Abstract
Our study aimed to investigate the clinical characteristics of PD patients stratified by OH status before and after levodopa challenge to explore the hypothesis that OH might serve as a clinical marker for the body-first subtype of PD. Supine and standing blood pressure were measured in a large cross-sectional cohort of PD patients at the OFF status before and after levodopa challenge test (LCT). Based on OH status, patients were divided into three groups: spontaneous OH (SOH), only levodopa-induced OH (LOH) and non-OH (NOH). Clinical characteristics and associated factors were compared among the groups. A total of 928 patients with a mean age of 62.4 years and average disease duration of 7.9 years were included. There were 224 (24.1%) patients with SOH, 321 (34.6%) with LOH, and 383 (41.3%) with NOH. Compared to NOH, both SOH and LOH were associated with older age, motor fluctuations, and probable rapid eye movement sleep behavior disorder (pRBD). In addition, OH was more associated with cardiovascular and digestive dysfunction, disease severity and worse quality of life. Results of the current study suggest that PD patients developed OH which is more likely to comorbid with RBD, severe autonomic dysfunction and motor fluctuations, consistent with the body-first subtype of PD.
Similar content being viewed by others
Introduction
Parkinson’s disease (PD) is characterized mainly by loss of dopaminergic neurons residing in the substantia nigra and thought to be a primary brain disorder. Recently, it has be recognized that PD is highly heterogeneous and probably consists of several subtypes due to the nature of wide spreading of α-synuclein (α-syn) aggregations and non-motor symptoms1,2. Borghammer et al. hypothesized that PD comprises two subtypes including body-first subtype in which pathological α-syn originates from the peripheral nervous system (PNS) and brain-first subtype in which α-syn initiates in the central nervous system (CNS)3,4. PD patients with body-first and brain-first subtypes have various clinical characteristics4. However, there is currently no definite marker available that can clearly differentiate between body-first and brain-first subtype of PD.
Most of previous literatures distinguished these two subtypes of PD based on rapid eye movement sleep behavior disorder (RBD)-status5,6. However, according to α-syn origin and connectome model (SOC model) proposed4, α-syn pathology propagates from enteric to cardiovascular autonomic system, then to brain. Therefore, digestive, and cardiovascular autonomic dysfunctions are also the major characteristics of PD patients with body-first subtype. Orthostatic hypotension (OH), as the hallmark feature of cardiovascular and autonomic dysfunction, had been reported to be highly related to RBD7,8,9. The combination of OH and RBD reflects a ‘malignant’ phenotype of PD with early cognitive impairment and postural instability10. Multicenter prospective and large population-based studies also demonstrated that OH was a prodromal biomarker of α-synucleinopathies11,12,13. Therefore, OH may be used as a potential clinical marker for body-first subtype of PD. However, there are currently no clinical studies have specifically investigated this hypothesis.
OH often eludes detection in PD patients through single-shot measurements, frequently escaped from the notice of clinicians. As we all know, levodopa stands as the cornerstone and primary drug choice for PD patients14. Numerous studies have reported levodopa-induced OH in PD patients, particularly those with autonomic dysfunction15,16,17. Levodopa challenge test (LCT) is commonly used for evaluating responsiveness of levodopa in PD patients18, and simultaneously, blood pressure (BP) measurements can reveal OH induced by the loading dose of levodopa, shedding light on potential cardiovascular dysfunction. Hence, we aimed to investigate clinical subtypes of PD based on OH status (spontaneous, only levodopa-induced OH and non-OH), and associated clinical characteristics in a large cohort of patients with mid-later stage PD. We hypothesized that PD patients with spontaneous OH (SOH) and only levodopa-induced OH (LOH) were with similar clinical features and were both associated with the cardinal clinical features of PD patients with body-first subtypes.
Results
Demographic and clinical characteristics
The demographic and clinical characteristics were described in Table 1. The mean age of the subjects was 62.4 (±8.9) years old with a mean disease duration of 7.9 (±5.4) years. More than half of the subjects were with H&Y stage ≤2.5 (57.2%), motor fluctuations (57.4%), and FOG (47.7%). Almost one-quarter of patients (24.9%) had dyskinesias. The mean score of MDS-UPDRS-III was 54.2. More than half of the patients were classified as PIGD, and 30% patients fulfilled the criteria for the TD subtype based on motor symptoms.
Classification of PD subjects based on OH status
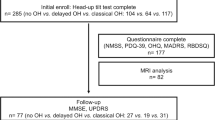
PD subjects were divided into three groups according to OH status. There were 545 patients (58.7%) had OH, among whom, 224 (24.1%) were found with SOH, 321 (34.6%) with LOH, and 383 (41.3%) with NOH. Most of the SOH patients (179, 80.0%) remained OH status during LCT (Fig. 1), and there were no significant differences in clinical characteristics between LOH and non-LOH subgroups among these SOH patients except the formers were with higher dose of LED, more dyskinesias, anxiety, and depression (Supplementary Table 1). Whereas only dyskinesias (OR 2.70, 1.01–7.21) remained an independent risk factor for LOH in the multivariate logistic regression model (Supplementary Table 2).
Motor and non-motor symptoms between OH subtypes
In general, both SOH and LOH patients had older age, longer disease duration, higher LED usage, and MDS-UPDRS III scores compared with NOH patients (Table 1). Patients with motor fluctuations, dyskinesias, and FOG were found more likely to develop OH, particularly for LOH. However, the distribution of different subtypes of PD based on motor symptoms (e.g., TD and PIGD) was not different among the three groups.
As to non-motor symptoms, we found that PD patients with OH, especially those with SOH were more likely to develop pRBD, hallucination, cognitive impairment, constipation, and severe cardiovascular and digestive dysfunction as compared to those without (Table 1). Importantly, only patients with LOH were more susceptible to SH (Table 1). Multivariate logistic regression analyses finally proved that compared to NOH subgroup, both SOH and LOH groups were independently associated with a higher risk of older age (SOH OR 1.03, 1.00-1.06 and LOH OR 1.03, 1.00-1.05), motor fluctuations (OR 1.80, 1.07-3.04 and OR 2.07, 1.31-3.29) and pRBD (OR 2.96, 1.87-4.69 and OR 1.76, 1.19-2.61). In addition, SOH and LOH subgroup were also more likely to be affected by cardiovascular dysfunction (OR 1.12, 1.02-1.24) and digestive dysfunction (OR 1.07, 1.03-1.11), respectively (Table 2).
OH subtypes and quality of life
As shown in Table 3, patients with SOH and LOH achieved significantly higher scores in MDS-UPDRS total, part I, part II, part III as compared to NOH. These associations remained significant after adjusting for age and disease duration. PDQ-39 total scores and sub-scores differed across these three OH groups as well. To be more specific, SOH and LOH patients generally showed worse in total scores, mobility, activities of daily living, emotion and cognition sub-scores of PDQ-39 compared with NOH. In addition, SOH patients showed worse in stigma, communication and bodily discomfort sub-scores as compared to NOH, and worse in emotion and cognition score as compared to LOH. The associations between PDQ-39 and OH subtypes remained significant, except for bodily discomfort, even after adjusting for age and disease duration.
Discussion
The current study has investigated the subtypes of PD based on OH status and associated clinical features in a large cohort of PD patients. It demonstrated that PD patients can be divided into SOH, LOH, and NOH subtypes. Generally, PD patients with OH, comprising SOH and LOH, shared similar clinical characteristics, including older age, higher probability of cardiovascular and digestive dysfunctions, motor fluctuations, and pRBD, while LOH was also associated with SH. Both two kinds of OH patients experienced worse quality of life compared to NOH, particularly in mobility, activities of daily living, emotion, and cognition. While patients with SOH had more stigma and communication problems.
Our results demonstrated that 58.7% PD patients develop OH, which is strongly associated with pronounced cardiovascular and digestive dysfunctions. Patients with OH alongside gastrointestinal, genitourinary, or sudomotor abnormalities could receive a diagnosis of pure autonomic failure (PAF)19. According to longitudinal studies, a significant proportion of those with PAF eventually develop PD, DLB, or MSA11, establishing PAF as another prodromal synuclein disease state similar to iRBD. So early identification of nOH is crucial, however, detecting OH in the early stages of the disease is challenging, many individuals with OH may not exhibit postural intolerance and other symptoms, leading clinicians to overlook blood pressure monitoring, or they may find it difficult to detect delayed OH, which could represent the early form of OH, due to inadequate orthostatic measurement time. Also, OH is not easily identified through a single blood pressure measurement. In our cohort, we found that SOH occurred in 22.6% of early-stage (HY 1-2.5) and 26.1% of mid-later stages (HY 3-5) PD patients, which are similar to previous studies, whose OH occurred in 19.5% of early-stage and 32.8% of mid-later stage PD patients20. Importantly, our research highlights that the use of the LCT significantly improved the detection rate of OH. When the LOH was included, the prevalence of OH notably increased to 54.6% in early-stage and 64.1% in mid-later stage PD patients. The induction of OH by levodopa21 suggests that this medication may further disrupt postural BP regulation, thereby revealing potential cardiovascular dysfunction. We utilized LCT to induce OH, and multiple BP measurements after administering levodopa may help uncover potential OH episodes and identify those patients with potential autonomic dysfunction.
Our results also demonstrated the presence of RBD in PD was strongly associated with OH in the cohort, which were the same to previous cross-sectional studies9,10,22,23,24. It could be explained by studies of Kashihara et al. 25 which demonstrated almost all individuals with RBD show significant cardiac sympathetic denervation before any damage occurs to the nigrostriatal dopamine system. Our research also showed that besides RBD, OH patients have more severe postural instability, gait disturbance, and cognitive impairment, especially the SOH group. The same to our result, a longitudinal study reported the combination of OH and RBD (“OH-RBD cluster”) was associated with the malignant phenotype of PD characterized by more rapid progression of cognitive deficits and postural instability10. SOC model research has firmly established that RBD is an important clinical marker for body-first subtype of PD5,6. Besides RBD, the body-first subtype of PD experienced more non-motor symptoms (e.g. cognitive impairment, OH), rapid disease progression and worse quality of daily life in previous studies4,26. This is similar to our study, which showed that patients with OH, in addition to being associated with RBD, had higher MDS-UPDRS II and III scores, higher levels of cognitive impairment, and poorer quality of life. Therefore, our results suggested that OH, has the potential to become a noteworthy clinical marker for a body-first subtype of PD.
Previous studies about OH in PD patients failed to differentiate between populations with SOH and those with LOH20,27. Our study further proved that the presence of RBD in PD was strongly associated with both SOH (OR: 2.96, 95%CI, 1.87-4.69) and LOH (OR: 1.76, 95%CI, 1.19-2.61) in the cohort. Both SOH and LOH groups showed prominent impairment in motor symptoms and worsened quality of life, particularly in mobility, activities of daily living, emotion, and cognition impairment. Pablo-Fernandez et al. had reported that OH was associated with a more rapid disease progression and shorter survival in patients with an autopsy-confirmed diagnosis of PD28. In addition, anxiety and depression were independent risk factors for levodopa-induced OH in PD patients with SOH, further suggesting that patients with OH may have poor prognosis, in accordance with body-first subtype29. Another finding worth noting was that, compared to LOH patients, SOH ones demonstrated a significantly higher risk of RBD, but lower risk of digestive dysfunction. This implies that it is meaningful to differentiate SOH and LOH subgroup in future research about the PAF or SOC model. In the univariate analysis, cognitive impairment exhibited significant differences; however, the multiple regression analysis revealed no discernible disparity in cognitive impairment between the groups. It is potentially attributable to the population of the current study is a surgery-set cohort that cover all stages of PD patients rather than a pure early-stage PD population.
There are some limitations in our study. Our patients were mainly recruited from a prospective surgery-set cohort with comprehensive DBS therapy assessment. In the study of clinical biomarkers, the earlier stage of PD patients might be better candidates. Because the SOC model suggests the two subtypes are prone to showing more differences of clinical and imaging indicators in early-stage of PD patients and may converge because of the increasing amounts and dissemination of α-syn pathology during the progression of disease. However, our cohort that contained the whole stage of PD patients with 57.2% of 1-2.5 H-Y stage patients, might be more suitable for the real-world observative study. Further study will be focus on more early or prodromal phase PD populations to confirm our findings. Secondly, we primarily utilized questionnaires and scales to assess clinical symptoms of patients, lacking more objective examinations for confirming PD diagnosis and delineating disease progression. For instance, polysomnography diagnoses RBD, FDOPA PET and DaT SPECT evaluate motor symptom asymmetry, and MIBG assesses cardiac sympathetic function. However, more examination might lead to a reduction in the study population. Sub studies in a more precise cohort with PET or blood biomarker examination will be our future work. The current study is imperative for subsequent studies to validate these findings and future longitudinal study with a longer follow-up is also warranted.
In conclusion, this cross-sectional study with a large cohort of advanced-stage PD patients revealed that over half of PD patients could develop OH which is associated with pRBD, autonomic dysfunction, disease severity, cognitive and psychological symptoms, and worse quality of daily life. These clinical characteristics are consistent with the body-first subtype of PD. OH may be a noteworthy clinical marker of the body-first subtype of PD, and LCT may be a potential test to uncover this phenotype.
Methods
Study population
1353 patients who underwent LCT at the Parkinson and Movement Disorder Center of Xuanwu Hospital Capital Medical University and Beijing Ruian Rehabilitation Hospital from June 2017 to January 2020 were included, and the majority of the subjects were under the pre-operative evaluation. 928 patients fulfilled the diagnosis of “clinically probable PD” or “clinically established PD” according to Movement Disorders Society (MDS) Clinical Diagnostic Criteria for PD30 and had complete BP measurements were enrolled. All participants provided written informed consent which has been approved by the Research Ethics Committee of Xuanwu Hospital Capital Medical University.
LCT
Each patient took a regular morning levodopa equivalent dose (LED) plus 50% for the LCT after 12 hours drug-free overnight. LED was calculated using the method of Claire L et al. 31. Movement Disorders Society version of Unified Parkinson’s Disease Rating Scale Part III (MDS-UPDRS-III)32 and supine-to-standing test (STS) was performed according to guideline33 before and after levodopa administration at 30-min, 1-hour, 2-hour, 3-hour and 4-hour. BP was measured repeatedly in supine position and standing position for 3 min (Omron HEM-7124; Kyoto, Japan) in each STS.
OH assessment
OH was defined as a BP decrease no less than 20 mmHg in systolic blood pressure (SBP) and/or no less than 10 mmHg in diastolic blood pressure (DBP) for subjects without supine hypertension (SH)34, and no less than 30 mmHg in SBP and/or no less than 15 mmHg in DBP for subjects with SH after 3 mins of standing35. SH was defined as SBP ≥ 150 mmHg or DBP ≥ 90 mmHg in the supine position36.
PD patients were categorized into groups: spontaneous OH (SOH), only levodopa-induced OH (LOH) and non-OH (NOH). SOH was defined as OH occurred at baseline evaluation before levodopa intake; LOH was defined as OH occurred at any time points after taking levodopa among patients without SOH. NOH was defined as OH not occurred at any timepoints both before and after taking levodopa.
Clinical assessment
All patients received standardized assessments conducted by qualified examiners before the test. Demographic information including age, gender, and disease duration were collected. To assess the asymmetry of disease onset, we queried the history of unilateral versus bilateral symptom onset37. The non-motor symptoms of the cardiovascular system and digestive system were evaluated by item 1 and 6 of the Non-Motor Symptom Scale (NMSS), respectively38. Non-motor symptoms in daily living were assessed by MDS-UPDRS Part I (MDS-UPDRS-I). MDS-UPDRS Part II (MDS-UPDRS-II) was used to assess the impact of motor symptoms on activities of daily living, which was also evaluated with the 39-item Parkinson’s Disease Questionnaire (PDQ-39)39.
Anxiety40, depression41, probable RBD (pRBD)42, and excessive daytime sleepiness (EDS)43 were defined as the total scores of Hamilton Anxiety Rating Scale (HAMA) ≥ 740, Hamilton Depression Rating Scale (HAMD-17) ≥ 841, The Hong-Kong version of REM sleep behavior disorder questionnaire (RBDQ-HK) > 1842 and Epworth Sleepiness Scale (ESS) > 643, respectively. Sleep quality was assessed by the Parkinson disease sleep scale (PDSS)44. Cognitive impairment was defined as total scores of Mini-mental State Examination (MMSE) ≤ 26 and Montreal cognitive assessment (MoCA)<2645. Constipation was defined if NMSS-21 > 0. Hallucination was defined as MDS-UPDRS-II item 2 ≥ 1. Hyposmia was defined as 6-item Hyposmia Rating Scale <22.546.
Clinical subtypes
Patients were classified as exhibiting freezing of gait (FOG) based on a score ≥ 1 on the MDS-UPDRS-II item 13. Motor fluctuations and dyskinesias were defined as MDS-UPDRS-IV item 3 ≥ 1 and item 1 ≥ 1, separately. The revised Hoehn & Yahr (H&Y) scale was used for disease staging47. Based on H&Y, PD patients were divided into the following three subgroups: early-stage (H&Y ≤ 2.5), middle-stage (H&Y = 3) and advanced stage (H&Y > 3). The ratio of the mean tremor scores (MDS-UPDRS-II item 10 and MDS-UPDRS-III items 15 -18 divided by 11) to the mean postural instability and gait difficulty (PIGD) scores (MDS-UPDRS-II items 12 -13 and MDS-UPDRS-III items 10 -12 divided by 5) was used to define tremor dominant (TD) (ratio ≥ 1.15), PIGD (ratio ≤ 0.9), and indeterminate (ratios > 0.9 and < 1.15) subtypes of patients with PD48.
Statistical analysis
Data were first tested for normality. Then different subgroups were compared for clinical features with parametric (analysis of variance, student’s t-test, chi-square test, and Fisher’s exact test) or non-parametric statistical tests (Kruskal-Wallis and Mann-Whitney test) as appropriate. For multiple comparisons, Bonferroni’s correction was applied.
To explore the clinical features of subtype groups, multinomial logistic analyses were performed. Clinical features with P < 0.05 in univariate analysis were chosen to be entered into the multivariate regression model. Backward stepwise logistic regression was performed, with P-to-remove set at 0.10. Correlations between the LOH in patients with SOH and clinical features were evaluated by binary logistic regression analysis.
To assess the differences in the dependent variables (PDQ-39 and MDS-UPDRSI-IV) while adjusting for age and disease duration, multiple linear regression was employed. The independent variables were the OH groups: SOH, LOH, and NOH.
All statistical analyses were performed using IBM SPSS Statistics for Windows (version 26.0, IBM Corp). Two-tailed P values < 0.05 were considered significant.
Data availability
Anonymized data generated during the current study are available from the corresponding author upon reasonable request.
References
Sauerbier, A., Jenner, P., Todorova, A. & Chaudhuri, K. R. Non motor subtypes and Parkinson’s disease. Parkinson. Relat. Disord. 22, S41–S46 (2016).
Marras, C. & Chaudhuri, K. R. Nonmotor features of Parkinson’s disease subtypes. Mov. Disord.: Off. J. Mov. Disord. Soc. 31, 1095–1102 (2016).
Borghammer, P. & Van Den Berge, N. Brain-First versus Gut-First Parkinson’s disease: a hypothesis. J. Parkinson’s Dis. 9, S281–S295 (2019).
Borghammer, P. The α-Synuclein Origin and Connectome Model (SOC Model) of Parkinson’s disease: Explaining motor asymmetry, non-motor phenotypes, and cognitive decline. J. Parkinson’s Dis. 11, 455–474 (2021).
Horsager, J. et al. Brain-first versus body-first Parkinson’s disease: a multimodal imaging case-control study. Brain: J. Neurol. 143, 3077–3088 (2020).
Knudsen, K. et al. Asymmetric Dopaminergic dysfunction in brain-first versus body-first Parkinson’s disease subtypes. J. Parkinson’s Dis. 11, 1677–1687 (2021).
Kim, J. S. et al. Orthostatic hypotension and cardiac sympathetic denervation in Parkinson disease patients with REM sleep behavioral disorder. J. Neurol. Sci. 362, 59–63 (2016).
Pilotto, A. et al. Orthostatic hypotension and REM sleep behaviour disorder: impact on clinical outcomes in α-synucleinopathies. J. Neurol. Neurosurg. Psychiatry 90, 1257–1263 (2019).
Yin, K. et al. REM sleep behavioral disorder may be an independent risk factor for orthostatic hypotension in Parkinson’s disease. Aging Clin. Exp. Res. 34, 159–166 (2022).
Fereshtehnejad, S. M. et al. New clinical subtypes of parkinson disease and their longitudinal progression: a prospective cohort comparison with other phenotypes. JAMA Neurol. 72, 863–873 (2015).
Kaufmann, H. et al. Natural history of pure autonomic failure: A United States prospective cohort. Ann. Neurol. 81, 287–297 (2017).
Postuma, R. B., Gagnon, J. F., Pelletier, A. & Montplaisir, J. Prodromal autonomic symptoms and signs in Parkinson’s disease and dementia with Lewy bodies. Mov. Disord. 28, 597–604 (2013).
Schrag, A. et al. Prediagnostic presentations of Parkinson’s disease in primary care: a case-control study. Lancet Neurol. 14, 57–64 (2015).
Pursiainen, V. et al. Blood pressure and heart rate in Parkinsonian patients with and without wearing-off. Eur. J. Neurol. 14, 373–378 (2007).
Fabbri, M. et al. Response of non-motor symptoms to levodopa in late-stage Parkinson’s disease: Results of a levodopa challenge test. Parkinson. Relat. Disord. 39, 37–43 (2017).
Li, K. et al. Subthalamic nucleus stimulation and levodopa modulate cardiovascular autonomic function in Parkinson’s disease. Sci. Rep. 7, 7012 (2017).
Haapaniemi, T. H. et al. Levodopa, bromocriptine and selegiline modify cardiovascular responses in Parkinson’s disease. J. Neurol. 247, 868–874 (2000).
Saranza, G. & Lang, A. E. Levodopa challenge test: indications, protocol, and guide. J. Neurol. 268, 3135–3143 (2021).
Coon, E. A., Singer, W. & Low, P. A. Pure autonomic failure. Mayo Clin. Proc. 94, 2087–2098 (2019).
Hiorth, Y. H. et al. Orthostatic hypotension in Parkinson disease: A 7-year prospective population-based study. Neurology 93, e1526–e1534 (2019).
Liu, Z. et al. Acute effect of levodopa on orthostatic hypotension and its association with motor responsiveness in Parkinson’s disease: Results of acute levodopa challenge test. Parkinson. Relat. Disord. 115, 105860 (2023).
Arnaldi, D. & De Carli, F. Prediction of cognitive worsening in de novo Parkinson’s disease: Clinical use of biomarkers. Mov. Disord. 32, 1738–1747 (2017).
Sommerauer, M. et al. Evaluation of the noradrenergic system in Parkinson’s disease: an 11C-MeNER PET and neuromelanin MRI study. Brain: J. Neurol. 141, 496–504 (2018).
Postuma, R. B. et al. Cardiac autonomic denervation in Parkinson’s disease is linked to REM sleep behavior disorder. Mov. Disord. 26, 1529–1533 (2011).
Kashihara, K., Imamura, T. & Shinya, T. Cardiac 123I-MIBG uptake is reduced more markedly in patients with REM sleep behavior disorder than in those with early stage Parkinson’s disease. Parkinson. Relat. Disord. 16, 252–255 (2010).
Pavelka, L. et al. Body-first subtype of parkinson’s disease with probable REM-sleep behavior disorder is associated with non-motor dominant phenotype. J. Parkinson’s Dis. 12, 2561–2573 (2022).
Palma, J. A. et al. Orthostatic hypotension in Parkinson disease: how much you fall or how low you go?. Mov. Disord. 30, 639–645 (2015).
De Pablo-Fernandez, E. et al. Association of autonomic dysfunction with disease progression and survival in Parkinson disease. JAMA Neurol. 74, 970–976 (2017).
Pagano, G. et al. REM behavior disorder predicts motor progression and cognitive decline in Parkinson disease. Neurology 91, e894–e905 (2018).
Postuma, R. B. et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 30, 1591–1601 (2015).
Tomlinson, C. L. et al. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov. Disord. 25, 2649–2653 (2010).
Goetz, C. G. et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov. Disord. 23, 2129–2170 (2008).
Juraschek, S. P. et al. Association of history of dizziness and long-term adverse outcomes with early vs later orthostatic hypotension assessment times in middle-aged adults. JAMA Intern. Med. 177, 1316–1323 (2017).
Lahrmann, H. et al. EFNS guidelines on the diagnosis and management of orthostatic hypotension. Eur. J. Neurol. 13, 930–936 (2006).
Freeman, R. et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin. Auton. Res. 21, 69–72 (2011).
Gibbons, C. H. et al. The recommendations of a consensus panel for the screening, diagnosis, and treatment of neurogenic orthostatic hypotension and associated supine hypertension. J. Neurol. 264, 1567–1582 (2017).
Postuma, R. B. & Gagnon, J. F. Symmetry of Parkinson’s disease and REM sleep: one piece of the puzzle. Ann. Neurol. 69, 905 (2011).
Martinez-Martin, P. et al. International study on the psychometric attributes of the non-motor symptoms scale in Parkinson disease. Neurology 73, 1584–1591 (2009).
Jenkinson, C. et al. The Parkinson’s Disease Questionnaire (PDQ-39): development and validation of a Parkinson’s disease summary index score. Age Ageing 26, 353–357 (1997).
Matza, L. S. et al. Identifying HAM-A cutoffs for mild, moderate, and severe generalized anxiety disorder. Int. J. Methods Psychiatr. Res. 19, 223–232 (2010).
Zimmerman, M. et al. Severity classification on the Hamilton Depression Rating Scale. J. Affect. Disord. 150, 384–388 (2013).
Shen, S. S. et al. Validation study of REM sleep behavior disorder questionnaire-Hong Kong (RBDQ-HK) in east China. Sleep. Med. 15, 952–958 (2014).
Marinus, J. et al. Assessment of sleep and sleepiness in Parkinson disease. Sleep 26, 1049–1054 (2003).
Chaudhuri, K. R. et al. The Parkinson’s disease sleep scale: a new instrument for assessing sleep and nocturnal disability in Parkinson’s disease. J. Neurol., Neurosurg., psychiatry 73, 629–635 (2002).
Chun, C. T., Seward, K. & Patterson, A. Evaluation of available cognitive tools used to measure mild cognitive decline: a scoping review. Nutrients. 13, 3974 (2021).
Millar Vernetti, P. et al. Validation of a new scale to assess olfactory dysfunction in patients with Parkinson’s disease. Parkinson. Relat. Disord. 18, 358–361 (2012).
Goetz, C. G. et al. Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: status and recommendations. Mov. Disord. 19, 1020–1028 (2004).
Stebbins, G. T. et al. How to identify tremor dominant and postural instability/gait difficulty groups with the movement disorder society unified Parkinson’s disease rating scale: comparison with the unified Parkinson’s disease rating scale. Mov. Disord. 28, 668–670 (2013).
Acknowledgements
The authors thank all the nurses and patients for their dedication and contributions to this study. This study was supported by grants from the National Key R&D Program of China (No. 2021YFC2501200, No.2018YFC1312001, No. 2020YFC2007304), National Natural Science Foundation of China (No. 82201401) and Xuanwu Youth Development Project (No. QNPY2021011).
Author information
Authors and Affiliations
Contributions
S.M. and X.W. served as the primary contributors to the writing process, while X.W. and Y.L. were responsible for data collection and collation. C.H. conducted the statistical analysis. Z.T., W.M., C.H. and P.C. participated in the thorough review and verification of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mei, S., Wang, X., Mao, W. et al. Orthostatic Hypotension: a clinical marker for the body-first subtype of patients with Parkinson’s Disease. npj Parkinsons Dis. 10, 173 (2024). https://doi.org/10.1038/s41531-024-00787-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41531-024-00787-y