Abstract
Evidence-guided regimens for advanced gastric cancer (AGC) in patients with performance status 2 (PS 2) are limited. Here, we proposed a structured therapeutic framework termed “performance status-matched strategy”, and further conducted the APICAL-GC trial (NCT04278222). This open-label, single-arm phase II study evaluated the efficacy and safety of anlotinib combined with toripalimab among 24 treatment-naïve AGC patients with PS 2. The primary outcome was the objective response rate (ORR), with secondary endpoints including disease control rate (DCR), duration of response (DoR), progression-free survival (PFS), overall survival (OS), and safety profile. This trial met its prespecified endpoints, demonstrating an ORR of 58.3% (95%CI 36.6–77.9) with a DoR of 12.1 months (range: 1.43–48.5), and a DCR of 95.8% (95%CI 78.9–99.9). Median PFS reached 7.33 months (95%CI 3.83–17.1), while median OS was 15.9 months (95%CI 7.73–23.2). Treatment-related adverse events (TRAEs) of any grade occurred in 21 patients (87.5%), with grade-3 TRAEs observed in 7 patients (29.2%). No grade-4/5 TRAEs were reported. These findings provide a rationale for anlotinib plus toripalimab as a promising chemotherapy-free option for the first-line treatment of AGC patients with PS 2 under the performance status-matched strategy, showing comparable anticancer activity and a lower occurrence rate of TRAEs.
Similar content being viewed by others
Introduction
The treatment of gastric cancer remains a formidable challenge in oncology. According to the latest cancer statistics in 2024, gastric cancer is the fifth most common malignancy worldwide and the fifth leading cause of cancer-related deaths, with almost one million new cases diagnosed annually and an estimated 659,805 deaths each year1. The unique anatomical and functional features of the stomach contribute significantly to the clinical presentation of gastric cancer. The pivotal role of the stomach in digestion and nutrient absorption means that gastric cancer often results in severe clinical symptoms and nutritional deficiencies, making patients more likely to present with a poor performance status (PS)2. Despite the lack of specific epidemiological data, the phase II GO trial, which investigated reduced-intensity chemotherapy among older and frail patients with gastroesophageal cancer, revealed that ~30–60% of these patients had a PS of 2, highlighting the significant prevalence of this vulnerable population3. The deterioration in their general condition not only impacts their quality of life but also limits their treatment options. Historically, patients with a PS of 2 are often excluded from clinical trials4. This selection bias has resulted in a paucity of evidence-based treatment strategies for patients with PS 2. Previously, these patients received reduced doses of chemotherapy or active palliative supportive care; however, the efficacy was limited because the average overall survival (OS) was only ~3 months3. Therefore, there is a critical need to develop and validate therapeutic approaches tailored to this vulnerable subgroup. Addressing this gap is essential for improving the clinical outcomes and quality of life of gastric cancer patients with a PS of 2.
The identification of immune checkpoint inhibitors has prompted the development of cancer immunotherapies, reinvigorating and potentially expanding preexisting anticancer immune responses5. Immunotherapy has emerged as a promising treatment for advanced gastric cancer (AGC). CheckMate-649, ORIENT-16, RATIONALE-305, and KEYNOTE-859 have demonstrated that a PD-1 inhibitor plus chemotherapy as a first-line regimen could sufficiently improve the clinical outcomes6,7,8,9. However, these trials also excluded patients with PS 2 because of intolerance to aggressive treatment regimens. With a deeper understanding of the cancer-immunity cycle, emerging evidence has shown that immunotherapy may be more effective in combination with agents that target other steps of the cancer-immunity cycle10. Antiangiogenic agents can directly or indirectly reprogram the immunosuppressive environment into an immunostimulatory microenvironment, whereas immunotherapy can induce the normalization of tumor vasculature11. Consequently, combining immunotherapy with an antiangiogenic agent has been proposed to have a synergistic antitumor effect12,13. The combination of a PD-1 inhibitor with a multi-target tyrosine kinase inhibitor (TKI) stands out as a prominent approach for the treatment of AGC, as shown in the REGONIVO trial and EPOC1706 trial14,15. This combination strategy showed fewer adverse events than traditional chemotherapy, suggesting its suitability for patients who are unable to tolerate chemoimmunotherapy, although patients with PS 2 were also excluded from these trials.
Based on the treatment intensity and safety profiles of the aforementioned two strategies, we propose a structured therapeutic framework termed “performance status-matched strategy” (Fig. 1), which systematically integrates contemporary therapeutic advances with established clinical practice where PS assessment routinely guides treatment decisions. In this strategy, patients with a PS of 0–1 are eligible for more aggressive treatments, such as chemoimmunotherapy; however, patients with a PS score of 2 are often intolerant to this aggressive approach, leaving them in a therapeutic dilemma during the era of traditional chemotherapy. With the advent of non-cytotoxic antitumor agents, particularly immunotherapy, a mild treatment regimen that is highly effective with low toxicity may represent a more appropriate option for patients with PS 2. Specifically, the combination of antiangiogenic agents with immunotherapy may offer an optimized balance between clinical efficacy and reduced toxicity, such as anlotinib plus toripalimab. A retrospective study demonstrated that anlotinib monotherapy would be a feasible third-line or later therapy in AGC16. A phase Ib/II trial showed that toripalimab monotherapy has a promising antitumor activity and a manageable safety profile in chemo-refractory AGC patients17. The combination of anlotinib and toripalimab has demonstrated favorable tolerability and promising efficacy in the treatment of advanced biliary tract cancer and nasopharyngeal carcinoma18,19. These findings provide a strong rationale for exploring the potential synergistic effects of this combination therapy in AGC.
In this work, we conducted a prospective, single-arm, phase II trial to assess the efficacy and safety of anlotinib in combination with toripalimab among patients with AGC who have a performance status of 2. This trial met its prespecified primary endpoint, demonstrating an objective response rate (ORR) of 58.3% (95%CI 36.6–77.9), exceeding the expected ORR of 40% during trial design.
Results
Patient demographics and baseline characteristics
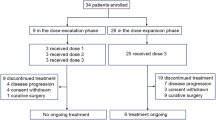
In the first phase of this trial, 14 treatment-naïve patients with AGC with PS 2 were initially enrolled, with eight achieving an objective response. Subsequently, 10 patients were included in the study. In total, 40 gastric cancer patients were screened between April 24, 2020, and July 1, 2024, resulting in 24 patients being enrolled and treated, constituting the intention-to-treat (ITT) population (Fig. 2). All patients received first-line treatment with anlotinib and toripalimab. The median age was 67.5 years (range 41–89 years), 83.3% were male, and all patients had a PS of 2. Of these patients, tumor-related factors accounted for PS 2 in 22 patients, while the remaining two cases were attributed to non-tumor-related causes, specifically advanced age (89 and 81 years old, respectively). 62.5% had more than two sites of metastasis, and 11 (45.8%) had liver metastases. All participants were microsatellite stable (MSS)/proficient mismatch repair (pMMR), and none of them harbored HER2 amplification. Ten patients had PD-L1 positivity (CPS >1), and the median tumor mutational burden (TMB) was 8.38 muts/Mb (range 3.35–27.4) (Table 1).
Treatment and follow-up
All eligible patients underwent at least one post-baseline radiological assessment. The data cut-off for both the efficacy and safety analyses was August 20, 2024, with a median follow-up duration of 33.3 months (95% CI: 13.0–50.1). At the cut-off, eight patients were still undergoing treatment, while 15 discontinued treatments due to disease progression. Additionally, one patient underwent radical surgery for gastric cancer.
Efficacy outcomes
The primary outcome of this trial is ORR. Among the 24 patients eligible for efficacy analysis, 14 exhibited a confirmed partial response, yielding an ORR of 58.3% (95% CI: 36.6–77.9) with a DCR of 95.8% (95% CI: 78.9–99.9) in the ITT cohort (Fig. 3A). Of the 14 responders, the median time to respond was 2.93 months (range: 1.33–9.53 months), and six patients continued to show ongoing responses at the data cut-off. Eight out of 14 responders had progressive disease, with a median duration of response of 12.1 months (range: 1.43–48.5). Tumor shrinkage per RECIST v1.1 was observed in 91.7% (22/24) of the patients in the ITT cohort (Fig. 3A).
Waterfall plot showing the maximum percent change in tumor-targeted lesion size from baseline in each patient as measured by the Response Evaluation Criteria in Solid Tumors (A). The upper dashed line indicates a 20% increase in the tumor burden (PD), and the lower dashed line indicates a 30% decrease in the tumor burden (PR). Asterisks indicate the presence of a new lesion. The Kaplan–Meier curve for progression-free survival (PFS) (B) and overall survival (OS) (C). The data cut-off date for clinical activity was August 20, 2024, and the median follow-up interval was 33.3 months. Vertical lines denote censored patients. The blue area shows the 95% confidence intervals (CI) for the PFS and OS curves. Source data are provided as a Source Data file.
PFS events due to disease progression occurred in 16 of 24 patients. The median PFS was 7.33 months (95% CI: 3.83–17.1) (Fig. 3B). The 6-month and 12-month PFS rates were 58.5 and 39.0%, respectively. OS events were noted in 14 patients, with a median OS of 15.9 months (95% CI: 7.73–23.2) (Fig. 3C). The 12-month and 24-month OS rates were 57.2 and 22.5%, respectively. The subgroup analysis suggested a positive correlation between efficacy and age (Fig. 4 and Supplementary Table 1). Older patients (>65 years, n = 15) demonstrated a higher ORR (70.6 vs. 28.6%), longer median PFS (15.1 months, 95% CI: 4.78–30.0 vs. 3.83 months, 95% CI: 1.30–7.57, P = 0.01) and median OS (22.1 months, 95% CI: 8.80–22.1 vs. 7.73 months, 95% CI: 1.30–23.2) than younger patients (≤65 years, n = 9). Similarly, patients without liver metastases (n = 13) had a significantly higher ORR of 69.2% than those with liver metastases (n = 11, ORR = 45.5%); however, there was no significant difference in the mPFS (7.40 months, 95% CI: 2.77–17.1 vs. 7.33 months, 95% CI: 3.73–30.0) and mOS (13.2 months, 95% CI: 6.80–22.1 vs. 22.0 months, 95% CI: 4.10–23.2) (Supplementary Fig. 1).
A Overall response rate (ORR), B progression-free survival (PFS), and C overall survival (OS) stratified by older patients (>65 years, n = 17) and younger patients (≤65 years, n = 7). P value, two-sided Fisher exact test for ORR analysis; log-rank test for PFS and OS analysis. Source data are provided as a Source Data file.
Safety profile
In the safety analysis, 21 of the 24 patients (87.5%) experienced at least one treatment-related adverse event (TRAE) (Table 2). Common TRAEs include diarrhea, hypothyroidism, hypertension, and hand-foot syndrome (HFS). Seven patients had grade-3 TRAEs, the most common being elevated ALT or AST levels (16.67%) and myelosuppression (12.50%). No grade 4 or 5 TRAEs or treatment-related deaths occurred. Most TRAEs were managed with an anlotinib dose reduction or appropriate medication. Anlotinib dose reductions occurred in three patients due to grade 3 TRAEs, and one patient interrupted toripalimab treatment due to grade 3 immune-related hepatitis.
Exploratory analyses
Gene sequencing data were available for 21 of the 24 eligible patients with gastric cancer. Gene sequencing revealed that TP53 was the most frequently altered gene, occurring in 90.5% of patients, followed by FAT4 (42.9%), CCNE1 (33.3%), and EPHA2 (23.8%) (Fig. 5). The enrichment analysis demonstrated that responders (PR + CR) were more likely to harbor FAT4 mutations (67 vs. 22%, P = 0.08) than non-responders (PD + SD). Patients with FAT4 mutations had a longer PFS and OS than those without FAT4 mutation (mPFS: 17.1 months vs 5.3 months, P = 0.03; mOS: 22.1 months vs 11.3 months, P = 0.18). Signaling pathway enrichment analysis showed that responders had a higher proportion of Hippo pathway alterations (66.6 vs. 22.2%, P = 0.08), WNT pathway alterations (75.0 vs. 33.3%, P = 0.09), and a lower proportion of base excision repair alterations (0 vs. 33.3%, P = 0.06) than non-responders (Supplementary Fig. 2). Multiplex immunofluorescence further confirmed that CD3+ T cells, PD-1+CD8+ T cells, CD20+ B cells, and CD56 dim NK cells were significantly enriched in the tumor microenvironment of the responders (Supplementary Figs. 3–6). In addition, no significant differences were observed in tissue TMB and PD-L1 expression levels.
Oncoprint of concomitant mutation in 21 treatment-naïve advanced gastric cancer patients in ITT (A). The association between FAT4 mutation, and clinical benefit from anlotinib plus toripalimab (B). P value, two-sided Fisher exact test for ORR analysis; log-rank test for PFS and OS analysis. Source data are provided as a Source Data file.
Discussion
Patients with AGC often present with a poor PS at the initial diagnosis because of the direct and indirect effects of the tumors, including gastrointestinal obstruction, malnutrition, and systemic hormonal inflammatory responses. This subgroup represents a significant unmet need for the treatment of AGC, highlighting the critical importance of tailored therapeutic strategies for this population. Multiple large-scale phase III trials focusing exclusively on AGC patients with PS 0–1 have established the role of immunotherapy combined with chemotherapy as the standard first-line treatment, but patients with PS 2 would be intolerable to this aggressive regimen. Other pilot trials have demonstrated the high efficacy and low toxicity of antiangiogenic agents and immunotherapy6,7,8,14,15. Based on the effectiveness and safety of these two therapeutic strategies, we propose a new optimal treatment paradigm called the “performance status-matched strategy” to achieve more precise stratified treatment for AGC. In this trial, we attempt to determine an optimal treatment option for PS 2 AGC using the performance status-matched strategy. To our knowledge, the present APICAL-GC trial is the first to prospectively assess the combination of immunotherapy and antiangiogenic agents in AGC patients with a PS of 2. In this trial, anlotinib plus toripalimab as a first-line regimen has exhibited promising efficacy with an ORR recorded in 58.3% of patients, and the mPFS and mOS of 7.33 and 15.9 months, respectively. These efficacy data are comparable to those of chemoimmunotherapy in patients with PS 0–1, although APICAL-GC only included patients with PS 2, with a lower rate of PD-L1 expression than prior trials. This regimen was safe and well tolerated by these patients, and most TRAEs were manageable with dose reductions, interruptions, or supportive care.
First-line treatment for AGC has rapidly transitioned towards the broad adoption of concurrent chemoimmunotherapy based on a series of clinical trials, such as ATTRACTION-4, CheckMate-649, ORIENT-16, RATIONALE-305, and KEYNOTE-8596,7,8,9,20. However, patients with a PS of 2 were excluded from these trials. One of the underlying reasons for this exclusion could be at least partially due to safety21. Indeed, it cannot be neglected that patients with poor PS have poor tolerance to chemotherapy and a high risk of toxicity. Grade 3–4 TRAEs were observed in 54–59.8% of patients with PS 0–1 AGC who received first-line chemoimmunotherapy6,8,20, suggesting that this strategy with high toxicity is not optimal for patients with PS 2. Although the researchers have attempted to use a dose-reduced chemotherapy regimen, the side effects of chemotherapy cannot be ignored. For example, the GO2 trial, a phase III randomized clinical trial, found that in frail and elderly patients, even with reduced doses of chemotherapy, 13–24% required further dose reductions, and 20–29% had to discontinue treatment due to adverse effects3. Therefore, it is essential and urgent to explore a well-tolerated regimen for patients with PS 2.
Previously, the CheckMate-153 trial indicated that the safety profile of immunotherapy for patients with an ECOG PS ≥2 is consistent with that of the overall population22. Recently, the IPSOS trial demonstrated that atezolizumab monotherapy is more effective than single-agent chemotherapy in advanced lung cancer patients with poor PS (≥2), age ≥70 years, a large number of comorbidities, and other contraindications, who are intolerant to first-line platinum-containing dual-drug chemotherapy23. These data suggest that patients with an ECOG PS of 2 could benefit from immunotherapy-based therapy. Given the potential efficacy of immunotherapy and the intolerability of chemotherapy in patients with a poor PS, an immunotherapy combination strategy without chemotherapy, such as immunotherapy plus antiangiogenic agents, may be the optimal regimen. REGONIVO trial reported an ORR of 44% with an mPFS of 5.6 months in patients with AGC who received regorafenib plus nivolumab14. Subsequently, the EPOC1706 trial showed that lenvatinib plus pembrolizumab as a first or second-line regimen could provide an ORR of 69% with an mPFS of 7.1 months in patients with AGC15. Updated findings from a phase II trial presented at the 2023 ESMO Congress demonstrated remarkable antitumor activity of fruquintinib plus sintilimab in advanced gastric cancer, achieving ORR of 72.2% in first-line and 33.3% in second-line settings, with corresponding mPFS of 11.0 months and 10.5 months, respectively24. Except for surprising efficacy, a significantly lower risk of AEs was observed, making this combination strategy a potential treatment for frail patients (PS 2). Thus, we explored the possibility of anlotinib plus toripalimab for AGC patients with PS 2 using the performance status-matched strategy in the APICAL-GC trial. This trial demonstrated that 58.3% of patients with PS 2 achieved significant tumor shrinkage, with an mPFS of 7.33 months. These efficacy data are comparable with those from previous reports from not only the EPOC 1706 and REGONIVO trials but also first-line chemoimmunotherapy trials, although only patients with PS 2 were included in the APICAL-GC trial. Compared to previous dose-reduced chemotherapy regimens among patients with PS 225, anlotinib plus toripalimab yielded better antitumor activity and a favorable safety profile. This trial supported anlotinib plus toripalimab as a potential first-line treatment option for patients with AGC and a PS of 2.
The importance of considering age as a factor associated with the immunotherapy response has been emphasized. A previous study found that patients >60 years old responded more efficiently to PD-1 inhibitors than younger patients26. Subsequent studies have demonstrated that immunotherapy can prolong survival in elderly patients with advanced cancer, regardless of age27,28; however, this conclusion has been questioned. Some studies found that elderly patients did not benefit from anti-PD-1/PD-L1 agents in terms of survival, especially in patients aged 75 years and older29. Recently, increasing evidence has suggested that age-dependent benefits vary among different immunotherapy types and histology28,30. In AGC, older patients often present with a poor PS. In the APICAL-GC trial, the median age was 67.5 years (range: 41–89 years). The age distribution was more skewed toward older patients than in previously reported immunotherapy-related trials. Interestingly, our trial showed that older patients obtained more clinical benefits from this regimen than younger patients, even with a PS of 2. This result offers a highly promising treatment option for elderly AGC patients with PS 2. Traditionally, elderly patients cannot tolerate standard chemotherapy owing to limited efficacy and significant AEs. Our chemotherapy-free regimen represents a new therapeutic breakthrough for this patient population. Elderly patients often have a poor physical condition, and the excellent performance of this regimen in elderly patients further suggests the value of performance status-matched strategies. Although the underlying mechanisms responsible for age-dependent efficacy differences remain to be fully elucidated, the difference in the causes of PS 2 between these two groups may be a potential factor. PS deterioration in non-elderly patients is more likely due to aggressive tumor biology, whereas in elderly patients, it may be more commonly associated with age or comorbidities. In our cohort, non-elderly patients had a higher proportion of two metastatic sites or more, a greater frequency of PD-L1 negative expression, and a higher incidence of peritoneal metastasis compared to elderly patients, although these differences were not statistically significant.
The APICAL-GC trial also explored efficacy-predictive biomarkers for this regimen. PD-L1 has been demonstrated to predict the response to immunotherapy in patients with gastric cancer31,32. Compared to trials focused on first-line chemoimmunotherapy, the PD-1 expression level among patients from APICAL-GC is relatively lower. For example, 81 and 60% of patients from CheckMate-649 had a CPS > 1 and CPS >5, respectively7. However, only nine patients had a CPS >1, and only two patients had a CPS >5 in the APICAL-GC trial. Despite this low PD-L1 expression, the APICAL-GC trial still demonstrated the significant efficacy of this regimen, further underscoring its effectiveness and adaptability in patients with PS 2; however, no significant differences in efficacy stratified by PD-1 expression were observed. Interestingly, the FAT4 mutation has also been identified as a potential predictive biomarker for anlotinib plus toripalimab. This finding is consistent with that reported in patients with colorectal cancer who received immunotherapy combined with an antiangiogenic agent33,34. As expected, the exploratory analysis demonstrated that patients with gastric cancer who benefit more from sintilimab plus anlotinib often exhibit higher immune cell infiltration in the tumor microenvironment.
This study has some limitations that should be acknowledged. First, the nature of this single-center phase II trial, while providing valuable hypothesis-generating data, necessitates confirmation through multi-institutional randomized controlled trials to validate the generalizability of the findings. Second, while the response evaluation was performed by a masked independent radiologists’ team without central review, the inclusion of an independent central assessment in future trials would enhance the objectivity of efficacy evaluation. Third, the efficacy differences stratified by age and liver metastasis were derived from subgroup analysis. Its reliability and accuracy warrant further validation. Meanwhile, the findings from exploratory analysis are also worthy of further research. Notwithstanding these considerations, the positive results of this study suggest the potential and feasibility of this treatment approach in AGC patients with a PS of 2. The subsequent large-sample randomized controlled trials would be conducted to validate these results.
In conclusion, this trial yielded the rationale for anlotinib plus toripalimab as a promising chemotherapy-free option for the first-line treatment of AGC in patients with PS 2, which was shown to encourage anticancer activity with manageable toxicity. This study also demonstrated the feasibility of implementing a performance status-matched strategy in the population with PS 2. These findings demonstrate the importance of the development of anlotinib plus toripalimab as a first-line treatment for AGC, which could be extended to patients with AGC with PS 0–1 in future trials.
Methods
Study design and participants
This trial adhered to the Declaration of Helsinki and Good Clinical Practice Guidelines and was approved by the Institutional Review Board of Shanghai Changzheng Hospital (approval number: 2019SL036). All patients provided written informed consent prior to enrollment.
This open-label, single-arm, phase II study, conducted at Shanghai Changzheng Hospital, aimed to evaluate the efficacy and safety of anlotinib combined with toripalimab in patients with AGC with PS 2 at the Shanghai Changzheng Hospital (NCT04278222). The key inclusion criteria were as follows: (1) patients aged older than18 years with histologically confirmed, unresectable, and metastatic gastric cancer (AJCC-TNM stage IV adenocarcinoma of the stomach); (2) PS 2 (PS was assessed using Eastern Cooperative Oncology Group performance score); (3) no prior systemic anticancer treatment, with relapse or metastasis occurring more than 12 months after adjuvant chemotherapy; (4) at least one measurable lesion as defined by the Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST 1.1); and (5) adequate organ function (hemoglobin (HB) ≥90 g/L, absolute neutrophil count (ANC) ≥1.5 × 109/L, platelet count (PLT) ≥80 × 109/L, bilirubin (BIL) <1.5 times the upper limit of normal (ULN), alanine transaminase (ALT) and aspartate transaminase (AST) <2.5 × ULN; and in the case of liver metastases, BIL <3 × ULN, ALT and AST <5 × ULN; serum creatinine (Cr) ≤1.5 × ULN). The exclusion criteria included the following: (1) HER2 positive AGC (defined as immunohistochemistry 3+ or immunohistochemistry 2+/fluorescence in situ hybridization-positive or immunohistochemistry 2+/silver in situ hybridization-positive); (2)mismatch repair deficient (dMMR)/microsatellite instability-high (MSI-H) status; (3) prior treatment with antiangiogenics or immune checkpoint inhibitors; (4) known or clinically suspected brain metastases, autoimmune disease, or organ transplantation; (5) use of glucocorticoids (>10 mg prednisone daily) or immunosuppressive agents; (6) history of another malignancy within the past 5 years, except for treated dermal basal cell or squamous cell carcinoma or cervical carcinoma in situ; and (7) significant concomitant diseases deemed by the investigator to preclude participation.
Procedures
Eligible patients received toripalimab intravenously at a dose of 240 mg on day 1 and anlotinib orally at a dose of 12 mg daily from days 1–14 of each 3-week cycle. Treatment was continued until disease progression, death, intolerable toxicity, or consent withdrawal occurred. Toxicity management involved supportive care, dose reduction of anlotinib, and interruption of anlotinib until the adverse events (AEs) were grade 2 or lower. The dose reduction steps for anlotinib were initially 10 mg daily and then 8 mg daily, if necessary. Treatment with anlotinib was terminated if the 8 mg dose was not tolerated. Dose modifications for toripalimab were not permitted, and toripalimab was interrupted in cases of grade 3 or higher immune-related AEs. Patients with intolerable AEs leading to delay or cessation of one medication continued the treatment.
Assessment
Tumor response was evaluated using computed tomography (CT) or magnetic resonance imaging (MRI) at baseline and every 6 weeks until disease progression according to RECIST version 1.1 by a masked independent radiologists’ team. Patients were examined every 3 weeks for hematology, serum chemistry, urinalysis, and Eastern Cooperative Oncology Group (ECOG) PS. AEs were assessed using the National Cancer Institute Common Terminology Criteria for Adverse Events, version 5.0. Prior to enrollment, formalin-fixed paraffin-embedded (FFPE) tissue samples were analyzed to determine the baseline molecular characteristics, including MMR status, molecular alteration profile, PD-L1 expression, and tumor immune-microenvironment. PD-L1 expression was defined as the combined positive score (CPS). Molecular alterations were assessed using a customized next-generation sequencing panel (3D Medicine, Shanghai, China). The tumor immune-microenvironment was evaluated by multiplex immunofluorescence (mIF) with an Akoya OPAL Polaris 7-Color Automation IHC kit.
NGS detection for molecular alterations
FFPE tissue sections were first evaluated using H&E staining to ensure a tumor cell content of at least 20%. The selected sections were then deparaffinized and digested. Genomic DNA was extracted using the ReliaPrep™ FFPE gDNA Miniprep System (Promega) and quantified with the Qubit™ dsDNA HS Assay Kit (Thermo Fisher Scientific). Then, DNA extracts underwent shearing to produce ~250 bp fragments. Libraries were constructed using the KAPA Hyper Prep Kit (KAPA Biosystems). The quality and size distribution of these libraries were assessed using a Qubit 3.0 fluorometer (Thermo Fisher Scientific) and a LabChip GX Touch HT Analyzer (PerkinElmer). Indexed libraries were subjected to hybridization with a custom NGS panel targeting 733 cancer-related genes. The xGen® Hybridization and Wash Kit (IDT) was used for the hybridization enrichment process. After hybridization, the concentration and fragment size distribution of the final library were determined using the same quantification and analysis tools. Subsequently, the captured libraries were sequenced on the NovaSeq 6000 platform (Illumina) with 100 bp paired-end reads and a mean sequencing depth of 1000. The raw sequencing data were aligned to the hg19 reference genome. Variants were called within the targeted regions using an in-house developed variant detection model based on a binomial test. Tumor mutational burden was calculated as the count of non-synonymous somatic SNVs and indels in the examined coding regions, excluding known driver mutations.
Tumor microenvironment (TME) analysis by multiplex immunofluorescence (mIF)
FFPE tissue slides were deparaffinized and sequentially incubated with primary antibodies targeting CD163, CD68, PD-1, PD-L1, CD3, CD4, CD8, CD56, CD20, FOXP3, pan-CK, or S100. Secondary antibodies and corresponding Opal fluorophores were then applied, followed by DAPI staining for nuclei. Negative controls without fluorophores were included to assess autofluorescence. Multiplex-stained slides were scanned using a Vectra Polaris Quantitative Pathology Imaging System (Akoya Biosciences) at 20 nm intervals from 440 to 780 nm. Images were superimposed and imported into inForm v.2.4.8 (Akoya Biosciences) for quantitative analysis. Tumor parenchyma and stroma were differentiated via Pan-CK staining. Cell population quantities were expressed as the number of stained cells per square millimeter and as the percentage of positively stained cells among all nucleated cells. Utilizing AP-TIME image analysis software (3D Medicines Inc.), images were imported and subjected to rigorous examination.
Outcomes
The primary outcome was the objective response rate (ORR), which was determined by the investigator using RECIST version 1.1. Secondary endpoints included the disease control rate (DCR), duration of response (DoR), progression-free survival (PFS), OS, and safety. ORR was defined as the proportion of patients who achieved a complete or partial response. The DCR included complete response, partial response, and stable disease as the best overall responses. PFS was measured from enrollment date to disease progression or death from any cause, whereas OS was measured from enrollment to death from any cause. Exploratory analyses included identifying efficacy-related biomarkers by comparing molecular characteristics between responders (CR + PR) and non-responders (SD + PD), as well as between long-PFS (PFS > mPFS) and short-PFS (PFS ≤ mPFS) groups.
Statistical analysis
The sample size was estimated using Simon’s minimax two-stage design. The response rate for anti-PD-1 antibody plus an antiangiogenic TKI ranged from 44.0 to 69.0% in patients with gastric cancer with PS 0–1, while the response rate for traditional chemotherapy among patients with AGC with PS 2 was ~20–30%25,35,36. An ORR of 40% was expected with the combination of anlotinib and toripalimab. The null hypothesis posited a true ORR of 20% against an alternative of 40% with 80% power and a 10% type I error rate. Fourteen patients were recruited for the first stage; if fewer than two responses were obtained, the study was terminated. Otherwise, an additional ten patients were accrued. The study was deemed positive if more than nine responders were observed among the 24 patients. Descriptive summaries of patient characteristics, safety data, and antitumor activities are provided. The ORR and DCR with 95% confidence intervals (CIs) were calculated using the Clopper and Pearson methods, Kaplan–Meier curve and log-rank test were used to evaluate PFS and OS. All patients who received at least one treatment dose were included in the intention-to-treat (ITT) cohort for the analysis of efficacy and safety. Fisher’s exact test was used for exploratory analyses of the ORR and other binary outcomes among the subgroups, Wilcoxon test is employed for comparing continuous data. P values were calculated using a two-sided method, and statistical significance was set at P < 0.05. All statistical analyses were conducted using the R software (https://www.r-project.org/, version 20.0.3; Belgium).
Data availability
The genomic data generated in this study have been deposited National Genomics Data Center (NGDC) under the accession code HRA011539. A copy of genomic data has also been deposited into the Genome Variation Map (GVM) database under accession code GVM001023. The individual de-identified participant data and genomic data are available for scientific purposes by sending requests to the corresponding author (Yuan-Sheng Zang, [email protected]) within 5 years after this paper’s publication. The remaining data are available within the Article, Supplementary Information, or Source Data file. Source data are provided with this paper.
Code availability
Custom code for data processing and analysis is available https://doi.org/10.5281/zenodo.15362698. (https://github.com/gn123761/-APICAL-GC).
References
Siegel, R. L., Giaquinto, A. N. & Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 74, 12–49 (2024).
Pressoir, M. et al. Prevalence, risk factors and clinical implications of malnutrition in French Comprehensive Cancer Centres. Br. J. Cancer 102, 966–971 (2010).
Hall, P. S. et al. Efficacy of reduced-intensity chemotherapy with oxaliplatin and capecitabine on quality of life and cancer control among older and frail patients with advanced gastroesophageal cancer: the GO2 phase 3 randomized clinical trial. JAMA Oncol. 7, 869–877 (2021).
Talarico, L., Chen, G. & Pazdur, R. Enrollment of elderly patients in clinical trials for cancer drug registration: a 7-year experience by the US Food and Drug Administration. J. Clin. Oncol. 22, 4626–4631 (2004).
Morad, G., Helmink, B. A., Sharma, P. & Wargo, J. A. Hallmarks of response, resistance, and toxicity to immune checkpoint blockade. Cell 184, 5309–5337 (2021).
Xu, J. et al. Sintilimab plus chemotherapy for unresectable gastric or gastroesophageal junction cancer: the ORIENT-16 randomized clinical trial. JAMA 330, 2064–2074 (2023).
Janjigian, Y. Y. et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet 398, 27–40 (2021).
Qiu, M. Z. et al. Tislelizumab plus chemotherapy versus placebo plus chemotherapy as first line treatment for advanced gastric or gastro-oesophageal junction adenocarcinoma: RATIONALE-305 randomised, double blind, phase 3 trial. BMJ 385, e078876 (2024).
Rha, S. Y. et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for HER2-negative advanced gastric cancer (KEYNOTE-859): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 24, 1181–1195 (2023).
Chen, D. S. & Mellman, I. Oncology meets immunology: the cancer-immunity cycle. Immunity 39, 1–10 (2013).
Khan, K. A. & Kerbel, R. S. Improving immunotherapy outcomes with anti-angiogenic treatments and vice versa. Nat. Rev. Clin. Oncol. 15, 310–324 (2018).
Fukumura, D., Kloepper, J., Amoozgar, Z., Duda, D. G. & Jain, R. K. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat. Rev. Clin. Oncol. 15, 325–340 (2018).
Tian, L. et al. Mutual regulation of tumour vessel normalization and immunostimulatory reprogramming. Nature 544, 250–254 (2017).
Fukuoka, S. et al. Regorafenib plus nivolumab in patients with advanced gastric or colorectal cancer: an open-label, dose-escalation, and dose-expansion phase Ib trial (REGONIVO, EPOC1603). J. Clin. Oncol. 38, 2053–2061 (2020).
Kawazoe, A. et al. Lenvatinib plus pembrolizumab in patients with advanced gastric cancer in the first-line or second-line setting (EPOC1706): an open-label, single-arm, phase 2 trial. Lancet Oncol. 21, 1057–1065 (2020).
Nie, C. et al. Clinical study of anlotinib as third-line or above therapy in patients with advanced or metastatic gastric cancer: a multicenter retrospective study. Front. Oncol. 12, 885350 (2022).
Wang, F. et al. Safety, efficacy and tumor mutational burden as a biomarker of overall survival benefit in chemo-refractory gastric cancer treated with toripalimab, a PD-1 antibody in phase Ib/II clinical trial NCT02915432. Ann. Oncol. 30, 1479–1486 (2019).
Zhou, M. et al. A phase II study to evaluate the safety and efficacy of anlotinib combined with toripalimab for advanced biliary tract cancer. Clin. Transl. Immunol. 13, e1483 (2024).
Zhang, Y. et al. Toripalimab plus anlotinib in patients with recurrent or metastatic nasopharyngeal carcinoma: a multicenter, single-arm phase 2 trial (TORAL). Cell Rep. Med. 5, 101833 (2024).
Kang, Y. K. et al. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 23, 234–247 (2022).
Dall’Olio, F. G. et al. ECOG performance status ≥2 as a prognostic factor in patients with advanced non small cell lung cancer treated with immune checkpoint inhibitors-A systematic review and meta-analysis of real world data. Lung Cancer 145, 95–104 (2020).
Spigel, D. R. et al. Safety, Efficacy, and patient-reported health-related quality of life and symptom burden with nivolumab in patients with advanced non-small cell lung cancer, including patients aged 70 years or older or with poor performance status (CheckMate 153). J. Thorac. Oncol. 14, 1628–1639 (2019).
Lee, S. M. et al. First-line atezolizumab monotherapy versus single-agent chemotherapy in patients with non-small-cell lung cancer ineligible for treatment with a platinum-containing regimen (IPSOS): a phase 3, global, multicentre, open-label, randomised controlled study. Lancet 402, 451–463 (2023).
Wei, X. et al. Fruquintinib plus sintilimab in patients(pts)with either treatment-naïve or previously treated advanced gastric or gastroesophageal junction(GEJ)adenocarcinoma:results from a multicenter,single-arm phase II study. ESMO Abstr 1519P (2023).
Hacibekiroglu, I. et al. Comparative analysis of the efficacy and safety of oxaliplatin plus 5-fluorouracil/leucovorin (modified FOLFOX6) with advanced gastric cancer patients having a good or poor performance status. Asian Pac. J. cancer Prev. 16, 2355–2359 (2015).
Kugel, C. H. 3rd et al. Age correlates with response to anti-PD1, reflecting age-related differences in intratumoral effector and regulatory T-cell populations. Clin. Cancer Res. 24, 5347–5356 (2018).
Ciccarese, C. et al. The anticancer efficacy of immune checkpoint inhibitors according to patients’ age: a systematic review and meta-analysis. J. Immunother. 43, 95–103 (2020).
Ninomiya, K. et al. Influence of age on the efficacy of immune checkpoint inhibitors in advanced cancers: a systematic review and meta-analysis. Acta Oncol. 59, 249–256 (2020).
Nie, R. C. et al. Efficacy of anti-PD-1/PD-L1 monotherapy or combinational therapy in patients aged 75 years or older: a study-level meta-analysis. Front. Oncol. 11, 538174 (2021).
Choucair, K. et al. Immune checkpoint inhibitors: the unexplored landscape of geriatric oncology. Oncologist 27, 778–789 (2022).
Nakamura, Y., Kawazoe, A., Lordick, F., Janjigian, Y. Y. & Shitara, K. Biomarker-targeted therapies for advanced-stage gastric and gastro-oesophageal junction cancers: an emerging paradigm. Nat. Rev. Clin. Oncol. 18, 473–487 (2021).
Ahn, S. & Kim, K. M. PD-L1 expression in gastric cancer: interchangeability of 22C3 and 28-8 pharmDx assays for responses to immunotherapy. Mod. Pathol.34, 1719–1727 (2021).
Chen, M. et al. FAT4 mutation as a potential predictive biomarker for immunotherapy combined with anti-angiogenic therapy in MSS metastatic colorectal cancer. J. Clin. Oncol. https://doi.org/10.1200/JCO.2022.1240.1216_suppl.e15504 (2022).
Wang, D. et al. FAT4 overexpression promotes antitumor immunity by regulating the β-catenin/STT3/PD-L1 axis in cervical cancer. J. Exp. Clin. cancer Res. 42, 222 (2023).
Hall, P. S. et al. A randomised phase II trial and feasibility study of palliative chemotherapy in frail or elderly patients with advanced gastroesophageal cancer (321GO). Br. J. Cancer 116, 472–478 (2017).
Lee, K. W. et al. A phase 3 randomized clinical trial to compare efficacy and safety between combination therapy and monotherapy in elderly patients with advanced gastric cancer (KCSG ST13-10). Cancer Res. Treat. 55, 1250–1260 (2023).
Acknowledgements
We thank the patients and their families for participating in the trial. This work was supported by Chinese National Natural Science Funding (82172710, grant to Y.-S.Z.); Innovation Clinical Research Project of Shanghai Changzheng Hospital (2020YLCYJ-Z03, grant to Y.-S.Z.); Shanghai Public Health Outstanding Academic Leader Program (GWVI-11.2-XD22, grant to Y.-S.Z.); Shanghai Oriental Talents Program (grant to Y.-S.Z.). The funder has no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. Additionally, this study was supported by unconditioned grants from Shanghai Junshi Biosciences Co., Ltd. (Shanghai, China) for toripalimab and Chia Tai Tianqing Pharmaceutical Group Co., Ltd. (Lianyungang, Jiangsu, China) for anlotinib hydrochloride. The sponsors provided the drugs but not been involved in the study design, data collection, data analysis, or manuscript writing.
Author information
Authors and Affiliations
Contributions
Y.-S.Z., X.-D.J., K.L. and B.-D.Q. designed the study. S.-Q.C., X.Z., X.-P.D., Y.W., Z.W., Y.L., L.S., C.-Y.Y. and D.-M.S. recruited patients for this study. S.-Q.C., X.Z. and B.-D.Q. collected data. K.L., B.-D.Q., X.-D.J., N.G. and Y.-S.Z. did the analyses. K.L., B.-D.Q. and Y.-S.Z. interpreted the data and wrote the manuscript. All authors gave final approval to submit for publication. All authors have agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All these authors listed read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics
The study was approved by the institutional review board of the ethics committee of Shanghai Changzheng Hospital.
Informed consent
Written informed consent was obtained from all patients before any study-related procedures performed. Both the collection of all data in this study and the adoption of the materials comply with the Declaration of Helsinki and the relevant provisions of the guidelines.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, K., Qin, BD., Chen, SQ. et al. Anlotinib plus toripalimab as a first-line treatment in patients with advanced gastric cancer and performance status 2: the phase II APICAL-GC trial. Nat Commun 16, 5069 (2025). https://doi.org/10.1038/s41467-025-60317-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-60317-z