Abstract
Nitrogen (N) is an essential macronutrient for plant development and, ultimately, yield. Identifying the genetic components and mechanisms underlying N use efficiency in maize (Zea mays L.) is thus of great importance. Nitrate (NO3−) is the preferred inorganic N source in maize. Here we performed a genome-wide association study of shoot NO3− accumulation in maize seedlings grown under low-NO3− conditions, identifying the ferredoxin family gene ZmFd4 as a major contributor to this trait. ZmFd4 interacts and co-localizes with nitrite reductases (ZmNiRs) in chloroplasts to promote their enzymatic activity. Furthermore, ZmFd4 forms a high-affinity heterodimer with its closest paralogue, ZmFd9, in a NO3−-sensitive manner. Although ZmFd4 exerts similar biochemical functions as ZmFd9, ZmFd4 and ZmFd9 interaction limits their ability to associate with ZmNiRs and stimulate their activity. Knockout lines for ZmFd4 with decreased NO3− contents exhibit more efficient NO3− assimilation, and field experiments show consistently improved N utilization and grain yield under N-deficient conditions. Our work thus provides molecular and mechanistic insights into the natural variation in N utilization, instrumental for genetic improvement of yield in maize and, potentially, in other crops.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
118,99 € per year
only 9,92 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
Gene sequences and amino acid sequences used in this Article were collected from Maize GDB (maize; https://www.maizegdb.org/), Gramene (rice; http://ensembl.gramene.org/index.html) and TAIR10 (Arabidopsis; https://www.arabidopsis.org/). All data supporting the findings of this study are available within the main text or the supplementary information files. The reporting summary for this article is presented as a supplementary information file. Source data are provided with this paper.
References
Arnao, M. B., Hernández-Ruiz, J. & Cano, A. Role of melatonin and nitrogen metabolism in plants: implications under nitrogen-excess or nitrogen-low. Int. J. Mol. Sci. 23, 15217 (2022).
Vidal, E. A. et al. Nitrate in 2020: thirty years from transport to signaling networks. Plant Cell 32, 2094–2119 (2020).
Liu, Q. et al. Improving crop nitrogen use efficiency toward sustainable green revolution. Annu. Rev. Plant Biol. 73, 523–551 (2022).
Krapp, A. et al. Nitrate transport and signalling in Arabidopsis. J. Exp. Bot. 65, 789–798 (2014).
Xu, G., Fan, X. & Miller, A. J. Plant nitrogen assimilation and use efficiency. Annu. Rev. Plant Biol. 63, 153–182 (2012).
Liu, X., Hu, B. & Chu, C. Nitrogen assimilation in plants: current status and future prospects. J. Genet. Genomics 49, 394–404 (2022).
Hu, B. et al. Variation in NRT1.1B contributes to nitrate-use divergence between rice subspecies. Nat. Genet. 47, 834–838 (2015).
Fan, X. et al. A putative 6-transmembrane nitrate transporter OsNRT1.1b plays a key role in rice under low nitrogen. J. Integr. Plant Biol. 58, 590–599 (2016).
Wang, W. et al. Expression of the nitrate transporter gene OsNRT1.1A/OsNPF6.3 confers high yield and early maturation in rice. Plant Cell 30, 638–651 (2018).
Huang, X. et al. Natural variation at the DEP1 locus enhances grain yield in rice. Nat. Genet. 41, 494–497 (2009).
Sun, H. et al. Heterotrimeric G proteins regulate nitrogen-use efficiency in rice. Nat. Genet. 46, 652–656 (2014).
Gao, Z. et al. The indica nitrate reductase gene OsNR2 allele enhances rice yield potential and nitrogen use efficiency. Nat. Commun. 10, 5207 (2019).
Wu, J. et al. Rice NIN-LIKE PROTEIN 4 plays a pivotal role in nitrogen use efficiency. Plant Biotechnol. J. 19, 448–461 (2021).
Yu, J. et al. Enhanced OsNLP4-OsNiR cascade confers nitrogen use efficiency by promoting tiller number in rice. Plant Biotechnol. J. 19, 167–176 (2021).
Zhang, Z. S. et al. Rice NIN-LIKE PROTEIN 3 modulates nitrogen use efficiency and grain yield under nitrate-sufficient conditions. Plant Cell Environ. 45, 1520–1536 (2022).
Wang, Q. et al. Genetic variations in ARE1 mediate grain yield by modulating nitrogen utilization in rice. Nat. Commun. 9, 735 (2018).
Ma, X. et al. Linking glucose signaling to nitrogen utilization by the OsHXK7-ARE4 complex in rice. Dev. Cell 58, 1489–1501 (2023).
Hanke, G. & Mulo, P. Plant type ferredoxins and ferredoxin-dependent metabolism. Plant Cell Environ. 36, 1071–1084 (2013).
He, L. et al. Primary leaf-type ferredoxin 1 participates in photosynthetic electron transport and carbon assimilation in rice. Plant J. 104, 44–58 (2020).
Fukuyama, K. Structure and function of plant-type ferredoxins. Photosynth. Res. 81, 289–301 (2004).
Guan, X. et al. FdC1 and leaf-type ferredoxins channel electrons from photosystem I to different downstream electron acceptors. Front. Plant Sci. 9, 410 (2018).
Li, P. et al. A genetic relationship between nitrogen-use efficiency and seedling root traits in maize as revealed by QTL analysis. J. Exp. Bot. 66, 3175–3188 (2015).
Rodrigues, M. C. et al. Genotypic variation and relationships among nitrogen-use efficiency and agronomic traits in tropical maize inbred lines. Genet. Mol. Res. 16, gmr16039757 (2017).
Liu, F. et al. The long non-coding RNA T5120 regulates nitrate response and assimilation in Arabidopsis. New Phytol. 224, 117–131 (2019).
Chen, K. E., Chen, H. Y., Tseng, C. S. & Tsay, Y. F. Improving nitrogen-use efficiency by manipulating nitrate remobilization in plants. Nat. Plants 6, 1126–1135 (2020).
Wang, Y. et al. The nitrate transporter NRT2.1 directly antagonizes PIN7-mediated auxin transport for root growth adaptation. Proc. Natl Acad. Sci. USA 120, e2221313120 (2023).
Yang, X. et al. Characterization of a global germplasm collection and its potential utilization for analysis of complex quantitative traits in maize. Mol. Breed. 28, 511–526 (2011).
Li, H. et al. Genome-wide association study dissects the genetic architecture of oil biosynthesis in maize kernels. Nat. Genet. 45, 43–50 (2013).
Kimata, Y. & Hase, T. Localization of ferredoxin isoproteins in mesophyll and bundle sheath cells in maize leaf. Plant Physiol. 89, 1193–1197 (1989).
Matsumura, T. et al. A nitrate-inducible ferredoxin in maize roots. Genomic organization and differential expression of two non-photosynthetic ferredoxin isoproteins. Plant Physiol. 114, 653–660 (1997).
Onda, Y. et al. Differential interaction of maize root ferredoxin: NADP+ oxidoreductase with photosynthetic and non-photosynthetic ferredoxin isoproteins. Plant Physiol. 123, 1037–1045 (2000).
Hanke, G. T., Kimata-Ariga, Y., Taniguchi, I. & Hase, T. A post genomic characterization of Arabidopsis ferredoxins. Plant Physiol. 134, 255–264 (2004).
Xing, H. L. et al. A CRISPR/Cas9 toolkit for multiplex genome editing in plants. BMC Plant Biol. 14, 327 (2014).
Mao, H. et al. A transposable element in a NAC gene is associated with drought tolerance in maize seedlings. Nat. Commun. 6, 8326 (2015).
Wang, X. et al. Genetic variation in ZmVPP1 contributes to drought tolerance in maize seedlings. Nat. Genet. 48, 1233–1241 (2016).
Zhang, X. et al. Genetic variation in ZmTIP1 contributes to root hair elongation and drought tolerance in maize. Plant Biotechnol. J. 18, 1271–1283 (2020).
van Heerwaarden, J. et al. Genetic signals of origin, spread, and introgression in a large sample of maize landraces. Proc. Natl Acad. Sci. USA 108, 1088–1092 (2011).
Kistler, L. et al. Multiproxy evidence highlights a complex evolutionary legacy of maize in South America. Science 362, 1309–1313 (2018).
Uhlén, M., Forsberg, G., Moks, T., Hartmanis, M. & Nilsson, B. Fusion proteins in biotechnology. Curr. Opin. Biotech. 3, 363–369 (1992).
Gräslund, S. et al. Protein production and purification. Nat. Methods 5, 135–146 (2008).
Sekhon, R. S. et al. Genome-wide atlas of transcription during maize development. Plant J. 66, 553–563 (2011).
Sekhon, R. S. et al. Transcriptional and metabolic analysis of senescence induced by preventing pollination in maize. Plant Physiol. 159, 1730–1744 (2012).
Walley, J. et al. Integration of omic networks in a developmental atlas of maize. Science 353, 814–818 (2016).
Soltabayeva, A. et al. Early senescence in older leaves of low nitrate-grown Atxdh1 uncovers a role for purine catabolism in N supply. Plant Physiol. 178, 1027–1044 (2018).
Carillo, P. & Rouphael, Y. Nitrate uptake and use efficiency: pros and cons of chloride interference in the vegetable crops. Front. Plant Sci. 13, 899522 (2022).
Thomas, H. & de Villiers, L. Gene expression in leaves of Arabidopsis thaliana induced to senesce by nutrient deprivation. J. Exp. Bot. 47, 1845–1852 (1996).
Diaz, U. et al. Leaf yellowing and anthocyanin accumulation are two genetically independent strategies in response to nitrogen limitation in Arabidopsis thaliana. Plant Cell Physiol. 47, 74–83 (2006).
Wu, Q. et al. Transcription factor ZmEREB97 regulates nitrate uptake in maize (Zea mays) roots. Plant Physiol. 196, 535–550 (2024).
Zhuo, M., Sakuraba, Y. & Yanagisawa, S. Dof1.7 and NIGT1 transcription factors mediate multilayered transcriptional regulation for different expression patterns of NITRATE TRANSPORTER2 genes under nitrogen deficiency stress. New Phytol. 242, 2132–2147 (2024).
Martin, A. et al. Two cytosolic glutamine synthetase isoforms of maize are specifically involved in the control of grain production. Plant Cell 18, 3252–3274 (2006).
Cai, H. et al. Overexpressed glutamine synthetase gene modifies nitrogen metabolism and abiotic stress responses in rice. Plant Cell Rep. 28, 527–537 (2009).
Brauer, E. K. et al. Reappraisal of nitrogen use efficiency in rice overexpressing glutamine synthetase1. Physiol. Plant. 141, 361–372 (2011).
He, X. et al. The nitrate-inducible NAC transcription factor TaNAC2-5A controls nitrate response and increases wheat yield. Plant Physiol. 169, 1991–2005 (2015).
Lee, S. et al. Concurrent activation of OsAMT1;2 and OsGOGAT1 in rice leads to enhanced nitrogen use efficiency under nitrogen limitation. Plant J. 103, 7–20 (2020).
Wang, Q. et al. The Ghd7 transcription factor represses ARE1 expression to enhance nitrogen utilization and grain yield in rice. Mol. Plant 14, 1012–1023 (2021).
Huang, H. et al. Disease resistance to bacterial pathogens affected by the amount of ferredoxin-I protein in plants. Mol. Plant Pathol. 8, 129–137 (2007).
Lin, Y. et al. Overexpression of ferredoxin, PETF, enhances tolerance to heat stress in Chlamydomonas reinhardtii. Int. J. Mol. Sci. 14, 20913–20929 (2013).
Liu, J. et al. A deficiency in chloroplastic ferredoxin 2 facilitates effective photosynthetic capacity during long-term high light acclimation in Arabidopsis thaliana. Plant J. 76, 861–874 (2013).
Wang, M. et al. The major leaf ferredoxin Fd2 regulates plant innate immunity in Arabidopsis. Mol. Plant Pathol. 19, 1377–1390 (2018).
Lu, M. et al. Rice ferredoxin OsFd4 contributes to oxidative stress tolerance but compromises defense against blight bacteria. Crop J. 11, 1937–1942 (2023).
Zhu, S., Bin, S., Wang, W., Lu, S. & Yang, W. Exogenous arachidonic acid affects fucoxanthin biosynthesis and photoprotection in Phaeodactylum tricornutum. Mar. Drugs 20, 644 (2022).
Ji, D. et al. NADP+ supply adjusts the synthesis of photosystem I in Arabidopsis chloroplasts. Plant Physiol. 189, 2128–2143 (2022).
Yin, B., Li, T., Zhang, S., Li, Z. & He, P. Sensitive analysis of 33 free amino acids in serum, milk, and muscle by ultra-high performance liquid chromatography-quadrupole-orbitrap high resolution mass spectrometry. Food Anal. Methods 9, 2814–2823 (2016).
Bradbury, P. J. et al. TASSEL: software for association mapping of complex traits in diverse samples. Bioinformatics 23, 2633–2635 (2007).
Takahashi, M., Sasaki, Y., lda, S. & Morikawa, H. Nitrite reductase gene enrichment improves assimilation of NO2 in Arabidopsis. Plant Physiol. 126, 731–741 (2001).
Rozas, J. et al. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol. Biol. Evol. 34, 3299–3302 (2017).
Hudson, R. R. Generating samples under a Wright–Fisher neutral model of genetic variation. Bioinformatics 18, 337–338 (2002).
Acknowledgements
We thank X. Yang for providing published inbred lines and helping with data analysis; and S. Huang, C. Jiang, G. Bi, C. Liu and S. Zhang for helpful discussions. The transgenic maize lines were generated by the Center for Crop Functional Genomics and Molecular Breeding of China Agricultural University. This work was supported by grants from the National Key Research and Development Program of China (2021YFF1000500 to J.Z.), the National Natural Science Foundation of China (32170265 and 32441022 to J.Z.), the Chinese Universities Scientific Fund (2024TC084 to J.Z.), the Pinduoduo-China Agricultural University Research Fund (PC2024B01005 to J.Z.), the Hainan Provincial Natural Science Foundation of China (323CXTD379 to J.Z.), and the Central Guidance on Local Science and Technology Development Fund of Shanxi Province (YDZJSX2024D040 to C.T. and J.Z.).
Author information
Authors and Affiliations
Contributions
J.Z. designed the study. G.J., G.C. and Z.Z. performed most of the experiments. C.T. helped with field testing. Y.W. performed subcellular ___location analysis. J.L. performed ITC binding measurement. K.Z. performed the stable nitrogen isotope analysis with an isotope-ratio mass spectrometer. Xiaoyun Zhao performed LC–MS/MS analysis. Xiaoming Zhao performed the maize transformation. G.J., G.C., Z.Z., C.T., Y.W., J.L., Z.L., L.S., W.Y., Y.G., J.F., Z.G. and J.Z. analysed the data. G.J., J.F., Z.G. and J.Z. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Plants thanks Chengcai Chu, Xiangdong Fu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 ZmNCRG1 confers natural variation in shoot NO3− contents under low-NO3− conditions.
a, Ranked distribution of shoot NO3− contents across 340 maize inbred lines. All inbred lines were grown under 0.05 mM NO3− conditions for 15 d, and the shoot tissues were collected for NO3− content measurements. b, Local Manhattan plot showing the GWAS results in the region of ZmNCRG1 for shoot NO3− contents from seedlings grown under 0.05 mM NO3− conditions. An 800-bp genomic region (Chr2: 59.7228–59.7236 Mb) is shown. The horizontal dashed line represents the Bonferroni-adjusted significance threshold (−log10(P) = 5). Two adjacent SNPs (Chr2_59723461 and Chr2_59723508) showing significant association with shoot NO3− contents are highlighted in red. The locations of the leading SNPs (Chr2_59723461 and Chr2_59723508) and the structure of ZmNCRG1 (Zm00001d003797) are shown. Blue box represents the coding region.
Extended Data Fig. 2 Mutation of ZmFd4 does not affect fresh or dry weight and root-to-shoot NO3− distribution.
a, Genomic structure of the ZmFd4 locus and sequence analysis of ZmFd4 in three knockout mutants generated via CRISPR/Cas9. Blue box represents the coding region. The target sequences are shown in blue font, and the protospacer adjacent motif (PAM) is in orange font. Zmfd4-c1 harbors two 1-bp insertions (after 150 and 208 bp downstream of the ATG translation start codon, respectively); Zmfd4-c2 carries a complex mutation pattern with an 18-bp deletion (134–151 bp) and a 1-bp insertion (after position 208 bp); and Zmfd4-c3 harbors a 58-bp deletion (151–208 bp). b, c, Fresh weight (b) and dry weight (c) of the wild type and Zmfd4 mutants grown under 4 mM NO3− (NN) or 0.05 mM NO3− (LN) conditions. Data are shown as means ± s.d. (b, n = 21 plants; c, n = 19 plants). d, NO3− contents analyzed in the roots of 15-d-old wild-type and Zmfd4 seedlings grown under 4 or 0.05 mM NO3− conditions. Data are shown as means ± s.d. (n = 3 biologically independent samples). e, Shoot/root 15NO3− concentration ratio of the wild type and Zmfd4 mutants grown under 4 or 0.05 mM NO3− conditions. Plants were exposed to 15NO3− for 24 h. Data are shown as means ± s.d. (n = 6 biologically independent samples).
Extended Data Fig. 3 Genotyping of ZmFd4 in NIL plants generated by introgressing ZmFd4 allele from inbred line CIMBL144 into Dan340.
Genotyping the BC4F2 segregating plants carrying a homozygous ZmFd4CIMBL144 or ZmFd4Dan340 allele or heterozygous ZmFd4CIMBL144/ZmFd4Dan340.
Extended Data Fig. 4 Chloroplast-localized ZmFd4 interacts with ZmNiR2 and does not affect photosynthetic capacity.
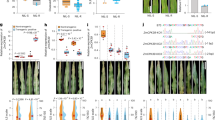
a, Subcellular localization of ZmFd4. Confocal images show the overlap of the ZmFd4-GFP signal with chlorophyll autofluorescence in maize protoplasts. Scale bars, 10 μm. b, Y2H assay showing that ZmFd4 interacts with ZmNiR2. c, In vitro pull-down assay showing that MBP-ZmNiR2 purified from E. coli can pull down GST-ZmFd4. d, LCI assay showing that ZmFd4 interacts with ZmNiR2 in N. benthamiana leaves. e, Co-IP assay showing that ZmFd4 associates with ZmNiR2 in maize protoplasts. f, g, Colocalization of ZmNiR1 (f) and ZmNiR2 (g) with chloroplast-localized ZmFd4 in N. benthamiana leaves. Scale bars, 10 μm. h–j, Chlorophyll fluorescence parameters of 15-d-old seedlings grown under 4 or 0.05 mM NO3− conditions: Fv/Fm (h), Y(II) (i), and Y(I) (j). Data are shown as means ± s.d. (h, i, n = 14 independent assays; j, n = 20 independent assays). k, P700 redox kinetics triggered by far-red illumination in 15-d-old seedlings grown under 4 or 0.05 mM NO3− conditions. l, Total C contents in the shoots of wild type and Zmfd4 mutants. Data are shown as means ± s.d. (n = 6 biologically independent samples). m, C/N content ratio of the wild type and Zmfd4 mutants. Data are shown as means ± s.d. (n = 6 biologically independent samples). n, Relative transcript levels of ZmNiRs in wild type and Zmfd4 mutants, as determined by RT–qPCR. Total RNA was isolated from shoots of 15-d-old seedlings grown under 0.05 mM NO3− conditions. Data are shown as means ± s.d. (n = 6 biologically independent samples). o, Shoot NO2− contents of wild-type and Zmfd4 plants grown under 0.05 mM NO3− conditions for 15 d. Data are shown as means ± s.d. (n = 3 biologically independent samples). A two-sided t-test was used to determine statistical significance.
Extended Data Fig. 5 ZmFds interact with ZmNiR2 and regulate NiR activity.
a, Y2H assay showing that ZmFd1, ZmFd2, ZmFd7, and ZmFd9 interact with ZmNiR2. b, In vitro pull-down assay showing that MBP-ZmNiR2 purified from E. coli can pull down GST-ZmFd1, GST-ZmFd2, GST-ZmFd4, GST-ZmFd7, and GST-ZmFd9. c, LCI assay showing that ZmFd1, ZmFd2, ZmFd7, and ZmFd9 interact with ZmNiR2 in N. benthamiana leaves. d, Co-IP assay showing that ZmFd9 associates with ZmNiR2 in maize protoplasts. e, Ability of recombinant His-ZmFds to stimulate NiR activity based on the consumption of NO2− by ferredoxin-dependent NiR in wild-type leaf extracts. Data are shown as means ± s.d. (n = 4 biologically independent samples). f, Immunoblot assay for the indicated fusion proteins in the LCI assays related to Fig. 5j, using an antibody recognizing full-length firefly LUC that reacts with N- (upper bands) and C-terminal (lower bands) firefly LUC fragments.
Extended Data Fig. 6 ZmFd4 and ZmFd9 compete with each other.
a, In vitro pull-down assay showing that ZmFd9 diminishes the interaction between ZmFd4 and ZmNiR2, and ZmFd4 also interferes with the interaction between ZmFd9 and ZmNiR2. Numbers below the lanes indicate the relative band intensities normalized to loading controls. b, Co-IP assay showing that ZmFd9 inhibits the association of ZmFd4 with ZmNiR2, and ZmFd4 weakens the association of ZmFd9 with ZmNiR2. Numbers below the lanes indicate the relative band intensities normalized to loading controls. c, LCI assay showing that ZmFd9 inhibits the interaction of ZmFd4 with ZmNiR2 (left image), and ZmFd4 also represses the interaction of ZmFd9 with ZmNiR2 (right image) in N. benthamiana leaves. Application of 4 mM NO3− attenuates the competition of ZmFd4 and ZmFd9 for ZmNiR2. Quantification of relative LUC activity is shown on the left and right graphs. Data are shown as means ± s.d. (n = 3 independent assays). Different lowercase letters indicate significant differences (P < 0.05; one-way ANOVA with Fisher’s LSD test). d, e, Immunoblot analyses of the indicated fusion proteins in the LCI assays related to Fig. 6c (d) and Extended Data Fig. 6c (e). An antibody recognizing full-length firefly LUC reacts with N- (lower bands) and C-terminal (upper bands) firefly LUC fragments. f, Effect of co-incubating ZmFd4 and ZmFd9 on the activity of ZmNiRs, as analyzed in an in vitro system. The concentration of recombinant ZmFds and ZmNiRs used for enzymatic assays was 12 µM. Data are shown as means ± s.d. (left graph: n = 4 biologically independent samples; right graph: n = 6 biologically independent samples). Different lowercase letters indicate significant differences (P < 0.05; one-way ANOVA with Fisher’s LSD test). g, Effect of ZmFd9 on NiR activity, as analyzed in a semi-in vitro system employing enzyme extracts derived from the wild type and Zmfd4 mutant, along with recombinant GST-ZmFd9. The concentration of ZmFd9 used for enzymatic assays was 12 µM. Data are shown as means ± s.d. (n = 6 biologically independent samples). Different lowercase letters indicate significant differences (P < 0.05; one-way ANOVA with Fisher’s LSD test). h, Redox potential of recombinant ZmFd4 and ZmFd9. Cyclic voltammograms were measured in 50 μM solutions of ZmFds versus a standard calomel electrode.
Extended Data Fig. 7 Characteristic analysis of different transgenic maize lines.
a, Genomic structure of the ZmFd9 locus and sequence analysis of ZmFd9 in three knockout mutants generated via CRISPR/Cas9. Blue box represents the coding region. The target sequences are in blue font, and the PAM is in orange font. Zmfd9-c1 harbors two 1-bp insertions (after 45 and 104 bp downstream of the ATG translation start codon, respectively); Zmfd9-c2 carries a 74-bp deletion (42–115 bp); and Zmfd9-c3 harbors two 1-bp insertions (after 42 and 103 bp, respectively). All three mutant alleles are predicted to produce truncated ZmFd9 proteins. b, Representative photographs of wild-type and Zmfd9 plants grown under 4 or 0.05 mM NO3− conditions for 15 d. Scale bars, 10 cm. c–e, Relative ZmNiR transcript levels (c), NiR activity (d), and shoot NO3− contents (e) in wild-type and Zmfd9 plants grown under 0.05 mM NO3− conditions. Data are shown as means ± s.d. (c, d, n = 6 biologically independent samples; e, n = 3 biologically independent samples). A two-sided t-test was used to determine statistical significance. f, Effect of ZmFd4 on NiR activity, as analyzed in a semi-in vitro system employing enzyme extracts derived from the wild type and Zmfd9 mutant, along with recombinant GST-ZmFd4. The concentration of ZmFd4 used for enzymatic assays was 12 µM. Data are shown as means ± s.d. (n = 4 biologically independent samples). Different lowercase letters indicate significant differences (P < 0.05; one-way ANOVA with Fisher’s LSD test). g, Genomic structure of the ZmFd4 and ZmFd9 loci and sequence analysis of the target sites in two double knockout mutant lines generated via CRISPR/Cas9. Blue boxes represent the coding regions. The target sequences are in blue font, and the PAM is in orange font. Zmfd4 Zmfd9-c1 harbors a 52-bp deletion (bases 441–492) in ZmFd4, and a C-to-T substitution (at 423 bp; synonymous mutation) and a replacement of a 9-bp fragment (439–447 bp) with a 10-bp sequence in ZmFd9. Zmfd4 Zmfd9-c2 carries a 2-bp deletion (after 446 bp) in ZmFd4 and a 1-bp insertion (after 445 bp) in ZmFd9. Both lines are predicted to produce frameshift proteins of ZmFd4 and ZmFd9. h–j, Relative ZmNiRs transcript levels (h), NiR activity (i), and shoot NO3− contents (j) in wild-type and Zmfd4 Zmfd9 plants grown under 0.05 mM NO3− conditions. Data are shown as means ± s.d. (n = 3 biologically independent samples). A two-sided t-test was used to determine statistical significance. k, Representative photographs of wild-type and Zmfd4 Zmfd9 plants grown under 4 or 0.05 mM NO3− conditions for 14 d. Scale bars, 10 cm. l, Relative ZmNiR2 transcript levels in wild-type and ZmNiR2 overexpression plants grown under 4 mM NO3− conditions. Data are shown as means ± s.d. (n = 3 biologically independent samples). A two-sided t-test was used to determine statistical significance. m, o, NiR activity (m) and shoot NO3− contents (o) in wild-type and ZmNiR2-OE plants grown under 0.05 mM NO3− conditions. Data are shown as means ± s.d. (m, n = 6 biologically independent samples; o, n = 3 biologically independent samples). A two-sided t-test was used to determine statistical significance. n, Representative photographs of wild-type and ZmNiR2-OE plants grown under 4 or 0.05 mM NO3− conditions for 16 d. Scale bars, 10 cm.
Extended Data Fig. 8 Field tests of agronomic traits of Zmfd4 knockouts.
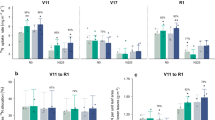
a–l, Plant height (a), ear leaf length (b), ear leaf width (c), days to heading (d), days to anthesis (e), days to silking (f), anthesis-silking interval (g), ear length (h), ear diameter (i), kernel length (j), kernel width (k), and kernel thickness (l) of the wild type and Zmfd4 mutants grown under normal- (NN) or low-N (LN) field conditions. Data are shown as means ± s.d. (a–i, n = 10, 9, 10, 10, 9, 10 plants for columns in order from left to right; j–l, n = 5 biologically independent samples).
Supplementary information
Supplementary Information
Supplementary Tables 1–4.
Supplementary Data
Supplementary Datasets 1–9.
Source data
Source Data Fig. 4
Unprocessed blots.
Source Data Fig. 5
Unprocessed blots.
Source Data Fig. 6
Unprocessed blots.
Source Data Extended Data Fig. 3
Unprocessed gels.
Source Data Extended Data Fig. 4
Unprocessed blots.
Source Data Extended Data Fig. 5
Unprocessed blots.
Source Data Extended Data Fig. 6
Unprocessed blots.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jia, G., Chen, G., Zhang, Z. et al. Ferredoxin-mediated mechanism for efficient nitrogen utilization in maize. Nat. Plants 11, 643–659 (2025). https://doi.org/10.1038/s41477-025-01934-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-025-01934-w