protein folding, contributing to the stability of protein structures, maintenance of cellular redox balance, and physiological functions of cells1,2. The homeostasis of intracellular disulfides is predominantly maintained through endogenous nicotinamide adenine dinucleotide phosphate (NADPH)3. A recent discovery highlights that the blockade of NADPH supply results in an excessive accumulation of disulfides, identifying a form of programmed cell death, termed disulfidptosis4. This mode of cell death occurs under glucose starvation that would cut off the pentose phosphate pathway (PPP), the primary route for producing NADPH5,6. Notably, tumor cells, with heightened demand for energy and nutrients for rapid growth7; exhibit increased vulnerability to disulfidptosis compared to normal cells. A distinctive characteristic feature of this elevated energy demand in tumor cells is the heightened expression of solute carrier family 7 member 11 (SLC7A11), which facilitates cystine import to maintain cellular redox homeostasis and survival8. However, the enhanced cystine import leads to the generation of abundant disulfides, escalating the need for NADPH for maintaining cellular redox homeostasis9. Consequently, downregulation of endogenous NADPH levels expedites disulfide stress in tumor cells, presenting a promising avenue for tumor disulfidptosis therapy. In addition, cutting off the glucose supply to inhibit new NADPH generation is anticipated to enhance tumor disulfidptosis.
Single-atom nanozymes (SAEs) are characterized by atomically dispersed active metal sites10. SAEs exhibit high enzymatic activities by mimicking the metal active centers in natural enzymes10,11. Due to the maximal atom utilization, SAEs exhibit higher catalytic activity than conventional nanozymes12,13. Beyond catalysis, SAEs offer advantages, such as controllable preparation, high stability, and efficient cellular internalization, making them suitable for biomedical applications14. In spite of these advantages, the sole use of SAE may not meet therapeutic requirements. Therefore, modification of SAE with natural macromolecules has emerged as a promising approach for improving bio-applicability of inorganic nanomaterials15. In addition, conjugation of natural enzymes brings additional biological functions to SAE, which would increase the therapeutic potential of the hybridized materials. Moreover, this conjugation could also increase the chance of using certain natural enzymes, such as the highly toxic glucose oxidase (GOx)16. Therefore, a rational combination of SAE with natural enzymes could synergize their biological functions and reduce their limitations for biomedical applications.
In this work, we developed a nanozyme hybrid for regulating the cellular energy supply and reducing power, aiming to induce glucose starvation and NADPH diminishing, the two key features of disulfidptosis. The nanozyme hybrid integrates a copper single-atom nanozyme (CuSAE) with natural glucose oxidase (GOx). CuSAE, mimicking NADPH oxidase, effectively converts NADPH to NADP+ and generates superoxide anions (O2•-). Simultaneously, GOx catalyzes glucose oxidation, producing gluconic acid and hydrogen peroxide (H2O2), which hinders the generation of NADPH by consuming the glucose16. GOx/CuSAE hybrid synergizes the two enzymatic activities of NADPH oxidase and glucose oxidase, resulting in efficient blockade of disulfide reduction and triggers disulfidptosis of tumor cells. Furthermore, CuSAE also possesses peroxidase (POD) and glutathione oxidase (GSHOx) mimicking activities, which can catalyze the reaction of H2O2, the product of GOx-catalyzed glucose oxidation, to generate hydroxyl radicals (•OH) and further enhance disulfidptosis. Collectively, this GOx/CuSAE hybrid nanoplatform provides a dual strategy to trigger tumor cell disulfidptosis (Fig. 1).
a Synthesis of GOx/CuSAE enzyme hybrid. Step 1: preparation of CuSAE by coating polydopamine (PDA) on SiO2 nanoparticles, followed by pyrolysis and hydrofluoric acid etching. Step 2: conjugation of GOx to CuSAE through DSPE-PEG-NH2. b Cellular response of GOx/CuSAE to induce disulfidptosis. GOx/CuSAE cuts off the intracellular pentose phosphate pathway by consuming glucose, blocking NADPH production, depleting existing NADPH, leading to abnormal accumulation of large excessive disulfide bonds in tumor cells and initiating disulfidptosis, featured by cytoskeleton shrinkage. Parts of the graphic elements were generated by Figdraw (www.figdraw.com).
Results
Preparation and characterization
Single-atom-dispersed CuSAE was synthesized on SiO2 supports as depicted in Fig. 1. Briefly, SiO2 nanospheric cores were initially synthesized using a tetraethyl orthosilicate (TEOS) sol-gel method17. Polydopamine (PDA)-coated SiO2 nanoparticles were obtained by incubating dopamine with SiO2 cores under an alkaline condition (pH 8.5), during which dopamine spontaneously polymerized on the surface of SiO2, forming PDA-coated nanoparticles (SiO2@PDA). Subsequently, Cu(II) precursors containing particles were prepared by coating PDA on SiO2 particles in the presence of copper(II) acetylacetonate, yielding SiO2@PDA@Cu. These Cu(II) precursors containing particles were then subjected to heating at 900 °C in a nitrogen atmosphere for 3 h to pyrolyze polydopamine into a carbon-nitrogen structure, resulting in SiO2@CN@Cu. Then the SiO2 cores were etched with hydrofluoric acid to generate hollow carbon/nitrogen spheres doped with Cu atoms, generating the single Cu atom nanozyme (CuSAE). The enzyme hybrid, denoted as GOx/CuSAE, was obtained by ligating the GOx protein to the amino group of DSPE-PEG-NH2 coated on CuSAE particles (see Supplementary information for details). The successful conjugation of GOx was verified by measuring protein on the particles using an SDS-PAGE method.
Transmission electron microscopy (TEM) images showed that the SiO2@CN@Cu particles exhibited a uniform spherical structure with a diameter of 260 nm (Fig. 2a). After the pyrolysis and etching processes, the resultant CuSAE particles showed a hollow morphology (Fig. 2b). High-resolution transmission electron microscopy (HRTEM) images indicated that the shell thickness was approximately 30 nm (Supplementary Fig. 1). In consistence with the atomically dispersed nature of Cu in CuSAE, no discernible clusters were observed in the particles. Inductively coupled plasma mass-spectrometry (ICP-MS) measurements indicated that the Cu content was only approximately 0.65% (w/w) in CuSAE. The corresponding energy dispersive X-ray spectroscopy (EDS) element mapping image demonstrates a uniform distribution of C, N, and Cu, confirming the presence of Cu active sites in the C/N-based supports (Fig. 2c). The X-ray diffraction (XRD) pattern of CuSAE exhibits weak and broad peaks at approximately 24° and 40–60° corresponding to characteristic diffractions of carbon (002) and (100)/(101)18. The diffraction peaks corresponding to crystalline Cu species were not identifiable, consistent with the existence of the single-atomic status of Cu atoms (Fig. 2d). X-ray photoelectron spectroscopy (XPS) spectra clearly showed signals of C 1s and N 1s elements (Fig. 2e, f). Due to the low content of Cu atoms, the Cu 2p peak appeared in a low signal-to-noise ratio (Supplementary Fig. 2).
a A representative TEM image of SiO2@PDA@Cu. Seven images were recorded and show similar results. b A representative TEM image of CuSAE. Images were recorded on three independent samples and show similar results. c A representative EDS element mapping of CuSAE. Three images were recorded and show similar results. d XRD spectrum of CuSAE. e, f XPS spectra of CuSAE; e the C 1s region, f the N 1s region. XPS measurements revealed graphitic-N, pyrrolic-N, and pyridinic-N as dominant nitrogen species in CuSAE. g HAADF-STEM image of CuSAE. White circles indicate single Cu atoms. Three images were recorded and show similar results. h, i X-ray absorption fine structure analysis of CuSAE; h Fourier-transformed EXAFS signal of CuSAE and reference samples; i XANES spectra at Cu K-edge of CuSAE and reference samples. Source data are provided as a Source Data file.
To probe the state of Cu species in CuSAE, high-angle annular dark field scanning transmission electron microscopy (HAADF-STEM) with aberration correction was performed (Fig. 2g). Numerous well-dispersed bright spots (indicated by white circles) were distinctly observed, clearly signifying the atomic dispersion of Cu species in the CN framework of CuSAE. For a comprehensive investigation of the coordination environment of Cu atoms, X-ray absorption spectroscopy measurements were conducted. Fourier transform extended X-ray absorption fine structure (EXAFS) analysis clearly indicated Cu–N signals at 1.5 Å, while Cu–O (2.5–3.1 Å) and Cu–Cu (2.24 Å) interactions can be excluded19,20,21. This result further confirmed the atomic dispersion of Cu species (Fig. 2h and Supplementary Figs. 3–7). The X-ray absorption near edge structure (XANES) spectrum of Cu K-edge reveals that the absorption edge of CuSAE is positioned between the absorption edges of Cu2O and CuO, suggesting the mixed cupreous and cupric states in the particles (Fig. 2i). These findings validate the successful synthesis of Cu single-atom-dispersed nanoparticles, providing the structural foundation for achieving a nanozyme with high catalytic properties.
The GOx conjugated hybrid nanozyme, featuring PEG linkers between protein and nanoparticles, was characterized. Dynamic light scattering (DLS) measurement indicated that the hydrated particle size of the nanoparticles increased from 250 to 359 nm after protein conjugation (Supplementary Fig. 8). Meanwhile, the surface charge underwent a transition from positive in CuSAE to negative in GOx/CuSAE (Supplementary Fig. 9). Further, sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was applied to analyze the protein components of the particles. The lanes of GOx/CuSAE show a protein band at the position same as that of GOx, confirming the successful conjugation of GOx in GOx/CuSAE (Supplementary Fig. 10). TEM imaging of GOx/CuSAE showed a layer of around the particles, indicting the coating on GOx on CuSAE (Supplementary Fig. 11). In addition, the hydrated particle size of GOx/CuSAE remained relatively unchanged in cell culture medium containing 10% fetal bovine serum for 72 h (Supplementary Fig. 12), suggesting reasonable stability of the nanoparticles. Furthermore, PEGylation and protein modification have been proposed as effective ways to reduce protein corona, which would improve the in vivo performance of the particles. SDS-PAGE assay confirmed this hypothesis on GOx/CuSAE particles (Supplementary Fig. 13).
The catalytic activity of the GOx/CuSAE hybrid nanoplatform
NADPH oxidase is a natural enzyme that catalyzes the conversion of NADPH to NADP+ while generating superoxide anion (O2•-)22. The NADPH oxidase-mimicking activity of CuSAE was assessed by monitoring the characteristic absorbance of NADPH (peak at 340 nm) during incubation with CuSAE. The decrease of NADPH absorbance upon incubation with CuSAE indicated that CuSAE can efficiently scavenge NADPH in a concentration-dependent manner (Fig. 3a). The measurement at different pH values indicated that the NADPH oxidase-mimicking activity of CuSAE further increased at low pH, suggesting that the CuSAE-mediated catalysis is more pronounced in acidic tumor microenvironments (TME) than in normal organs. The CuSAE-catalyzed NADPH production was further validated through high-performance liquid chromatography (HPLC) (Supplementary Fig. 14). The significant decrease of NADPH absorbance (340 nm) after incubation with CuSAE provides solid evidence of the NADPH oxide-mimicking activity of CuSAE (Fig. 3b), in comparison to the slight decrease in absorbance of NADPH in the absence of CuSAE (Supplementary Fig. 15). Furthermore, the O2•- generation during the oxidation of NADPH was verified by electron paramagnetic resonance (EPR) using a trapping agent 5,5-dimethyl-1-pyrroline N-oxide (DMPO). O2•- can combine with H+ and produce hydrogen peroxide free radicals (•OOH), which can be captured by DMPO, generating an EPR-detectable species DMPO-OOH23. After incubation of CuSAE with NADPH in a weakly acidic condition (pH 6.0), EPR spectra were recorded upon addition of DMPO. The six peaks, with nearly equal intensities at positions 1, 2, 4, and 6, and weaker peaks at positions 3 and 5 (Fig. 3c), correspond to the characteristic EPR signal of DMPO-OOH24,25. These results confirmed the generation of O2•- radicals in the CuSAE-catalyzed NADPH oxidation.
a, b Measuring NOx-like activity of CuSAE based on the conversion of NADPH. The experiment was performed by incubation of 200 μg/mL NADPH with different concentrations of CuSAE (0–100 μg/mL) (a) or different pH (7.4, 6.0, and 4.5) (b). After incubation for 30 min, the concentration of the remaining NADPH was measured based on its absorption at 340 nm. c EPR spectra detecting O2•- by using 1,3-diphenylisobenzofuran as a trapping agent. The experiment was performed on 100 μg/mL CuSAE and 200 μg/mL NADPH. d, e POD-like activity of CuSAE measured with TMB chromogenic assay at different pH (d) or different concentration of CuSAE (e). f EPR spectra detect •OH radical by using 1,3-diphenylisobenzofuran as a trapping agent. The assay was performed on 10 mM H2O2 and 60 μg/mL CuSAE. g GSHOx-like activity of CuSAE measured with DTNB assay. The assay was performed on GSH (0.15 mM) and different concentrations of CuSAE (0–90 μg/mL) in the presence of DTNB (2.5 mM). The peak at 412 nm indicates the reduced TNB from GSH reduction. h pH variation in the GOx/CuSAE-catalyzed oxidation of glucose. The reaction was performed by incubation of GOx/CuSAE (80 μg/mL) with different concentrations of glucose for 12 h. i Catalytic activities of free GOx and GOx/CuSAE after storage. GOx (80 μg/mL) and GOx/CuSAE (with 80 μg/mL GOx) were incubated in weakly acidic conditions (pH 6.0) at 37 °C for different times. After the incubation, their catalytic activity was analyzed by incubation with glucose (10 mM) for 2 h, and the generation of H2O2 production was measured titanium sulfate assay. Data are shown as mean ± s.d. from three independent measurements. For statistical analyses, One-way ANOVA Dunnett correction (a, d, e, and h), or Two-sided Student’s t-test (i) was used on the results from three independent experiments (n = 3). Source data are provided as a Source Data file.
The POD-like activity of CuSAE was verified with a colorimetric assay using a chromogenic probe 3,3’,5,5’-tetramethylbenzidine (TMB). The colorless TMB can be oxidized by •OH and generate blue ox-TMB with absorption at 652 nm26,27. Incubation of CuSAE with H2O2 in the presence of TMB clearly showed the production of ox-TMB; the absorption measurement indicated that the generation of •OH was a CuSAE concentration-dependent process (Fig. 3d, e and Supplementary Fig. 16). Meanwhile, the consumption of H2O2 in the CuSAE-catalyzed reaction was analyzed using titanium sulfate (Ti(SO4)2), a H2O2 chromogenic agent. The decreased absorbance confirmed the active consumption of H2O2 during the incubation with CuSAE (Supplementary Fig. 17). Additionally, the generation of •OH was directly measured by EPR with DMPO as a radical trapping agent; the typical signal (1:2:2:1) confirmed the production of •OH (Fig. 3f).
The GSHOx-like activity of CuSAE was verified using 5,5’-dithiobis (2-nitrobenzoic acid) (DTNB), the probe that can be reduced by GSH and generate TNB with absorption at 412 nm. The time-dependent measurement showed that the concentration of GSH decreased upon incubation with CuSAE (Fig. 3g). This result indicated that CuSAE possesses GSHOx-mimicking activity, leading to the consumption of GSH, which would exacerbate the imbalance of cellular redox.
GOx is a natural enzyme that catalyzes the oxidation of glucose to the product gluconic acid and H2O2; hence, the GOx activity of GOx/CuSAE hybrid nanoplatform was analyzed. Incubation of GOx/CuSAE with glucose led to a significant pH decrease of the reaction solution, which evidenced the generation of gluconic acid (Fig. 3h). Moreover, the titanium sulfate assay confirmed that the H2O2 production gradually increased during incubation of GOx/CuSAE in the glucose solution (Supplementary Fig. 18). These results indicated that the conjugation of GOx did not interfere with its catalytic activity in GOx/CuSAE, implying the glucose consumption and H2O2 generation activity of the hybrid nanozyme in cells.
The catalytic performance of GOx/CuSAE on glucose oxidation was assessed in different buffers simulating lysosomal, TME, and normal tissue environments. The H2O2 production catalyzed by GOx/CuSAE remained nearly unaffected by changes in pH, though GOx alone exhibited increased activity under weak acidic conditions (Supplementary Fig. 19). This result suggests that the enzymatic activity of GOx/CuSAE for H2O2 generation is not influenced by pH alteration, ensuring consistent performance in cells. Remarkably, the immobilization of GOx on CuSAE significantly improves the stability of the natural enzyme. While the catalytic activity of GOx obviously decreased after storage at 37 °C for 48 h, GOx/CuSAE retained its activity without a noticeable reduction under the same conditions (Fig. 3i). This highlights the superior practical applicability of GOx/CuSAE. Taken these in vitro enzymatic assays together, we can conclude that GOx/CuSAE can effectively exert its enzyme-like ability to reduce the amount of NADPH, GSH, and glucose, producing various reactive oxygen species (ROS). This capability holds great potential for inducing cellular disulfide accumulation, enhancing oxidative stress, and ultimately triggering cell disulfidptosis.
DFT studies on the activity of CuSAE
To elucidate the NADPH oxidase-like catalytic processes of the single-atom catalyst CuSAE, density functional theory (DFT) calculations were performed to analyze the adsorption energies of reactants, free energy changes during the catalytic reaction process, and electronic density of states (DOS) of CuSAE. The conversion of the same species on the carbon-nitrogen (C–N) substrates in the absence of copper ions was also calculated for comparison. EXAFS analysis of the scattering interaction between the Cu center and the first shell of N atoms in CuSAE revealed that Cu atoms are coordinated with 4.4 ± 0.4 atoms of N with an average bond length of 1.936 ± 0.007 Å (Supplementary Fig. 3, 4 and Supplementary Table 1). This result indicated that the coordination structure in CuSAE is predominantly Cu–N4 (Supplementary Fig. 20).
The NADPH oxidase-mimicking catalytic process was built up according to literature28, showing the conversion of NADPH to NADP+ and the concomitant reduction of O2 to O2•− (Fig. 4a). The DFT calculation result indicated that the adsorptions of O2 and NADPH exhibit lower energies on CuSAE (−1.1087 eV and −0.7631 eV, respectively) relative to those on the C–N substrates (−0.641 eV and −0.4213 eV, respectively) (Supplementary Fig. 21 and 22). This indicates that Cu atoms enhance the interaction of C–N substrates with the reactant O2, hence creating highly active sites for the catalytic process. Furthermore, DOS analysis demonstrated that the introduction of Cu atoms significantly influences the electronic structure, particularly resulting in new states near the Fermi level (Supplementary Fig. 23). This result suggests that CuSAE possesses a high availability of states for electron transfer during the catalytic reaction, thereby enhancing overall catalytic efficiency.
The Gibbs free energy diagram indicates that the active copper center enhances the affinity for O2 (Fig. 4b). On the other hand, it has been proposed that NADPH can be absorbed onto C–N substrates through π-π stacking interactions28. The DFT calculation indicates that absorption of NADPH to C–N substrates increases the system energy; however, the presence of Cu in CuSAE reduces the energy barrier associated with NADPH adsorption, suggesting that Cu promotes the NADPH binding to CuSAE. Therefore, NADPH could bind to CuSAE more favorably at the active Cu centers. Upon binding of both O2 and NADPH to CuSAE, NADPH is oxidized to NADP+ by donating electrons to the CuSAE, through which O2 acquires electrons and forms O2•- radicals. DFT calculations demonstrate that CuSAE exhibits lower free energy compared the C–N substrates throughout the catalytic process (Fig. 4b), indicating that the active Cu atoms in C–N substrates play a crucial role in facilitating electron transfer in NADPH oxidation. These DFT calculations provide mechanistic insights into the enzymatic activities of CuSAE, supporting the experimental observations of the NADPH oxidase activity and confirming the catalytic properties of the nanozyme.
GOx/CuSAE induces disulfidptosis of tumor cells
Encouraged by the remarkable catalytic performance of GOx/CuSAE, we evaluated the cellular response and therapeutic effects of this enzyme hybrid on tumor cells. The in vitro results of the GOx/CuSAE-induced depletion of NADPH and glucose presented strong implications of disulfidptosis of tumor cells. Hence, the cellular response associated with disulfidptosis was explored. SLC7A11, the key cystine transporter required for disulfidptosis, has been found to be overexpressed in many types of cancer29. Triple negative breast cancer 4T1 cells, a type of SLC7A11-positive cells, were employed in this study30.
At the cellular level, we first analyzed the behavior of nanozymes entering 4T1 cells using cell uptake experiments (Fig. 5a). The time-dependent cellular uptake of the nanozyme was examined (Supplementary Fig. 24) using red fluorophore-labeled CuSAE (RF@CuSAE). Fluorescence imaging clearly showed the internalization of CuSAE after 1.5 h of incubation, with continuous accumulation reaching higher fluorescence intensity after 5 h, and remained in the cells for up to 12 h. This indicates that the nanozyme can be effectively internalized into tumor cells.
a Cellular uptake of CuSAE. The assay was performed on 4T1 cells treated with red fluorophore (RF)-labeled RF@CuSAE for 5 h (in red fluorescence). Nucleuses were stained blue by Hoechst 33342. b Viability of 4T1 cells at 24 h post-treatment with CuSAE or GOx/CuSAE. Two-sided Student’s t-test was used in the statistical analysis on the data from three biologically independent experiments (n = 3), giving P = 0.0133 for the samples of 100 μg/mL CuSAE v.s. 0 μg/mL CuSAE, and P = 0.0027 for the samples of 100 μg/mL GOx/CuSAE v.s. 0 μg/mL GOx/CuSAE. c Glucose uptake levels in 4T1 cells treated with CuSAE and GOx/CuSAE (in 20 μg/mL CuSAE) for 24 h. One-way ANOVA, Dunnett correction was used in the statistical analysis on the results from three biologically independent experiments (n = 3), giving P = 0.0003 for the samples of GOx/CuSAE v.s. Control. d NADPH level in 4T1 cells treated with CuSAE or GOx/CuSAE (in 20 μg/mL CuSAE) for 24 h. NADPH level refers to the [NADPH]/[NADPtotal] ratio relative to the control group. One-way ANOVA, Dunnett correction was used in the statistical analysis on the results from three biologically independent experiments (n = 3), giving P = 0.0274 for the samples of GOx/CuSAE v.s. Control. e Western blotting analysis of disulfide formation of actin cytoskeleton proteins in 4T1 cells treated with CuSAE or GOx/CuSAE (in 20 μg/mL CuSAE) for 24 h. Reducing western blotting analyses were also performed by adding reducing agent β-mercaptoethanol to reduce disulfide bonds of the proteins. f 4T1 cells were treated with CuSAE or GOx/CuSAE (in 20 μg/mL CuSAE) for 24 h (red: F-actin, blue: nucleus). g Fluorescent staining of F-actin and membrane of the cells after being treated with CuSAE or GOx/CuSAE (in 20 μg/mL CuSAE) for 24 h (red: F-actin, green: membrane). h TEM imaging of 4T1 cells treated with GOx/CuSAE (in 20 μg/mL CuSAE) for 24 h. Data are shown as mean ± s.d. from three independent measurements. Experiments in (e–h) were repeated independently three times, with similar results. Source data are provided as a Source Data file.
Next, the lysosome escape assay was performed to further elucidate the behavior of CuSAE in cells. By staining lysosomes with green fluorescent LysoTracker, the endocytosis and lysosomal escape behavior of nanozymes can be monitored by fluorescence microscopy (Supplementary Fig. 25). Initially, red-fluorescent CuSAE was primarily co-localized with green-labeled lysosomes after 1.5 h of incubation. While increased cellular uptake of CuSAE occurred at 5 h, a stronger red signal was observed, showing partial lysosomal escape of CuSAE at this stage. After 12 h incubation, the red fluorescence from CuSAE was basically separated from the green fluorescence of lysosomes, indicating lysosomal escape of the majority of CuSAE into the cytoplasm.
A cellular viability assay indicated that GOx/CuSAE inhibited the cell proliferation in a concentration-dependent manner, with significantly greater inhibitory efficacy than CuSAE alone (Fig. 5b and Supplementary Fig. 26). This result confirmed the cytotoxicity of CuSAE to tumor cells, and incorporation of GOx clearly synergized inhibitory effect of CuSAE to tumor cells.
Disulfidptosis is characterized by a decrease in NADPH content and the accumulation of disulfide bonds in SCL7A11-positive cells under low glucose conditions4. Glucose analysis indicated that the cellular glucose levels significantly decreased after the treatment of GOx/CuSAE (Fig. 5c). This result aligns with the presence of GOx in the hybrid nanozyme and confirms its superior glucose-depleting ability. In addition, the treatment of CuSAE clearly reduced cellular NADPH level due to its NADPH oxidase activity (Fig. 5d). By comparison, GOx/CuSAE exhibited greater activity of NADPH depletion, indicating the synergistic effect of glucose decrease in NADPH depletion of the hybrid nanozyme.
NADPH decline typically causes aberrant accumulation of disulfides in cells; together with glucose starvation, these cellular alterations tend to induce disulfidptosis that is featured by cytoskeleton condensation and deformation5. Three cytoskeletal proteins associated with disulfidptosis, including actin, filamin A (FLNA), and Talin31,32,33, were analyzed, as excessive formation of disulfide bonds can cause aberrant oligomerization of proteins. Western blotting showed that three proteins formed oligomers in the cells treated with CuSAE, while the treatment of GOx/CuSAE resulted in a more pronounced effect (Fig. 5e), which is consistent with the increase of the total disulfide bond content in cells treated with CuSAE and GOx/CuSAE. The nearly fivefold increase clearly indicated the aberrant accumulation of disulfides in cells treated with GOx/CuSAE, and the occurrence of disulfidptosis (Supplementary Fig. 27). The presence of reducing agent, β-mercaptoethanol in the Western blotting analysis prevented the appearance of high molecular bands, confirming that the protein oligomerization was induced by disulfide bonds. This result clearly indicated that GOx/CuSAE induce disulfidptosis of the tumor cells, and GOx and CuSAE play synergistic roles in the cell energy supply blockade. It is noteworthy that, given that actin is a crucial component of cytoskeleton microfilaments, the abnormal intermolecular disulfide bonds in actin would interfere with the cytoskeleton structure and cell morphology. Fibros actin (F-actin) imaging showed that the filamentous structure of F-actin was disrupted by the treatment of CuSAE or GOx/CuSAE, evidenced by the contracted actin filament, distorted cytoskeleton, shrunken cell volume, and condensed morphology, and a more pronounced effect was observed on GOx/CuSAE treatment (Fig. 5f).
The fine structural change of cells, the other feature of disulfidptosis, was explored in cells treated with GOx/CuSAE. Co-staining of F-actin and cell membrane showed that GOx/CuSAE-induced contraction of the actin cytoskeleton and shrinkage of the cell membrane. Notably, the actin cytoskeleton showed a greater degree of contraction than the cell membrane, clearly separating from the cytoplasmic membrane (Fig. 5g), which is one of the most important typical characteristics of cell disulfidptosis4. Similar results were observed in the bio-TEM images (Fig. 5h and Supplementary Fig. 28), providing intuitive evidence for the structural changes in disulfidptotic cells.
In cells with high SLC7A11 expression, NADPH depletion triggers disulfidptosis. This process induces the formation of intermolecular and/or intramolecular disulfide bonds in the sulfhydryl groups of active cysteine residues in redox-sensitive proteins, including cytoskeletal proteins like actin. The abnormal disulfide bond formation in these cytoskeletal proteins leads to the contraction of F-actin and its detachment from the plasma membrane, ultimately causing the disintegration of the actin network and cell death. To highlight the critical role of SLC7A11, SLC7A11 low-expressing cells were generated using siRNA interference (SLC7A11low), and SLC7A11-high expressing cells were obtained by transfecting SLC7A11 plasmid (SLC7A11high) to increase the SLC7A11 level in cells (Supplementary Fig. 29).
First, we analyzed whether the cellular glucose level is affected by the alteration of SLC7A11 levels. The results indicated that no detectable differences were observed on the glucose levels among the SLC7A11 low expression (SLC7A11low), SLC7A11 overexpression (SLC7A11high), and regular (SLC7A11control) 4T1 cells (Supplementary Fig. 30). Under such a condition, treatments with CuSAE and GOx/CuSAE were applied to these types of cells. The cellular glucose levels were not influenced by the treatment of CuSAE, but clearly decreased upon the treatment of GOx/CuSAE. Notably, all three types of cells exhibited similar glucose levels in these treatments, indicating that SLC7A11 expression did not influence the GOx-induced glucose consumption (Supplementary Fig. 30). This result indicates that alteration of the SLC7A11 level does not influence the function of GOx in cells. Thus, the effect of SLC7A11 expression on disulfidptosis can be analyzed independently.
Then, the effect of SLC7A11 level on the GOx/CuSAE-induced disulfidptosis was verified via cell viability assay. GOx/CuSAE clearly exhibited higher cytotoxicity on tumor cells with increased SLC7A11 expression, and lower cytotoxicity on tumor cells with decreased SLC7A11 expression (Fig. 6a, b). This result confirms the crucial role of SLC7A11 in the GOx/CuSAE-induced cell death, which is consistent with disulfidptosis. The SLC7A11 level is positively correlated with the intracellular cystine levels, a key factor in inducing disulfidptosis.
a, b Viability of 4T1 cells with different SLC7A11 expression levels treated with CuSAE (a) and GOx/CuSAE (b) for 24 h. Two-sided Student’s t-test was used in the statistical analysis on the data from three biologically independent experiments (n = 3), giving P = 0.0004 for the samples of SLC7A11control v.s. SLC7A11low treated with 100 μg/mL CuSAE, P < 0.0001 for the samples of SLC7A11control v.s. SLC7A11high treated with 100 μg/mL CuSAE, and P < 0.0001 for the samples of SLC7A11control v.s. SLC7A11high and the samples of SLC7A11control v.s. SLC7A11high treated with 100 μg/mL GOx/CuSAE. c Western blotting of actin of 4T1 cells with different SLC7A11 expression levels treated with GOx/CuSAE (in 20 μg/mL CuSAE) for 24 h. “+“ refers to protein samples of cells treated with GOx/CuSAE. 1, 2, 3 refer to SLC7A11control, SLC7A11low and SLC7A11high cells respectively. d F-actin imaging of 4T1 cells with different SLC7A11 expression levels treated with CuSAE and GOx/CuSAE (in 20 μg/mL CuSAE) for 24 h. e Fluorescent staining of F-actin and membrane of 4T1 cells with different SLC7A11 expression levels treated with CuSAE and GOx/CuSAE (in 20 μg/mL CuSAE) for 24 h. Experiments of (c–e) were repeated independently three times, with similar results. Data are shown as mean ± s.d., and all measurements are independent. Source data are provided as a Source Data file.
To further confirm the correlation between SLC7A11 expression and the disulfidptosis induced by GOx/CuSAE, we compared the degree of actin polymerization upon regulation of SLC7A11 levels. The results showed that the polymerization of actin and Talin 1 were moderate decreased in the SLC7A11low cells, and increased in the SLC7A11high cells (Fig. 6c and Supplementary Fig. 31). This result was also evidenced via fluorescence confocal analysis, which showed the positive correlations of the SLC7A11 expression level with the disulfidptosis-associated morphology changes, including the contraction of the cytoskeleton, cell membrane and the whole cells (Fig. 6d, e and Supplementary Fig. 32). These findings collectively confirm that SLC7A11 plays a significant role in mediating cell disulfidptosis upon the treatment of GOx/CuSAE, in agreement with the mechanism of disulfidptosis.
Different types of cell deaths were also verified using various inhibitors on the cells treated with GOx/CuSAE, including apoptosis inhibitor (Z-VAD-fmk), necrosis inhibitor (necrostatin-1, nec-1), autophagy inhibitor (chloroquine, CQ), and ferroptosis inhibitor (deferoxamine, DFO)34,35,36. The cell viability assays indicated that DFO slightly rescued the GOx/CuSAE-induced cell death, while other inhibitors did not reduce the cytotoxicity of GOx/CuSAE (Supplementary Fig. 33). This suggests that the weak ferroptosis caused by GOx/CuSAE is related to the enhanced ROS production in cells. The minor contribution of ferroptosis was further confirmed by using 4-hydroxy-TEMPO (Tempol) and Trolox; these ROS scavengers can only mildly rescue the GOx/CuSAE-induced the cell death (Supplementary Fig. 34). Consistent with the literature37, the cytotoxicity of disulfidptosis cannot be eliminated by inhibitors of other types of programmed cell death, but can be eliminated by reducing agent dithiothreitol (DTT), β-mercaptoethanol (β-Me), or tris-(2-carboxyethyl) -Phosphine (TCEP) (Supplementary Fig. 35). This result indicates that disulfidptosis the predominant type of cell death induced by GOx/CuSAE.
The presence of ferroptosis, albeit at a relatively low level, was further assessed by measuring the cellular response associated with ferroptosis. The intracellular ROS levels were evaluated using ROS probe 2,7-dichlorofluorescin diacetate (DCFH-DA). CuSAE clearly induced ROS generation in cells, and GOx/CuSAE significantly intensified this effect (Fig. 7a), which is positively correlated with their NADPH blockade and GSH scavenging activity. Overproduction of ROS typically damages mitochondria and lysosomes. Mitochondrial damage was observed in the GOx/CuSAE-treated cells with a decrease of polarized membrane and an increase of depolarized membrane, detected using a mitochondrial membrane potential probe JC-1, which showed green fluorescence in monomer form (J-monomer) in damaged mitochondria and red fluorescence in aggregate (J-aggregates) in normal mitochondria (Fig. 7b). Simultaneously, mitochondria shrinkage, a distinctive feature of ferroptosis38, was clearly observed in the GOx/CuSAE-treated cells (Fig. 7c). Furthermore, confocal microscopy showed the lysosomal damage on the GOx/CuSAE-treated cells by using a lysosomotropic dye acridine orange (AO), detected by the remarkable reduction of intact lysosomes in red fluorescence and increase of ruptured lysosome in green fluorescence (Fig. 7d)39.
a Intracellular ROS levels. 4T1 cells were treated with CuSAE or GOx/CuSAE (in 40 μg/mL CuSAE) for 6 h, and ROS was detected using DCFH-DA staining. b Fluorescence imaging to analyze mitochondrial depolarization of cells treated with CuSAE or GOx/CuSAE (in 20 μg/mL CuSAE) for 24 h. Mitochondria were stained with the JC-1 kit. c TEM imaging of the morphology of the cells treated with GOx/CuSAE (in 20 μg/mL CuSAE) for 24 h. The white arrows point to the mitochondria. d Lysosomal images of the cells treated with CuSAE or GOx/CuSAE (in 40 μg/mL CuSAE) for 6 h. Cells were stained with AO before imaging (red: lysosome, green: cytoplasm, and nucleus). e Western blotting analysis of GPX4 in the cells treated with CuSAE or GOx/CuSAE (in 20 μg/mL CuSAE) for 24 h. The blots of GPX4 and GAPDH were obtained from the same samples, run on separate gels with identical processing and the same molecular weight markers. The whole image is provided in Supplementary Fig. 36. f GSH level of the cells treated with CuSAE or GOx/CuSAE (in 20 μg/mL CuSAE) for 24 h. P < 0.0001 for the samples of GOx/CuSAE v.s. Control. g Lipid peroxide (LPO) measured by BODIPY-C11 stained in the cells treated with CuSAE or GOx/CuSAE (in 20 μg/mL CuSAE) for 12 h. (green: LPO, blue: nucleus). h MDA level of the cells treated with CuSAE or GOx/CuSAE (in 20 μg/mL CuSAE) for 12 h. P = 0.0002 for the samples of GOx/CuSAE v.s. Control. Data are shown as mean ± s.d. from three independent measurements. For both statistical analyses, One-way ANOVA, Dunnett correction was used on the results from three biologically independent experiments (n = 3). Experiments of (a–d) were repeated independently three times, with similar results. Source data are provided as a Source Data file.
Defect of GSH/GPX4 antioxidant pathway and generation of lipid peroxides (LPOs) are crucial features of ferroptosis38, although overexpressing SLC7A11 may inhibit ferroptosis by increasing GSH uptake40,41. Here we found that the treatment of GOx/CuSAE clearly decreased the GPX4 expression and GSH level in tumor cells (Fig. 7e, f and Supplementary Fig. 36). The oxidative stress resulting from the GSH/GPX4 defect is closely correlated with lipid peroxidation in cell membranes. By using a fluorescent LPO probe, BODIPY-C11, significantly increased LPO was detected in the cells treated with GOx/CuSAE (Fig. 7g). Moreover, excessive cellular ROS can also lead to the generation of malondialdehyde (MDA), another important marker of ferroptosis42. The measurement clearly showed the elevated MDA level in the GOx/CuSAE-treated cells (Fig. 7h). These results confirmed that ferroptosis occurred along with disulfidptosis in the GOx/CuSAE-induced cell death, and the dual lethal effect would lead to efficient inhibitory potential in tumor treatment.
To further verify the GOx/CuSAE-triggered cell death pathway, other types of cell death, including autophagy, apoptosis, and necrosis, were analyzed by using corresponding probes. The staining of monodansylcadaverine (MDC), a green fluorescent autophagosome probe, showed no detectable autophagy in cells treated with GOx/CuSAE (Supplementary Fig. 37). Flow cytometry results revealed that the PI-positive cells increased over time upon GOx/CuSAE treatment, indicating the occurrence of cell death. However, the majority of these cells were neither apoptotic (Annexin V-FITC positive) nor necrotic (PI and Annexin V-FITC double positive) (Supplementary Fig. 38 and 39). Together with the increased disulfide bond content, cell shrinkage, and low occurrence of autophagy, apoptosis, and necrosis, this result confirmed that disulfidptosis is the major cause of cell death of GOx/CuSAE.
Moreover, the energy and molecular metabolisms in tumor cells after GOx/CuSAE treatment were analyzed, aiming to uncover the specific events underlying glucose depletion, NADPH depletion, and disulfide accumulation. A hierarchical clustering analysis was performed using targeted quantitative liquid chromatography-tandem mass spectrometry (LC-MS/MS). The result demonstrated that 64 metabolites were substantially changed in the GOx/CuSAE-treated cells (Fig. 8a, P < 0.05, VIP > 1), showing that blocking energy supply significantly hampers multiple downstream synthesis pathways.
a Heat map showing intracellular levels of metabolites in GOx/CuSAEs-treated 4T1 cells (n = 3 biologically independent experiments per group). All these metabolites were clustered into the substrates cluster and the products cluster. Cluster method, K-means. b PCA analysis between the Control group and the GOx/CuSAE group of all the target metabolites determined in the assay. c Pathway enrichment analysis of differential metabolites. The X axis represents the impact factor of the pathway topology analysis, and the Y axis represents the P value of the pathway enrichment analysis (−ln P value). d Predicted metabolite sets enrichment analysis of differential metabolites. The X axis represents the impact factor of the pathway topology analysis, and the Y axis represents the P value of the pathway enrichment analysis (−log10 P value). e The correlation between disulfide, MDA, GSH, and GPX4 levels and various metabolites. The disulfide, MDA, GSH, and GPX4 levels were obtained from the results in Fig. 7 and Supplementary Fig. 27. The Pearson’s r is calculated with Excel 2016 using the Pearson function. Asterisks denote P-values (<0.001 ***; <0.01 **; <0.05 *). The correlation coefficients are given in the Table. For statistical analyses, Two-sided Student’s t-test (c–e) was used on the results from three biologically independent experiments (n = 3). Source data are provided as a Source Data file.
Analysis of the metabolic changes suggests that the inhibition of NADPH was not only through the classical pentose phosphate pathway (PPP), but also associated with compensatory synthesis pathways, including citric acid and malic acid pathways. This is evidenced by significantly decreased levels of citric acid (LogFC = −0.53, P = 0.036, where FC stands for fold change after the treatment) and malic acid (LogFC = −0.66, P = 0.002) in the tricarboxylic acid cycle (TCA). This result reveals that GOx/CuSAE can continuously and efficiently regulate NADPH to low levels by interfering with multiple metabolic pathways.
Next, the results indicated severe impacts of GOx/CuSAE treatment on the syntheses of amino acids, nucleotides, and fatty acids, probably due to reduced energy (NADPH and glucose) in cells that hindered amino acid biosynthesis. Particularly, the reduced levels of methionine (LogFC = −1.06, P = 0.0002) and S-adenosylhomocysteine (LogFC = −0.55, P = 0.033) indicate significant inhibition of the methionine cycle, consistent with disulfide accumulation. Decreases in other amino acids (glutamate, alanine, serine, valine, and phenylalanine) were also observed. Furthermore, GOx/CuSAE treatment also reduced the cellular levels of nucleotide and long-chain fatty acids; among them, significant decreases of AMP, GMP, and 10Z-nonadecenoic acid were detected.
Alterations of oxidative stress-related metabolites were observed on polyunsaturated fatty acids (PUFAs), including arachidonic acid (AA) and eicosapentaenoic acid (EPA), likely due to the POD-mimicking activity of CuSAE, and the result is associated with lipid peroxidation and severe oxidative damage to cells.
Principal component analysis (PCA) revealed that significant metabolic changes (53% variance) occurred in the cells with GOx/CuSAE treatment (Fig. 8b), highlighting its role in regulating cell fate through metabolic mechanisms. These changes occurred among several metabolic pathways, including aminoacyl-tRNA biosynthesis, arginine and proline metabolism, nitrogen metabolism, alanine, aspartate, and glutamate metabolism, and histidine metabolism (Fig. 8c). These alterations indicate that GOx/CuSAE treatment significantly interrupts cellular metabolic networks, disrupting cellular homeostasis of essential biomolecules. Additionally, significant alterations of metabolites were found to be involved in the key enzymatic activities, including pyruvate carboxylase, aldehyde dehydrogenase (phenylacetaldehyde, NADP/NAD), citrate synthase, and fatty-acyl-CoA elongation (n-C18:3CoA) (Fig. 8d). These results confirmed that GOx/CuSAE treatment impairs cellular energy balance and biosynthesis processes crucial for cell survival.
Next, the correlation between these metabolites and disulfidptosis was analyzed via Pearson Correlation Analysis. Significant associations were observed between these metabolic changes and biomarkers of disulfidptosis and oxidative stress, including disulfide and malondialdehyde (MDA), and reductions in glutathione (GSH) and glutathione peroxidase 4 (GPX4) (Fig. 8e). The data showed negative correlations between syntheses of amino acids and nucleotides and disulfide levels, confirming the disulfidptosis-associated changes of metabolites. In addition, PUFAs (such as AA and EPA) are positively correlated with MDA levels and negatively correlated with GSH and GPX4 levels, showing the ferroptosis-associated alteration of metabolites. These results indicate that GOx/CuSAE-induced metabolic disruptions contribute to disulfide accumulation and oxidative stress in cells, which collectively promote disulfidptosis with complementary effects of ferroptosis.
In vivo antitumor efficacy of GOx/CuSAE
The in vivo antitumor efficacy of GOx/CuSAE was evaluated in a 4T1-tumor-bearing murine model. First, the in vivo distribution of nanoparticles was verified via fluorescence imaging using the CuSAE loaded with red-fluorescent dye Cy7 (denoted as Cy7/CuSAE). The nanoparticles showed high accumulation in the tumor 8 h after administration, and the signal continuously increased during 48 h (Supplementary Fig. 40). Then, the tumor-bearing mice were randomly divided into four groups and administered with PBS, GOx, CuSAE, or GOx/CuSAE via intravenous injection (Fig. 9a). The tumor growth profiles indicated that the GOx/CuSAE treatment led to superior antitumor activity by significantly suppressing tumor growth, while GOx and CuSAE showed moderate antitumor effects (Fig. 9b, c). Measurements of tumor size and weight at the end of the experiment confirmed the inhibitory effects of GOx/CuSAE (Fig. 9d and Supplementary Figs. 41 and 42). Moreover, the mice that received treatment of GOx/CuSAE exhibited the highest survival rate in a 50-day monitoring (Supplementary Fig. 43), further supporting the prominent antitumor effect of GOx/CuSAE.
a Schematic illustration of the treatment schedule. BALB/c mice were subcutaneously inoculated with 8 × 105 4T1 cells. Mice were randomly divided into four groups and intravenously injected when the tumor grew to approximately 70 mm3, and injected intravenously with PBS, GOx (0.25 mg/kg), CuSAE (25 mg/kg), and GOx/CuSAE (0.25 mg GOx and 25 mg CuSAE /kg) every two days. The body weight and tumor volume of the mice were recorded every other day. Parts of the graphic elements were generated by Figdraw (www.figdraw.com). b Tumor volume of mice during the treatment. P < 0.0001 for mice treated with GOx/CuSAE v.s. Control (n = 6). c Tumor volume of each mouse during the treatment. d Tumor weight of mice after administration. P < 0.0001 for mice treated with GOx/CuSAE v.s. Control (n = 6). e H&E staining of cells of tumors. f Immunohistochemistry staining to detect Ki67-positive proliferative cells in the tumors after treatment. g Glucose level in tumors of mice after treatment. P < 0.0001 for mice treated with GOx/CuSAE v.s. Control (n = 3). h NADPH level in tumors of mice after administration. NADPH level refers to the [NADPH]/[NADPtotal] ratio relative to the PBS group. P < 0.0001 for mice treated with GOx/CuSAE v.s. Control (n = 3). i Immunohistochemistry staining to detect GPX4-positive cells in the tumors after treatment. Data are shown as mean ± s.d. from independent measurements. For all statistical analyses, One-way ANOVA, Dunnett correction was used on the results from biologically independent experiments. Source data are provided as a Source Data file.
The in vivo cell proliferation in tumors was evaluated with hematoxylin and eosin (H&E) staining, Ki67 measurement, and terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling (TUNEL) staining. H&E staining and Ki67 assay showed that GOx/CuSAE significantly inhibited the growth of tumor cells in vivo, while CuSAE demonstrated a moderate inhibitory effect (Fig. 9e, f). On the other hand, TUNEL staining indicated that GOx/CuSAE caused only a few apoptotic cells (Supplementary Fig. 44), consistent with the in vitro cell death analysis that apoptosis is not the major way in the GOx/CuSAE-induced cell death (Fig. S18)43.
To further confirm the occurrence of disulfidptosis in tumor cells in vivo, the cellular alterations in response to GOx/CuSAE treatment were analyzed. The levels of glucose and NADPH in tumors clearly decreased after receiving GOx/CuSAE treatment (Fig. 9g, h). Western blotting analysis indicated a substantial formation of actin oligomers in tumors from the mice treated with GOx/CuSAE (Supplementary Fig. 45), confirming the induction of disulfidptosis. By comparison, moderate effects were observed in the mice treated with GOx or CuSAE alone, underscoring the synergism of these two components in inducing disulfidptosis. These results indicate that the artificial enzyme hybrid GOx/CuSAE effectively induces tumor disulfidptosis. In addition, immunohistochemistry analysis demonstrated a significant reduction in the GPX4 level in tumors treated with GOx/CuSAE (Fig. 9i), confirming the presence of ferroptosis in tumor cells, which would synergize with the disulfidptosis-induced cell death in vivo.
The systemic toxicity of GOx/CuSAE was evaluated by measuring the body weight of mice during the administration. The mouse growth profiles showed that the treatment of GOx/CuSAE did not affect the body weight of mice (Supplementary Fig. 46). Histopathology analysis indicated that the treatment of GOx/CuSAE did not cause abnormalities of the main organs (Supplementary Fig. 47). These results confirm the low systemic toxicity of the GOx/CuSAE treatment. In-depth examination of blood biochemistry parameters, including blood urea nitrogen (BUN), alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (AKP), and creatinine (CRE) at different time points post-administration indicated that all parameters remained within reference ranges (Supplementary Fig. 48). This outcome suggests that the GOx/CuSAE did not cause significant nephrotoxicity and hepatotoxicity. The blood routine data did not show significant differences in the mice after administration (Supplementary Fig. 49). Moreover, an in vitro hemolysis assay showed that GOx/CuSAE had minimal influence on the erythrocyte membrane, as indicated by negligible absorbance of released hemoglobin in the supernatant (Supplementary Fig. 50). Collectively, these results underscore the excellent safety profile of GOx/CuSAE.
Discussion
Disulfidptosis is a recently identified form of programmed cell death that is initiated by depletion of endogenous NADPH under glucose starvation4. The therapeutic potential of disulfidptosis remains unexplored. This work demonstrated that disulfidptosis can be introduced for tumor therapy by using an enzyme hybrid GOx/CuSAE. The NADPH oxidase-mimicking activity of CuSAE and the glucose depletion activity of GOx demonstrate significant synergistic effects in inducing disulfidptosis. While CuSAE depletes existing NADPH, the incorporation of GOx consumes cellular glucose and inhibits the generation of new NADPH by blocking cellular energy metabolism through the pentose phosphate pathway. These complementary effects of CuSAE and GOx strengthen disulfidptosis induced by the material, showing the significance of the rational design of this artificial enzyme hybrid.
Single-atom nanozymes (SAEs) have demonstrated significant advantages in catalytic tumor therapy. CuSAE has been synthesized on different substrates, such as protein caged nanocrystals44, self-assembled nanostructures45, metal-organic framework (MOF), and zeolitic imidazolate framework (ZIF)46,47. These CuSAEs typically possess POD-mimicking activity, hence were used for promoting cellular oxidative stress via the generation of ·OH radicals in tumor cells. In this work, a copper single-atom nanozyme (CuSAE) exhibiting NADPH oxidase-mimicking activity has been synthesized. This activity fits perfectly with the mechanism for inducing tumor disulfidptosis by depleting existing NADPH in cells. Additionally, CuSAE also exhibits peroxidase (POD)- and glutathione oxidase (GSHOx)-mimicking activities, which elevate oxidative stress in tumor cells, thereby blocking the compensatory repair mechanisms and continuously activating tumor disulfidptosis. Mechanistic investigations confirmed the significant contribution of disulfidptosis in the GOx/CuSAE-induced cell death.
SLC7A11 is a cystine/glutamate antiporter that facilitates the cellular uptake of cystine and the release of glutamate, thereby promoting the synthesis of intracellular glutathione (GSH) and maintaining cellular redox homeostasis. SLC7A11 overexpression occurs in many types of cancer cells and is associated with cancer progression9. Mechanistic investigation reveals that SLC7A11 overexpression plays a crucial role in suppressing ferroptosis by supplying sufficient cysteine for glutathione biosynthesis9; while inhibition of SLC7A11 function could promote the efficacy of the ferroptosis-related tumor therapy48. Hence, ferroptosis, as an oxidative stress cancer therapy, is effective against SLC7A11low cancer cells, but less effective against SLC7A11high cancer cells. This feature brings a challenge to many nanozyme therapeutic systems that promote tumor cell death by inducing oxidative stress.
Although SLC7A11low cancer cells are not sensitive to the ferroptosis-based therapy, the recently discovered disulfidptosis offers an alternative strategy for the treatment of this type of cells. The SLC7A11-mediated cystine uptake imposes a high energy demand on cancer cells, since these cells consume large amounts of NADPH for reducing cystine to cysteine, a precursor for the synthesis of GSH. This effect, while decreasing oxidative stress, makes these SLC7A11high tumor cells highly dependent on the supply of glucose, the source of NADPH generation through the glucose-pentose phosphate (PPP) pathway. Under glucose starvation, NADPH in these cells cannot be efficiently synthesized, resulting in aberrant accumulation of disulfide bonds in actin cytoskeletal proteins, which induces collapse of the actin network, hence triggers disulfidptosis due to disulfide stress29. Therefore, introducing disulfidptosis could be achieved by modulating cellular glucose and NADPH levels, which is an advantage for the treatment of SLC7A11high tumor cells, though these cells are not sensitive to the ferroptosis-based therapy.
In this work, we designed a nanozyme hybrid GOx/CuSAE to induce disulfidptosis according to its mechanism via modulating cellular glucose and NADPH levels in tumor cells. GOx depletes intracellular glucose, thereby blocking NADPH synthesis via the PPP pathway. CuSAE demonstrates NADPH oxidase activity, depleting existing NADPH in cells. These two approaches synergize disulfidptosis through complementary effects. In the meantime, CuSAE also catalyzed the generation of ROS and depletion of GSH, which causes downregulation of GPX4 and results in ferroptosis in tumor cells. Measuring viability of cells with different SLC7A11 levels reveals that disulfidptosis plays a predominant role in the GOx/CuSAE-induced cell death, with concomitant moderate contribution of ferroptosis. This result has been confirmed by the different antagonistic effects on disulfidptosis and ferroptosis.
In summary, we have successfully developed an effective strategy for promoting tumor disulfidptosis therapy by using an enzyme hybrid (GOx/CuSAE) that integrates a copper single-atom nanozyme (CuSAE) with the natural glucose oxidase (GOx). GOx induces glucose starvation and elevates H2O2 levels in tumor cells by catalyzing the oxidation of glucose to generate gluconic acid and H2O2, which effectively cuts off the source of nicotinamide adenine dinucleotide phosphate (NADPH) by blocking the pentose phosphate pathway. Concurrently, CuSAE exhibits NADPH oxidase-mimicking activity that can deplete the existing cellular NADPH. This synergistic action endows the hybrid nanoplatform with a dual NADPH-inhibition function, which strengthens the blockade of disulfide reduction to trigger disulfidptosis of tumor cells. Furthermore, CuSAE possesses peroxidase (POD)- and glutathione oxidase (GSHOx)-mimicking activities, which catalyze cascade reactions by using H2O2, the product of GOx catalysis, as a substrate, to generate ·OH radical and deplete cellular GSH. This process elevates oxidative stress in cells and intensifies cell damage through the induction of moderate ferroptosis. In vivo results demonstrate that this combinational disulfidptosis/ferroptosis therapy remarkably inhibits tumor growth while causing minimal systemic toxicity. This work underscores that the potential of hybrid nanozymes as a viable approach for effective disulfidptosis therapy through multi-enzymatic intervention by inducing glucose starvation, depleting existing NADPH, and elevating cellular oxidative stress functions in tumor cells.
Methods
All animal procedures were performed in accordance with the guidelines for the Care and Use of Laboratory Animals of the University of Science and Technology of China and approved by the Animal Ethics Committee of the University of Science and Technology of China.
Materials characterizations
The morphology and element mapping of particles were observed by transmission electron microscope (TEM) (H-7650, Hitachi, Japan). The crystal structure measurement was performed on X-ray powder diffraction (XRD) using Cu-Kα radiation (λ = 0.1542475 nm) at 18 kW (MiniFlex 600, Rigaku, Japan). The measurements of composition and structure of samples were conducted on X-ray photoelectron spectroscopy (XPS) (ESCALAB 250Xi, Thermo Scientific, USA). The size distribution and zeta potential of particles were measured via a dynamic light scattering (DLS) detector (NanoBrook 90Plus PALS, Brookhaven, USA). Metal content was quantified on an inductively coupled plasma optical emission spectrometer (ICP-OES) (iCAP 7400, Thermo Scientific, USA).
Preparation of SiO2
SiO2 nanospheric cores were synthesized using a tetraethyl orthosilicate (TEOS) sol-gel method. Briefly, ethanol (147 mL), deionized water (20 mL), and TEOS (6 mL) were mixed under magnetic stirring at room temperature for 10 min. NH3·H2O (5 mL) was added dropwise to the above solution and was stirred for another 2 h. The product was isolated by centrifugation (8,000 g, 10 min) and washed with deionized water three times and with ethanol three times, respectively. Finally, the SiO2 nanospheres were dried for further use.
Preparation of SiO2@PDA@Cu
1.21 g Tris, 4 mg copper acetylacetone, and 150 mg SiO2 nanospheres were dissolved and dispersed in deionized water (80 mL) under sonication. 150 mg dopamine was added to the suspension, and the mixture was stirred for 12 h under an alkaline condition (pH 8.5) to generate the polydopamine (PDA)-coated SiO2 nanoparticles. The product was isolated by centrifugation (8000 g, 7 min) and washed with deionized water three times and with ethanol three times, respectively. The particles were dried for further use.
Preparation of CuSAE
The obtained SiO2@PDA@Cu was subjected to heating at 900 °C in a nitrogen atmosphere for 3 h to pyrolyze polydopamine into a carbon-nitrogen structure, resulting in SiO2@CN@Cu. Then the SiO2 cores were etched with hydrofluoric acid to generate hollow carbon/nitrogen spheres doped with Cu atoms, the single Cu atom nanozyme (CuSAE). The product was obtained by centrifugation (10,000 × g, 15 min) and washed with water and ethanol.
Preparation of GOx/CuSAE
CuSAE was PEGylated with DSPE-PEG3.4K-NH2 through a thin film hydration method. Specifically, CuSAE was mixed with an equal weight of DSPE-PEG3.4K-NH2 and dispersed in dichloromethane. After 15 min of ultrasonication, the solvent was removed by vacuum distillation, and then dispersed in aqueous buffer (pH 6.5). Activated GOx was obtained by incubation of 5 mg GOx, 2 mg EDC, and 2 mg NHS under stirring for 2 h. Then, this activated GOx was mixed with the PEGylated CuSAE and stirred for 12 h to obtain GOx/CuSAE.
Detection •OH radicals
TMB colorimetric assay was conducted to evaluate the generation of •OH. CuSAE in different concentrations was added to a buffer solution containing 0.1 mM H2O2 and 0.1 mM TMB, and the absorbance at 652 nm was measured. Ti(SO4)2 assay was applied to measure H2O2 depletion. 200 μg/mL CuSAE were incubated with 2 mM H2O2 in HAC-NaAC buffer (pH 6.0). After incubation for different times, the sample was centrifuged (10,000 × g, 15 min) and 50 μL supernatant was taken to mix with 100 μL Ti(SO4)2. The absorbance at 425 nm was measured. All UV-vis spectra were recorded on an Agilent 8453 instrument. For EPR determination, CuSAE was added to a HAC-NaAC buffer (pH 6.0) containing 1.0 mM H2O2 and 100 μM DMPO. After 60 ss of intense ultrasonication, the mixture was transferred into a quartz tube, and the EPR spectra were recorded on a JEOL JES-FA200 spectrometer.
GSH depletion
GSH levels were quantified using the DTNB assay. GSH (0.15 mM) was incubated with different concentrations of CuSAE in HAC-NaAC buffer (pH 6.0). After incubation for different times, the sample was centrifuged (10,000 × g, 15 min), and 200 μL supernatant was taken and mixed with 300 μL 2.5 mM DTNB. The absorbance at 412 nm was measured (8453, Agilent).
NADPH depletion
NADPH (200 μg/mL) was incubated with 80 μg/mL CuSAE in HAC-NaAC buffer (pH 6.0). After incubation for different times, the sample was centrifuged (10,000 × g, 15 min) and 200 μL supernatant was taken to measure the absorbance at 340 nm (8453, Agilent).
Detection of NADPH and NADP+ by liquid chromatography
NADPH (200 μg/mL) was incubated with 80 μg/mL CuSAE in HAC-NaAC buffer (pH 6.0). The supernatants were collected through centrifugation (10,000 × g, 15 min) for HPLC measurements (Agilent Technologies, 1200 Series, USA) equipped with a C18 column. 45 μL samples were injected and 0.6 mL/min flow rate was used. The wavelength of the detector was set at 340 nm.
Density functional theory (DFT) calculation
The density functional theory (DFT) calculation was performed using the software VASP 5.4.1. First-principles were employed for all Spin-polarization DFT calculations49,50; and Perdew-Burke-Ernzerhof (PBE) exchange-correlation function under the generalized gradient approximation (GGA) was used51. Projected augmented wave (PAW) potentials were used to describe the ionic cores52,53, and valence electrons were taken into account using a plane wave basis set with a kinetic energy cutoff of 500 eV. The GGA + U method was adopted in the calculations. The effective Hubbard U value of Cu was set at 5.018 eV. Partial occupancies of the Kohn−Sham orbitals were allowed using the Gaussian smearing method with a width of 0.05 eV. The electronic energy was considered self-consistent when the energy change was smaller than 10−5 eV. A geometry optimization was considered convergent when the energy change was smaller than 0.05 eV Å−1. The Brillouin zone integration was performed using 2 × 2 × 1 Monkhorst-Pack k-point sampling for the structure. Finally, the adsorption energies (Eads) were calculated with the equation of Eads = Ead/sub − Ead − Esub, where Ead/sub, Ead, and Esub are the total energies of the optimized adsorbate/substrate system, the adsorbate in the structure, and the clean substrate, respectively. The free energy was calculated using the equation: G = Eads + ZPE – TS, where G, Eads, ZPE, and TS are the free energy, total energy from DFT calculations, zero point energy, and entropic contributions, respectively, where T was set to 300 K.
Cell culture
Mouse 4T1 cells (Cat#TCM32) were obtained from the Cell Bank, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences. The cells were grown in Roswell Park Memorial Institute (RPMI) 1640 Medium (Gibco) containing 10% (v/v) fetal bovine serum (FBS, lonsera) under the standard culture conditions (37 °C, 5% CO2).
Cell uptake
4T1 cells were incubated in confocal culture plates (5 × 104 cells/plate) for 24 h, then RF@CuSAE was added to incubate with cells for different times. After washing with PBS three times, cells were stained with Hoechst 33342 for 20 min. The cells were analyzed with a confocal microscope (ZEISS880 ZEISS or Leica STED).
Lysosome escape
4T1 cells were incubated in confocal culture plates (5 × 104 cells/plate) for 24 h, then RF@CuSAE was added to incubate with cells for different times. After washing with PBS three times, cells were stained with Hoechst 33342 and Lysotracker Green for 20 min. The cells were analyzed with a confocal microscope (Leica STED, Leica).
In vitro cellular cytotoxicity assays
4T1 cells were seeded with 5000 cells per well in a 96-well plate. After 24 h incubation, the culture medium was replaced with new medium containing different formulations. The cells were incubated for 24 h before adding MTT. The cells were incubated for another 4 h, then 150 μL DMSO was added to dissolve formazan crystals. The absorbance was measured at 490 nm on a microplate reader (680 microplate reader, Bio-Rad).
For live/dead cell staining assay, 4T1 cells were incubated in a six-well culture plate (3 × 105 cells/well) for 24 h. After 24 h incubation with different formulations, the cells were washed three times with PBS, stained with FDA (10 μM) and PI (20 μM), and then analyzed using fluorescence microscopy (IX71, Olympus).
Intracellular glucose measurements
4T1 cells were incubated in a six-well culture plate (3 × 105 cells/well) for 24 h, followed by incubation with different formulations for 12 h. The cells were washed three times with PBS, and intracellular glucose levels were analyzed using glucose assay kits.
Intracellular NADPH measurements
4T1 cells were incubated in a six-well culture plate (3 × 105 cells/well) for 24 h, followed by incubation with different formulations for 24 h. The cells were washed three times with PBS, and intracellular NADPH levels were analyzed using NADP+/NADPH assay kits.
Fluorescent staining of actin filaments and the cellular membrane
4T1 cells were incubated in confocal culture plates (5 × 104 cells/plate) for 24 h, and then GOx/CuSAE was added to incubate with cells for 24 h. After washing with PBS three times and staining with Hoechst in culture medium for 20 min, cells were fixed for 30 min at room temperature with 4% paraformaldehyde in PBS, followed by washing three times with permeabilization buffer (0.5% Triton X-100 in PBS). The fixed cells were then cultured with permeabilization buffer (3% BSA, 0.5% Triton X-100 in PBS) containing Actin-Tracker Red (Alexa Fluor 594-labeled phalloidin). The samples were analyzed with a confocal microscope (ZEISS880 ZEISS or Leica STED).
Western blotting
4T1 cells were incubated in a six-well culture plate (4 × 105 cells/well) for 24 h, followed by incubation with different formulations for 24 h. The cells were washed three times with PBS and lysed to obtain proteins. Then, protein SDS-PAGE electrophoresis was carried out. DTT or β-Me was applied in the Western blot samples where a reducing agent is required (DTT for GPX4 and GAPDH, and β-Me for actin, FLNA, and Talin 1). Proteins were transferred to a PVDF membrane, then incubated with primary and secondary antibodies. Image Quant LAS 4000 (GE) was used for analyses. Image J was used for the quantification of the protein bands. Each protein band was separately scanned, and their grayscales were measured for quantification analyses. The polymerization levels of actin, FLNA, and Talin 1 were determined by the grayscale ratios of the non-reduced oligomer bands to the reduced monomer bands in the same group, with the ratio in the Control group normalized to 1. The expression levels of GPX4 were determined by Western blotting, measuring the grayscale ratios of the GPX4 band to the GAPDH band in each group, with the ratio in the Control group normalized to 1. All Western blotting experiments were performed in triplicate using biologically independent samples, and consistent results were obtained in the replication experiments. The used antibodies include anti-GPX4 (Abcam, Cat#ab125066, monoclonal antibody, EPNCIR144, Lot#GR3438547-8, 1/1,000 dilution), anti-GAPDH (Abcam, Cat#ab128915, monoclonal antibody, EPR6256, Lot#GR303514-13, 1/10,000 dilution), anti-Talin 1 (Abcam, Cat#ab108480, monoclonal antibody, 97H6, Lot#GR1018004-2, 1/2400 dilution), anti-Filamin A (Abcam, Cat#ab76289, monoclonal antibody, EP2405Y, Lot#GR1028508-2, 1/300,000 dilution), and anti-β-actin (proteintech, Cat#66009-1-Ig, monoclonal antibody, 2D4H5, Lot#10027682, 1/50,000 dilution). Unprocessed scans of the blots were provided in the Source Data file and the Supplementary Information.
Transmission electron microscopy
4T1 cells were cultured in 100 mm culture plates (2 × 106 cells/plate) for 24 h, then incubated with 20 μg/mL GOx/CuSAE for 24 h. The cells were fixed with 2.5% glutaraldehyde fixative solution, stained with lead citrate and uranyl acetate, and collected on copper grids. The images were recorded on a TEM spectrometer (H-7650, Hitachi).
Cell transfection
4T1 cells were cultured in 60 mm culture plates (2 × 106 cells/plate) for 24 h, then treated with siRNA or SLC7A11 containing plasmid in transfection reagent Lipofectamine 3000 for 48 h. The sequence of the siRNA is 5’- CCAGAUAUGCAUCGUCCUUTT-3’. The efficiency of cell transfection was determined by qPCR. The qPCR was performed with a transcriptor first strand cDNA synthesis kit and a LightMix® qPCR kit (Roche). The primer for gene amplification and qPCR is 5’- CCCAAGCTTATGGTCAGAAAGCCTGTTGTGTCCAC-3’.
Lysosome integrity detection
4T1 cells were incubated in a six-well culture plate (3 × 105 cells/well) for 24 h, followed by incubation with different formulations for 24 h. After three times washing with PBS, the cells were treated with AO (10 μM) to analyze lysosome integrity via fluorescence microscopy (IX71, Olympus).
JC-1 assay
4T1 cells were incubated in confocal culture plates (5 × 104 cells/plate) for 24 h, followed by incubation with different formulations for 24 h. After three times washing with PBS, the cells were treated with a JC-1 assay kit to analyze the mitochondrial membrane potential via confocal microscopy (Leica STED, Leica).
Intracellular ROS detection
4T1 cells were incubated in six-well culture plates (3 × 105 cells/well) for 24 h, followed by different formulations for 6 h. After three times washing with PBS, the cellular ROS levels were analyzed using an ROS assay kit via fluorescence microscopy (IX71, Olympus).
Lipid peroxide measurement
4T1 cells were incubated in confocal culture plates (5 × 104 cells/plate) for 24 h, then incubated with different formulations for 24 h. After three times washing with PBS, the lipid peroxidation was analyzed with BODIPY 581/591 C11 via confocal microscopy (Zeiss ZEISS880).
Intracellular GSH and disulfide levels
4T1 cells were incubated in a six-well culture plate (3 × 105 cells/well) for 24 h, followed by incubation with different formulations for 24 h. After three times washing with PBS, intracellular GSH levels and disulfide level were analyzed using GSH and GSSG assay kits, and disulfide level were analyzed using GSH and GSSG assay kits without protein clearance process.
Intracellular MDA level
4T1 cells were incubated in a six-well culture plate (3 × 105 cells/well) for 24 h, followed by incubation with different formulations for 24 h. After three times washing with PBS, and intracellular MDA levels were analyzed using MDA assay kits.
Autophagosome detection
4T1 cells were incubated in confocal culture plates (5 × 104 cells/plate) for 24 h, followed by incubation with different formulations for 12 h. After three times washing with PBS, an autophagy staining assay kits with MDC was used to detect autophagosome by confocal microscopy (ZEISS880, Zeiss).
Apoptosis and necrosis assay
4T1 cells were incubated in a six-well culture plate (3 × 105 cells/well) for 24 h, followed by incubation with different formulations. After three times washing with PBS, the cells were stained using Annexin V-FITC apoptosis detection kits.
Metabolomic analysis
4T1 cells were seeded in cell culture flasks (2 × 106 cells/flask) and incubated for 24 h. GOx/CuSAE (30 μg/mL) was added, and cells were incubated for another 24 h. After three times washing with PBS, the cells were collected for metabolomics analysis. LC-MS/MS analyses were conducted to evaluate the energy metabolism upon the CuSAE treatment.
Real-time biodistribution imaging
4T1 cells (1 × 106 cells per mouse) were injected subcutaneously into the right flank of each female BALB/c nude mouse. Mice injected with Cy7/CuSAE and the biodistribution was monitored by a small animal living imaging system (IVScope 8000, CLINX).
In vivo antitumor efficiency
All mice were fed ad lib and kept in a pathogen-free environment and at 21 ± 1 °C, in 40% to 70% humidity, and with a 12 h light/dark cycle (from 8 a.m. to 8 p.m.). All the tumor sizes of the experimental mice are within the maximal size (2000 mm3) permitted by the Animal Ethics Committee.
4T1 cells (1 × 106 per mouse) were subcutaneously injected into the right abdomen of each of the 44 female BALB/c mice (6 weeks old). When the tumors reached 70 mm3 on the 6th day, the mice were randomly divided into four groups and administered with PBS, GOx, CuSAE, or GOx/CuSAE through the tail vein injection. The drugs were administered once every 2 days, and tumor growth was monitored every 2 days. After 18 days, mice in each group were randomly taken and euthanized, and the tumors and main organs were harvested for immunohistochemical analyses. The survival analysis was monitored for a total of 50 days in the mice that received different treatments. Antibodies of anti-Ki67 (CST, CAT#12202, 1/200 dilution, monoclonal antibody, D3B5, Lot#5) and FITC-labeled Goat Anti-Rabbit IgG (H+L) (Beyotime, Cat#A0562, 1/200 dilution, secondary antibody) were used for Ki67 analysis and TUNEL analysis.
Hemolysis analysis of GOx/CuSAE
The mouse blood was collected from the supraorbital veins, and resuspended in saline after being centrifuged at 1000 × g for 10 min to separate the serum. The resuspended blood cells were washed twice with saline. The blood cells were incubated with different concentrations of GOx/CuSAE for 30 min. Then, the mixtures were centrifuged (3000 × g, 5 min) and the absorbance of the supernatant was measured at 405 nm.
Blood routine analysis
12 female BALB/c mice were randomly divided in four groups and treated with different formulations. The blood was collected on days 0, 1, 7, and 14 after the treatment. The blood routine test was performed on a hematology analyzer (XT-1800i) using whole blood.
Biochemistry parameter analysis
16 female BALB/c mice (6 weeks old) were randomly divided in four groups and treated with different formulations. The blood was collected on days 0, 1, 7, and 14 after the treatment, and the serum was isolated by centrifugation (1000 × g, 5 min) and stored at 4 °C. The serum biochemistry parameters were analyzed using the detection kits for CRE, AKP, ALT, BUN, and AST, respectively.
Statistics and reproducibility
Multiple images (at least 3 images) of micrographs are recorded and show similar results. All data are expressed as the mean ± standard error. p < 0.05 was considered to be statistically significant. The analyses were performed using the software Agilent Masshunter, VASP 5.4.1, VESTA, ImageJ, Origin 2019, and Excel 2016.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its supplementary information files. All the microscopy data generated in this study have been deposited in the Figshare database under accession code https://doi.org/10.6084/m9.figshare.28749758. The full image dataset is available from the corresponding author upon request. Source data are provided with this paper.
References
Kalyanaraman, H., Zhuang, S., Pilz, R. B. & Casteel, D. E. The activity of cGMP-dependent protein kinase Iα is not directly regulated by oxidation-induced disulfide formation at cysteine 43. J. Biol. Chem. 292, 8262–8268 (2017).
Carelli, S. et al. Cysteine and glutathione secretion in response to protein disulfide bond formation in the ER. Science 277, 1681–1684 (1997).
Liu, X. et al. Cystine transporter regulation of pentose phosphate pathway dependency and disulfide stress exposes a targetable metabolic vulnerability in cancer. Nat. Cell Biol. 22, 476–486 (2020).
Liu, X. et al. Actin cytoskeleton vulnerability to disulfide stress mediates disulfidptosis. Nat. Cell Biol. 25, 404–414 (2023).
Zheng, T., Liu, Q., Xing, F., Zeng, C. & Wang, W. Disulfidptosis: a new form of programmed cell death. J. Exp. Clin. Cancer Res. 42, 137 (2023).
Zheng, P., Zhou, C., Ding, Y. & Duan, S. Disulfidptosis: a new target for metabolic cancer therapy. J. Exp. Clin. Cancer Res. 42, 103 (2023).
Guo, W. et al. Dysregulated glutamate transporter SLC1A1 propels cystine uptake via Xc− for glutathione synthesis in lung cancer. Cancer Res 81, 552–566 (2021).
Jiang, Y. et al. Cysteine transporter SLC3A1 promotes breast cancer tumorigenesis. Theranostics 7, 1036–1046 (2017).
Koppula, P., Zhuang, L. & Gan, B. Cystine transporter SLC7A11/xCT in cancer: ferroptosis, nutrient dependency, and cancer therapy. Protein Cell 12, 599–620 (2021).
Cheng, J. et al. Boosting ferroptosis therapy with iridium single-atom nanocatalyst in ultralow metal content. Adv. Mater. 35, 2210037 (2023).
Huang, L., Chen, J., Gan, L., Wang, J. & Dong, S. Single-atom nanozymes. Sci. Adv. 5, eaav5490 (2019).
Wang, Z. & Wu, F.-G. Emerging single-atom catalysts/nanozymes for catalytic biomedical applications. Adv. Healthc. Mater. 11, 2101682 (2022).
Song, J. et al. CuO2-assisting-Zn single atom hybrid nanozymes for biofilm-infected wound healing. Chem. Eng. J. 474, 145706 (2023).
Zhang, S. et al. Single-atom nanozymes catalytically surpassing naturally occurring enzymes as sustained stitching for brain trauma. Nat. Commun. 13, 4744 (2022).
Jin, D. et al. Protein encapsulation of nanocatalysts: a feasible approach to facilitate catalytic theranostics. Adv. Drug Deliv. Rev. 192, 114648 (2023).
Wang, M. et al. Recent advances in glucose-oxidase-based nanocomposites for tumor therapy. Small 15, 1903895 (2019).
Uysal, B. Ö & Tepehan, F. Z. Controlling the growth of particle size and size distribution of silica nanoparticles by the thin film structure. J. Sol. Gel Sci. Technol. 63, 177–186 (2012).
Zhang, Z. et al. Atomically dispersed cobalt trifunctional electrocatalysts with tailored coordination environment for flexible rechargeable Zn–air rattery and self-Driven water splitting. Adv. Energy Mater. 10, 2002896 (2020).
Liu, T. et al. Ultrasmall copper-based nanoparticles for reactive oxygen species scavenging and alleviation of inflammation related diseases. Nat. Commun. 11, 2788 (2020).
Li, F. et al. Boosting oxygen reduction catalysis with abundant copper single atom active sites. Energy Environ. Sci. 11, 2263–2269 (2018).
Lu, X. et al. Bioinspired copper single-atom catalysts for tumor parallel catalytic therapy. Adv. Mater. 32, 2002246 (2020).
Augsburger, F. et al. Pharmacological characterization of the seven human NOX isoforms and their inhibitors. Redox Biol. 26, 101272 (2019).
Bannister, J. V. & Bannister, W. H. Production of oxygen-centered radicals by neutrophils and macrophages as studied by electron spin resonance (ESR). Environ. Health Perspect. 64, 37–43 (1985).
Li, F. et al. Boosting the hydrogen peroxide production over In2S3 crystals under visible light illumination by gallium ions doping and sulfur vacancies modulation. Chin. J. Catal. 55, 253–264 (2023).
Kohno, M., Mizuta, Y., Kusai, M., Masumizu, T. & Makino, K. Measurements of superoxide anion radical and superoxide anion scavenging activity by electron spin resonance spectroscopy coupled with DMPO spin trapping. Bull. Chem. Soc. Jpn. 67, 1085–1090 (2006).
Wu, S., Wang, P., Qin, J., Pei, Y. & Wang, Y. GSH-depleted nanozymes with dual-radicals enzyme activities for tumor synergic therapy. Adv. Funct. Mater. 31, 2102160 (2021).
Liu, M. et al. Hemin-caged ferritin acting as a peroxidase-like nanozyme for the selective detection of tumor cells. Inorg. Chem. 60, 14515–14519 (2021).
Wu, D. et al. Engineering Fe-N doped graphene to mimic biological functions of NADPH oxidase in cells. J. Am. Chem. Soc. 142, 19602–19610 (2020).
Liu, X., Zhuang, L. & Gan, B. Disulfidptosis: disulfide stress-induced cell death. Trends Cell Biol. 34, 327–337 (2023).
Lanzardo, S. et al. Immunotargeting of antigen xCT attenuates stem-like cell behavior and metastatic progression in breast cancer. Cancer Res. 76, 62–72 (2016).
Banerjee, S., Gardel, M. L. & Schwarz, U. S. The actin cytoskeleton as an active adaptive material. Annu. Rev. Condens. Matter Phys. 11, 421–439 (2020).
Franz, F. et al. Allosteric activation of vinculin by talin. Nat. Commun. 14, 4311 (2023).
Valentin, V. et al. Filamin A mutations: a new cause of unexplained emphysema in adults?. Chest 159, e131–e135 (2021).
Moretti, L., Kim, K. W., Jung, D. K., Willey, C. D. & Lu, B. Radiosensitization of solid tumors by Z-VAD, a pan-caspase inhibitor. Mol. Cancer Ther. 8, 1270–1279 (2009).
Mauthe, M. et al. Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion. Autophagy 14, 1435–1455 (2018).
Lv, Q. et al. Decitabine induces ferroptosis in myelodysplastic syndrome. Blood 134, 2995–2995 (2019).
Zhao, D. et al. Molecular landmarks of tumor disulfidptosis across cancer types to promote disulfidptosis-target therapy. Redox Biol. 68, 102966 (2023).
Jiang, X., Stockwell, B. R. & Conrad, M. Ferroptosis: mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 22, 266–282 (2021).
Zhu, Y. et al. Carrier-free self-assembly nano-sonosensitizers for sonodynamic-amplified cuproptosis-ferroptosis in glioblastoma therapy. Adv. Sci. 11, 2402516 (2024).
Meng, Y., Chen, X. & Deng, G. Disulfidptosis: a new form of regulated cell death for cancer treatment. Mol. Biomed. 4, 18 (2023).
Lei, H., Pei, Z., Jiang, C. & Cheng, L. Recent progress of metal-based nanomaterials with anti-tumor biological effects for enhanced cancer therapy. Exploration 3, 20220001 (2023).
Zhang, X. et al. Endogenous glutamate determines ferroptosis sensitivity via ADCY10-dependent YAP suppression in lung adenocarcinoma. Theranostics 11, 5650–5674 (2021).
Jin, D. et al. A leaking-proof theranostic nanoplatform for tumor-targeted and dual-modality imaging-guided photodynamic therapy. BME Front. 4, 0015 (2023).
Li, T. et al. A pH-activatable copper-biomineralized proenzyme for synergistic chemodynamic/chemo-immunotherapy against aggressive cancers. Adv. Mater. 35, 2210201 (2023).
Zhou, J. et al. Coordination-driven self-assembly strategy-activated Cu single-atom nanozymes for catalytic tumor-specific therapy. J. Am. Chem. Soc. 145, 4279–4293 (2023).
Chang, M. et al. Cu single atom nanozyme based high-efficiency mild photothermal therapy through cellular metabolic regulation. Angew. Chem. Int. Ed. 61, e202209245 (2022).
Yu, B. et al. Using Cu-based metal-organic framework as a comprehensive and powerful antioxidant nanozyme for efficient osteoarthritis treatment. Adv. Sci. 11, 2307798 (2024).
Jiang, L. et al. Ferroptosis as a p53-mediated activity during tumour suppression. Nature 520, 57–62 (2015).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B Condens. Matter 50, 17953–17979 (1994).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (22177109, 22377116, 52021002) (Y.L.), the National Key R&D Program of China (2020YFA0710700) (Y.L.), the Plans for Major Provincial Science & Technology Projects (202303a07020004) (Y.L.), USTC Research Funds of the Double First-Class Initiative (YD2060002502, YD9100002033) (Y.L.), the Research Personnel Cultivation Programme of Zhongda Hospital Southeast University (CZXM-GSP-RC160) (J.C.), and Zhongda Hospital Affiliated to Southeast University, Jiangsu Province High-Level Hospital Construction Funds (GSP-ZXY03) (J.C.). This work was partially carried out at the Instruments Center for Physical Science, University of Science and Technology of China. All animal procedures were performed in accordance with the guidelines for the Care and Use of Laboratory Animals of the University of Science and Technology of China, and approved by the Animal Ethics Committee of the University of Science and Technology of China.
Author information
Authors and Affiliations
Contributions
The manuscript was written through the contributions of all authors. All authors have given approval to the final version of the manuscript. W.Y. and D.J. contributed equally. Y.L. and J.C. conceived the idea and supervised the project. W.Y. and J.Y. synthesized and characterized materials. W.Y. performed the DFT studies. W.Y. and D.J. carried out the most cellular assays and mechanistic analyses. Y.Z. contributed to the metabolomics analysis. S.W. constructed the SLC7A11-encoding plasmid. M.L. contributed to the cell assays and the antitumor assays. Y.D. performed the HPLC assay. Y.Y. contributed to the antitumor assays. W.Y. wrote the first draft of the manuscript. Y.L. wrote the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Wei Jiang, Xiaoguang Liu, and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yu, W., Jin, D., Zhang, Y. et al. Provoking tumor disulfidptosis by single-atom nanozyme via regulating cellular energy supply and reducing power. Nat Commun 16, 4877 (2025). https://doi.org/10.1038/s41467-025-60015-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-60015-w