Abstract
Heterochromatic condensates (chromocenters) are critical for maintaining the silencing of heterochromatin. It is therefore puzzling that the presence of chromocenters is variable across plant species. Here we reveal that variations in the plant heterochromatin protein ADCP1 confer a diversity in chromocenter formation via phase separation. ADCP1 physically interacts with the high mobility group protein HMGA to form a complex and mediates heterochromatin condensation by multivalent interactions. The loss of intrinsically disordered regions (IDRs) in ADCP1 homologues during evolution has led to the absence of prominent chromocenter formation in various plant species, and introduction of IDR-containing ADCP1 with HMGA promotes heterochromatin condensation and retrotransposon silencing. Moreover, plants in the Cucurbitaceae group have evolved an IDR-containing chimaera of ADCP1 and HMGA, which remarkably enables formation of chromocenters. Together, our work uncovers a coevolved mechanism of phase separation in packing heterochromatin and silencing retrotransposons.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
118,99 € per year
only 9,92 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All sequencing data generated in this study are available on the NCBI GEO under accession no. GSE233265. Source data are provided with this paper.
References
Allshire, R. C. & Madhani, H. D. Ten principles of heterochromatin formation and function. Nat. Rev. Mol. Cell Biol. 19, 229–244 (2018).
Marsano, R. M., Giordano, E., Messina, G. & Dimitri, P. A new portrait of constitutive heterochromatin: lessons from Drosophila melanogaster. Trends Genet. 35, 615–631 (2019).
Jost, K. L., Bertulat, B. & Cardoso, M. C. Heterochromatin and gene positioning: inside, outside, any side? Chromosoma 121, 555–563 (2012).
Saksouk, N., Simboeck, E. & Dejardin, J. Constitutive heterochromatin formation and transcription in mammals. Epigenetics Chromatin 8, 3 (2015).
Morrison, O. & Thakur, J. Molecular complexes at euchromatin, heterochromatin and centromeric chromatin. Int. J. Mol. Sci. 22, 6922 (2021).
Janssen, A., Colmenares, S. U. & Karpen, G. H. Heterochromatin: guardian of the genome. Annu. Rev. Cell Dev. Biol. 34, 265–288 (2018).
Dimitri, P., Corradini, N., Rossi, F. & Verni, F. The paradox of functional heterochromatin. Bioessays 27, 29–41 (2005).
Feng, W. & Michaels, S. D. Accessing the inaccessible: the organization, transcription, replication, and repair of heterochromatin in plants. Annu. Rev. Genet. 49, 439–459 (2015).
Simon, L., Voisin, M., Tatout, C. & Probst, A. V. Structure and function of centromeric and pericentromeric heterochromatin in Arabidopsis thaliana. Front. Plant Sci. 6, 1049 (2015).
Jagannathan, M. & Yamashita, Y. M. Function of junk: pericentromeric satellite DNA in chromosome maintenance. Cold Spring Harb. Symp. Quant. Biol. 82, 319–327 (2017).
Marsano, R. M. & Dimitri, P. Constitutive heterochromatin in eukaryotic genomes: a mine of transposable elements. Cells 11, 761 (2022).
The Cold Spring Harbor Laboratory, Washington University Genome Sequencing Center & PE Biosystems Arabidopsis Sequencing Consortium. The complete sequence of a heterochromatic island from a higher eukaryote. Cell 100, 377–386 (2000).
Lippman, Z. et al. Role of transposable elements in heterochromatin and epigenetic control. Nature 430, 471–476 (2004).
Zhang, X. et al. Genome-wide high-resolution mapping and functional analysis of DNA methylation in Arabidopsis. Cell 126, 1189–1201 (2006).
Cokus, S. J. et al. Shotgun bisulphite sequencing of the Arabidopsis genome reveals DNA methylation patterning. Nature 452, 215–219 (2008).
Law, J. A. & Jacobsen, S. E. Establishing, maintaining and modifying DNA methylation patterns in plants and animals. Nat. Rev. Genet. 11, 204–220 (2010).
Zhang, H., Lang, Z. & Zhu, J. K. Dynamics and function of DNA methylation in plants. Nat. Rev. Mol. Cell Biol. 19, 489–506 (2018).
Jackson, J. P. et al. Dimethylation of histone H3 lysine 9 is a critical mark for DNA methylation and gene silencing in Arabidopsis thaliana. Chromosoma 112, 308–315 (2004).
Jacob, Y. et al. ATXR5 and ATXR6 are H3K27 monomethyltransferases required for chromatin structure and gene silencing. Nat. Struct. Mol. Biol. 16, 763–768 (2009).
Feng, S. & Jacobsen, S. E. Epigenetic modifications in plants: an evolutionary perspective. Curr. Opin. Plant Biol. 14, 179–186 (2011).
Underwood, C. J., Henderson, I. R. & Martienssen, R. A. Genetic and epigenetic variation of transposable elements in Arabidopsis. Curr. Opin. Plant Biol. 36, 135–141 (2017).
Kabi, M. & Filion, G. J. Heterochromatin: did H3K9 methylation evolve to tame transposons? Genome Biol. 22, 325 (2021).
Padeken, J., Methot, S. P. & Gasser, S. M. Establishment of H3K9-methylated heterochromatin and its functions in tissue differentiation and maintenance. Nat. Rev. Mol. Cell Biol. 23, 623–640 (2022).
Zemach, A., McDaniel, I. E., Silva, P. & Zilberman, D. Genome-wide evolutionary analysis of eukaryotic DNA methylation. Science 328, 916–919 (2010).
Grewal, S. I. S. The molecular basis of heterochromatin assembly and epigenetic inheritance. Mol. Cell 83, 1767–1785 (2023).
Fransz, P., De Jong, J. H., Lysak, M., Castiglione, M. R. & Schubert, I. Interphase chromosomes in Arabidopsis are organized as well defined chromocenters from which euchromatin loops emanate. Proc. Natl Acad. Sci. USA 99, 14584–14589 (2002).
Yelagandula, R. et al. The histone variant H2A.W defines heterochromatin and promotes chromatin condensation in Arabidopsis. Cell 158, 98–109 (2014).
Wang, J., Jia, S. T. & Jia, S. New insights into the regulation of heterochromatin. Trends Genet. 32, 284–294 (2016).
Alper, B. J., Lowe, B. R. & Partridge, J. F. Centromeric heterochromatin assembly in fission yeast—balancing transcription, RNA interference and chromatin modification. Chromosome Res. 20, 521–534 (2012).
Almouzni, G. & Probst, A. V. Heterochromatin maintenance and establishment: lessons from the mouse pericentromere. Nucleus 2, 332–338 (2011).
Wang, H., Dittmer, T. A. & Richards, E. J. Arabidopsis CROWDED NUCLEI (CRWN) proteins are required for nuclear size control and heterochromatin organization. BMC Plant Biol. 13, 200 (2013).
Pontvianne, F. & Grob, S. Three-dimensional nuclear organization in Arabidopsis thaliana. J. Plant Res. 133, 479–488 (2020).
Doğan, E. S. & Liu, C. Three-dimensional chromatin packing and positioning of plant genomes. Nat. Plants 4, 521–529 (2018).
Lindroth, A. M. et al. Dual histone H3 methylation marks at lysines 9 and 27 required for interaction with CHROMOMETHYLASE3. EMBO J. 23, 4286–4296 (2004).
Maison, C. et al. Higher-order structure in pericentric heterochromatin involves a distinct pattern of histone modification and an RNA component. Nat. Genet. 30, 329–334 (2002).
Soppe, W. J. et al. DNA methylation controls histone H3 lysine 9 methylation and heterochromatin assembly in Arabidopsis. EMBO J. 21, 6549–6559 (2002).
Strom, A. R. et al. Phase separation drives heterochromatin ___domain formation. Nature 547, 241–245 (2017).
Larson, A. G. et al. Liquid droplet formation by HP1alpha suggests a role for phase separation in heterochromatin. Nature 547, 236–240 (2017).
Wang, L. et al. Histone modifications regulate chromatin compartmentalization by contributing to a phase separation mechanism. Mol. Cell 76, 646–659.e6 (2019).
Sanulli, S. et al. HP1 reshapes nucleosome core to promote phase separation of heterochromatin. Nature 575, 390–394 (2019).
Zhao, S. et al. Plant HP1 protein ADCP1 links multivalent H3K9 methylation readout to heterochromatin formation. Cell Res. 29, 54–66 (2019).
Zhang, C. et al. Arabidopsis AGDP1 links H3K9me2 to DNA methylation in heterochromatin. Nat. Commun. 9, 4547 (2018).
Xu, L. & Jiang, H. Writing and reading histone H3 lysine 9 methylation in Arabidopsis. Front. Plant Sci. 11, 452 (2020).
Harris, C. J. & Jacobsen, S. E. ADCP1: a novel plant H3K9me2 reader. Cell Res. 29, 6–7 (2019).
Bizhanova, A. & Kaufman, P. D. Close to the edge: heterochromatin at the nucleolar and nuclear peripheries. Biochim. Biophys. Acta Gene Regul. Mech. 1864, 194666 (2021).
Padeken, J. & Heun, P. Nucleolus and nuclear periphery: velcro for heterochromatin. Curr. Opin. Cell Biol. 28, 54–60 (2014).
Cheng, S. et al. The Tarenaya hassleriana genome provides insight into reproductive trait and genome evolution of crucifers. Plant Cell 25, 2813–2830 (2013).
Launholt, D., Merkle, T., Houben, A., Schulz, A. & Grasser, K. D. Arabidopsis chromatin-associated HMGA and HMGB use different nuclear targeting signals and display highly dynamic localization within the nucleus. Plant Cell 18, 2904–2918 (2006).
Zhao, B., Xi, Y. P., Kim, J. Y. & Sung, S. B. Chromatin architectural proteins regulate flowering time by precluding gene looping. Sci. Adv. 7, abg3097 (2021).
Kotlinski, M. et al. Phylogeny-based systematization of Arabidopsis proteins with histone H1 globular ___domain. Plant Physiol. 174, 27–34 (2017).
Peng, S. et al. Phase separation at the nanoscale quantified by dcFCCS. Proc. Natl Acad. Sci. USA 117, 27124–27131 (2020).
Fang, X. et al. Arabidopsis FLL2 promotes liquid–liquid phase separation of polyadenylation complexes. Nature 569, 265–269 (2019).
Velez, G. et al. Evidence supporting a critical contribution of intrinsically disordered regions to the biochemical behavior of full-length human HP1gamma. J. Mol. Model. 22, 12 (2016).
Li, C. H. et al. MeCP2 links heterochromatin condensates and neurodevelopmental disease. Nature 586, 440–444 (2020).
Shin, Y. & Brangwynne, C. P. Liquid phase condensation in cell physiology and disease. Science 357, eaaf4382 (2017).
Ozturk, N., Singh, I., Mehta, A., Braun, T. & Barreto, G. HMGA proteins as modulators of chromatin structure during transcriptional activation. Front. Cell Dev. Biol. 2, 5 (2014).
Kishi, Y., Fujii, Y., Hirabayashi, Y. & Gotoh, Y. HMGA regulates the global chromatin state and neurogenic potential in neocortical precursor cells. Nat. Neurosci. 15, 1127–1133 (2012).
Stros, M., Launholt, D. & Grasser, K. D. The HMG-box: a versatile protein ___domain occurring in a wide variety of DNA-binding proteins. Cell. Mol. Life Sci. 64, 2590–2606 (2007).
Reeves, R. Molecular biology of HMGA proteins: hubs of nuclear function. Gene 277, 63–81 (2001).
Narita, M. et al. A novel role for high-mobility group A proteins in cellular senescence and heterochromatin formation. Cell 126, 503–514 (2006).
McCarthy, R. L. et al. Diverse heterochromatin-associated proteins repress distinct classes of genes and repetitive elements. Nat. Cell Biol. 23, 905–914 (2021).
Jagannathan, M., Cummings, R. & Yamashita, Y. M. A conserved function for pericentromeric satellite DNA. eLife 7, e34122 (2018).
Fyodorov, D. V., Zhou, B. R., Skoultchi, A. I. & Bai, Y. Emerging roles of linker histones in regulating chromatin structure and function. Nat. Rev. Mol. Cell Biol. 19, 192–206 (2018).
Crane-Robinson, C. Linker histones: history and current perspectives. Biochim. Biophys. Acta 1859, 431–435 (2016).
Stasevich, T. J., Mueller, F., Brown, D. T. & McNally, J. G. Dissecting the binding mechanism of the linker histone in live cells: an integrated FRAP analysis. EMBO J. 29, 1225–1234 (2010).
Sequeira-Mendes, J. et al. The functional topography of the Arabidopsis genome is organized in a reduced number of linear motifs of chromatin states. Plant Cell 26, 2351–2366 (2014).
Dekker, J. & Mirny, L. The 3D genome as moderator of chromosomal communication. Cell 164, 1110–1121 (2016).
Bonev, B. & Cavalli, G. Organization and function of the 3D genome. Nat. Rev. Genet. 17, 661–678 (2016).
Charbonnel, C. et al. The linker histone GH1-HMGA1 is involved in telomere stability and DNA damage repair. Plant Physiol. 177, 311–327 (2018).
Ahn, J. H. et al. Phase separation drives aberrant chromatin looping and cancer development. Nature 595, 591–595 (2021).
Boulay, G. et al. Cancer-specific retargeting of BAF complexes by a prion-like ___domain. Cell 171, 163–178.e19 (2017).
Hosaka, A. & Kakutani, T. Transposable elements, genome evolution and transgenerational epigenetic variation. Curr. Opin. Genet. Dev. 49, 43–48 (2018).
Li, C. et al. FastCloning: a highly simplified, purification-free, sequence- and ligation-independent PCR cloning method. BMC Biotechnol. 11, 92 (2011).
Yoo, S. D., Cho, Y. H. & Sheen, J. Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat. Protoc. 2, 1565–1572 (2007).
Lemoine, F. et al. NGPhylogeny.fr: new generation phylogenetic services for non-specialists. Nucleic Acids Res. 47, W260–W265 (2019).
Letunic, I. & Bork, P. Interactive Tree Of Life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 49, W293–W296 (2021).
Acknowledgements
We thank all members of the Sun laboratory for valuable discussion; C. Dean, H. Zhang, G. Li, Q. Zhang, Y. Guo and D. Ren for constructive comments on the work; Y. Qi and Z. Fu for assistance with growing plant materials; Q. Chen and X. Yang for sharing CRISPR-Cas9 vectors; L. Yang for guidance in the preparation and transformation of cucumber protoplasts; Y. Lv for help in painting plant models; the Core Facility of the Center of Biomedical Analysis (Tsinghua University) for assistance with mass spectrometry and confocal microscopy analysis; and Y. Li of the Cryo-EM Facility (Tsinghua University) for help in the immuno-TEM samples preparation. This work was supported by grants from the National Natural Science Foundation of China (31822028 and 91940306 to Q.S., 32070651 to W.Z.) and the Ministry of Science and Technology of China (2016YFA0500800 to Q.S.). The Sun Lab is supported by Tsinghua-Peking Center for Life Sciences, and W.Z. is supported by the China Postdoctoral Science Foundation Project (2019M660610) and the postdoctoral fellowship from Tsinghua-Peking Center for Life Sciences.
Author information
Authors and Affiliations
Contributions
Q.S. and W.Z. conceived the study. Q.S., P.L., H.L., C.C. and W.Z. designed the experiments. L.C. generated the pADCP1::ADCP1-eGFP/FLAG transgenic lines and performed the ADCP1 mass spectrometry analysis. K.L. analysed the TE distribution of plant species and calculated Gini coefficients. L.X. constructed the nucleosomal arrays. C.C. and J.J. quantitatively measured and analysed the ADCP1-HMGA-H3K9me3 puncta formation in vitro. X.L. conducted the protoplast truncated ADCP1 and HMGA Co-IP assays. A.J. performed ITC experiments and predicted the ADCP1-HMGA protein complex structure. W.Z. performed the rest of the experiments. W.Z. and Q.S. wrote the manuscript with input from all authors, and all authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Plants thanks Fredy Barneche and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Microscopic images of H3K9me2 and H3K27me1 immunostaining in nuclei of different plant species.
Colors indicated the signals of H3K9me2 (green), H3K27me1 (magenta), and DNA stain (DAPI, grey). At least 50 independent nuclei were observed each species, and the representative ones were imaged. Scale bars, 5 μm. The models of corresponding ADCP1 proteins are shown in the right.
Extended Data Fig. 2 Variations of TE distribution and chromocenter formation in plants.
a,b, The distribution of TEs in plant species with (a) and without (b) chromocenters. The green lines indicated the positions of TEs in the presented chromosomes. Blue-lined boxes showed the heterochromatin regions that concentrate the heterochromatic elements in chromocenter-containing species. c, Gini coefficient is calculated by the numbers of TEs in 100kb-windows to represent the differences in distributions of TEs across the chromosome. The species with and without chromocenters are separated by a black dotted line. Error bars denote standard deviation (SD) of chromosomes.
Extended Data Fig. 3 ADCP1 interacts specifically with HMGA but not with its paralogs.
a, Phylogenetic tree and ___domain architecture of Arabidopsis HMGA proteins. b, The sequence alignment of Arabidopsis HMGA proteins. The N-terminal GH1 ___domain is highlighted in blue, and the four AT-hooks are highlighted in orange. The HMGA peptides found by ADCP1 IP-MS were shown by green boxes. c, The BiFC assays in Arabidopsis show that ADCP1 interacts specifically with HMGA but not with its homologous proteins HMGA1, HMGA2 and HMGA4. Scale bar, 10 μm. d, BiFC assays in Arabidopsis using ___domain truncated ADCP1-YFPn shown in left and full-length HMGA-YFPc. Scale bar, 5 μm. c,d, At least 50 independent cells were observed in each experiment, and the representative ones were imaged. e, Arabidopsis protoplasts were used to co-express MYC-HMGA-GFP with full length or truncated FLAG-ADCP1-mCherry to conduct Co-IP assays. f, The prediction result of ADCP1-HMGA complex using AlphaFold2.
Extended Data Fig. 4 HMGA promotes ADCP1-depended chromocenter formation.
a, Transient expression of ADCP1-GFP in Col-0 protoplasts. The zoom-in images of the boxed area are shown in Fig. 2h. Scale bar, 5 μm. b, The ___location of ADCP1 in hmga mutant protoplasts. The zoom-in images of the boxed area are shown in Fig. 2i. Scale bar, 5 μm. c, The nucleus of Arabidopsis transgenic plant leaf expressing mCherry-HMGA. Right image is line scans at the position depicted by the white line. Scale bar, 5 μm. d, The nucleus of Arabidopsis transgenic plant root expressing ADCP1-GFP. Right image is line scans at the position depicted by the white line. Scale bar, 5 μm. e, The Arabidopsis mCherry-HMGA and ADCP1-GFP co-transgenic plants showed ADCP1 and HMGA were co-localized in the chromocenters. Right image is line scans at the position depicted by the white line. Scale bar, 5 μm. At least 50 independent nuclei were observed in each line, and the representative ones were imaged.
Extended Data Fig. 5 HMGA helps ADCP1 to localize in heterochromatic regions.
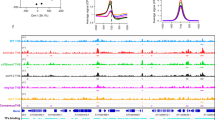
a, Scatter plots showing the weaken of ADCP1 binding capacity on H3K9me2-marked regions in hmga mutant. b, The classification of ADCP1-targeted TEs in Col-0 and hmga background, the classification of all TEs in TAIR10 annotation is displayed as a control. c, The HMGA binding motifs found by MEME-ChIP analysis. d, H3K9me2 (red), ADCP1 in wild type (green) and hmga (blue), and HMGA (purple) ChIP-seq enrichment along Arabidopsis chromosomes 2-5. e, Snapshots of H3K9me2 (red), ADCP1 in wild type (green) and hmga (blue), and HMGA (purple) ChIP-seq signals on Arabidopsis LTR-TEs sites. ChIP-seq signals are showed as reads per kilobase per million mapped reads (RPKM).
Extended Data Fig. 6 The phase separation activity of ADCP1 and HMGA.
a,b, In vitro phase separation assay of full-length ADCP1-GFP and mC-HMGA proteins with H3K9me3 NA. The images in panels and show the DAPI (a) and merged (b) signals, respectively. Scale bars, 20 μm. c,d, ADCP1 (c) and HMGA (d) ___domain architecture and predictor of natural disordered regions (PONDR) score for intrinsic disorder regions (IDRs) (black line), >0.5 is considered disordered. e, Full length and truncated ADCP1-GFP and mCherry-HMGA proteins used in phase separation assays. f, The puncta formed by ADCP1-HMGA-H3K9me3 NA were quantitatively measured by Fluorescence Correlation Spectroscopy (FCS) system. FCS curves (left) and fluorescence trajectories (right) of condensates formed by H3K9me NA with ADCP1 and HMGA are shown. g, Hydrodynamic radii calculated from FCS curves shown in Fig. 4g. Error bars denote standard deviation (SD) of replicates.
Extended Data Fig. 7 The ADCP1-HMGA recognition pair triggers the formation of chromocenter-like condensates in rice and maize protoplasts.
a, BiFC assays using ADCP1 and HMGA homologs from soybean and tobacco in Col-0 protoplasts. Scale bar, 5 μm. b, Schematic diagram of the ADCP1-HMGA pair expression in rice and maize protoplasts. The Arabidopsis, rice and maize plant models were created with BioRender.com. c,d, The distribution change of ADCP1-mCherry, HMGA-GFP, and DAPI signals in the nuclei of rice (c) and maize (d) protoplasts over time. e-g, Transformation of ADCP1 protein alone does not affect the pattern of DAPI staining in the nuclei of tobacco (e), rice (f), or maize (g). The images were taken at 72h after transformation. Scale bar, 5 μm. At least 50 independent nuclei were observed in each experiment, and the representative ones were imaged. h, Relative expression of LTR-TEs in tobacco 84h after co-transformed ADCP1-GFP and mCherry-HMGA. The NbActin3 gene was used as the internal reference. Error bars denote standard deviation (SD) of three replicates. Two-tailed t tests.
Extended Data Fig. 8 The structure feature of CsaADCP1.
a, Domain predication results of CsaADCP1 from SMART (http://smart.embl-heidelberg.de), and snapshots showed the hidden HMG-like ___domain in CsaADCP1. b, Sequence alignment of the hidden HMG-like ___domain in CsaADCP1 and HMG proteins. The sequences alignment shows HMG-like ___domain in CsaADCP1 protein owns similar structure features with HMG proteins. c, Three examples from tobacco infection assays using AT-hooks-truncated CsaADCP1. Scale bar, 5 μm. d, CRISPR mediated knock out the CsaADCP1 in cucumber to confirm its function in chromocenter formation. The right panels show the enlarged nuclei, and the numbers mean percentage of nuclei that display DAPI distribution patterns as in the figures. Scale bars, 5 μm.
Extended Data Fig. 9 Introduction of AtADCP1 and AtHMGA promotes heterochromatin condensation and TE silencing.
a, Formation of chromocenter-like condensates in the AtADCP1 and AtHMGA co-transgenic plants of tomato. At least 50 independent nuclei were observed, and the representative ones were imaged. Scale bar, 2 μm. Right image shows line scans at the position depicted by white line. b, Nuclei of tomato without transgene as a control. Scale bar, 2 μm. Right image shows line scans at the position depicted by white line. c, Heatmaps of H3K9me2 and AtADCP1 ChIP-seq enrichment around their binding sites. d,e, ChIPseq snapshots show the binding of H3K9me2 and AtADCP1 at LTR-TE regions of tomato transgenic plants. ChIP-seq signals are showed as reads per kilobase per million mapped reads (RPKM). f, Relative expression of ADCP1 targeted TEs in tomato transgenic plants. The SlActin7 gene was used as the internal reference. Error bars denote standard deviation (SD) of three replicates. Two-tailed t tests. g, H3K9me2 immunostaining in nucleus of the AtADCP1 and AtHMGA co-transgenic plants of tobacco. Colors indicated the signals of ADCP1 (green), HMGA (magenta), H3K9me2 (cyan), and DNA stain (DAPI, grey). Scale bar, 2 μm. h, FISH with the Copia481 repeats in nucleus of the AtADCP1 and AtHMGA co-transgenic plants of tobacco. Colors indicated the signals of Copia481 (magenta) and DNA stain (DAPI, grey). Scale bar, 2 μm.
Supplementary information
Supplementary Information
Supplementary Figs. 1 and 2, and Tables 1–4.
Supplementary Video 1
FRAP assay of the ADCP1 foci in ADCP1-GFP transgenic plant of wild-type background.
Supplementary Video 2
FRAP assay of the ADCP1 foci in ADCP1-GFP transgenic plant of hmga background.
Supplementary Video 3
FRAP assay of the HMGA foci in mCherry-HMGA transgenic plant of wild-type background.
Supplementary Video 4
FRAP of the ADCP1-HMGA complex foci in BiFC assay.
Source data
Source Data Figs. 2–6 and Extended Data Figs. 2, 4–7 and 9
Summary of all statistical source data.
Source Data Fig. 2
Unprocessed western blots of Fig. 2e.
Source Data Extended Data Fig. 3
Unprocessed western blots of Extended Data Fig. 3e.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, W., Cheng, L., Li, K. et al. Evolutional heterochromatin condensation delineates chromocenter formation and retrotransposon silencing in plants. Nat. Plants 10, 1215–1230 (2024). https://doi.org/10.1038/s41477-024-01746-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-024-01746-4