Abstract
Influenza B viruses pose a significant threat to global public health, leading to severe respiratory infections in humans and, in some cases, death. During the last 50 years, influenza B viruses of two antigenically distinct lineages (termed ‘Victoria’ and ‘Yamagata’) have circulated in humans, necessitating two different influenza B vaccine strains. In this study, we devised a novel vaccine strategy involving reciprocal amino acid substitutions at sites where Victoria- and Yamagata-lineage viruses differ, leading to the generation of ‘hybrid’ vaccine viruses with the potential to protect against both lineages. Based on antigenic characterization, we selected two candidates and assessed their protective efficacy in a ferret model. Notably, both recombinant HA proteins conferred enhanced protection against heterologous challenges compared to their respective wild-type antigens. These findings show the potential of our novel strategy to develop cross-lineage protective influenza B virus vaccines.
Similar content being viewed by others
Introduction
Infections by influenza A and B viruses are a major public health burden globally1. Most human infections are caused by influenza A viruses, but influenza B viruses are responsible for about one-third of all influenza cases, according to a study that included 149 countries and over 4.6 million influenza cases1. Influenza B virus was first detected in the 1940s and circulated as a single lineage until the mid-1970’s/early-1980’s, when two genetically and antigenically distinct lineages emerged: ‘Victoria’ (after B/Victoria/2/87) and ‘Yamagata’ (after B/Yamagata/16/88). Until the early 2000’s, viruses of the Victoria lineage were primarily restricted to Asia, whereas Yamagata-lineage viruses circulated worldwide. In the early 2000’s, viruses of the Victoria lineage started to spread worldwide, and influenza B viruses of both lineages co-circulated over the next decades (reviewed in ref. 2).
During the SARS-CoV-2 pandemic, travel restrictions and mitigation measures, such as physical distancing, handwashing, and mask-wearing, were implemented to reduce the spread of SARS-CoV-2. These measures also resulted in a major reduction in influenza cases. Since the discontinuation of the pandemic control measures, influenza A and influenza B/Victoria-lineage activity has rebounded, but no influenza B/Yamagata-lineage viruses have been reported since March 20203. Nevertheless, viruses of this lineage could still circulate at low levels in certain regions of the world and could re-emerge.
Traditionally, influenza vaccines have included three components, representing influenza A/H1N1, A/H3N2, and influenza B virus. However, vaccines directed against one of the influenza B virus lineages induce only low levels of cross-reactive antibodies against the other influenza B virus lineage. Between 2001 and 2011, the influenza B virus lineage selected for the human trivalent vaccine matched the dominant circulating lineages in only five of ten seasons (reviewed in refs. 4,5,6), resulting in low vaccine efficacy. Since 2012, the WHO has recommended the inclusion of both influenza B virus lineages in quadrivalent vaccine formulations; however, with the potential extinction of the Yamagata-lineage, recent recommendations are to remove it7.
Multiple efforts are underway to develop broadly protective vaccines against influenza A and B viruses. Antibodies recognizing the highly conserved stem region of HA are broadly reactive against several influenza A virus subtypes, or even against influenza A and B viruses (reviewed in ref. 8), but the stem epitopes are immunosubdominant. For influenza A viruses, several strategies have been explored to focus immune responses towards these immunosubdominant epitopes in the HA stem, including sequential immunizations with recombinant viruses possessing HA heads from different subtypes (to avoid immune-focusing on previously encountered HA head sequences)9,10,11,12,13,14,15. This strategy has also been explored for influenza B viruses16. In another study, broadly reactive antibodies were elicited in ferrets vaccinated with computationally optimized broadly reactive antigens (COBRAs)17. Recently, we developed ‘scrambled’ H3 HA with random mutations in immunodominant epitopes and found that immune responses were redirected towards conserved, immunosubdominant head epitopes18. However, whether these strategies would elicit protective antibodies in humans remains unknown. Here, we tested a different strategy: starting from representative Victoria- and Yamagata-lineage viruses, we introduced mutations into HA at amino acid positions at which the two viruses differ and selected mutants that shared antigenic characteristics of both lineages, theorizing that broadly reactive antibodies elicited by these antigens may provide protection against both lineages. Due to their increased range of protection, our novel vaccines may also provide benefits when there is an antigenic mismatch between the selected vaccine virus and the circulating strains, making this strategy attractive even if Yamagata-lineage viruses do not re-emerge.
Results
Generation of virus libraries and selection of mutant viruses
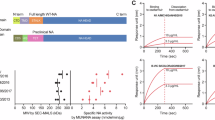
To generate influenza B vaccines that protect against viruses from both the Victoria- and Yamagata-lineages, we first compared the HA1 amino acid sequences of representative Victoria- and Yamagata-lineage viruses. We selected B/Phuket/3073/2013 (Phuket/Yam, the Yamagata-lineage vaccine strain since the 2017–2018 season) and B/Washington/02/2019 (Wash/Vic, the Victoria-lineage vaccine strain in the 2020–2022 southern hemisphere influenza season). The Wash/Vic and Phuket/Yam HA1 proteins differ by 39 amino acids in HA1, including a two-amino acid deletion in Wash/Vic (Fig. 1). Using commercial gene synthesis services, we synthesized two gene libraries (based on the Wash/Vic and Phuket/Yam HA1 sequences) that encoded either parental amino acid at each of the 37 respective amino acid positions (Table 1). No mutations were introduced at the amino acid deletion of Wash/Vic HA compared to Phuket/Yam HA. Thus, the synthetic HA1 gene libraries theoretically encoded 237 mutant HA genes. The amino acid changes in the Wash/Vic or Phuket/Yam HA1 regions could create structural and/or functional incompatibilities with HA2 or neuraminidase (NA). To account for this possibility, we employed molecular cloning techniques to first join the mutant Wash/Vic or Phuket/Yam HA1 fragments with Wash/Vic or Phuket/Yam HA2 sequences. We then used our established reverse genetics system19 to create virus libraries composed of the six internal genes of high-yield B/Yamagata/173 virus20, the NA genes of Wash/Vic or Phuket/Yam, and the Wash/Vic or Phuket/Yam HA libraries, resulting in eight virus libraries (Table 2). Forty-eight hours after transfecting cells with plasmids for virus library generation, the virus libraries were collected from plasmid-transfected human embryonic kidney (293 T) cells and inoculated onto hCK cells for plaque assays. Mutations at up to 37 amino acid positions of HA may result in many non-functional mutants; however, at the mutated positions, we started with only two amino acids that are known to be functional in the context of Wash/Vic or Phuket/Yam, respectively. Moreover, the strategy used here––reverse genetics followed by viral plaque assays––eliminated any non-viable mutants, which would not form virus plaques. Thus, while many mutants are theoretically possible, only viruses with a functional HA would be isolated from plaque assays.
a Shown are the HA amino acid differences between B/Phuket/3073/2013 (Phuket/Yam) and B/Washington/02/2019 (Wash/Vic). Dots indicate the same amino acid at this position; hyphens represent deletions at this position. b Three-dimensional structure of influenza B virus HA (Protein Structure Database, PDB code: 6FYW). Different HA trimers are colored in light green, blue, and beige. The amino acid differences in the HA1 subunits of Phuket/Yam and Wash/Vic are shown in red.
We isolated 384 virus plaques from Phuket/Yam HA1 virus libraries and 163 virus plaques from Wash/Vic HA1 libraries and established their HA sequences by Sanger sequencing. In total, we identified 217 different genotypes, 188 with a Phuket/Yam-based HA1 and 29 with a Wash/Vic-based HA1 (Supplementary Data 1).
All mutant viruses were tested with ferret sera raised against Wash/Vic and Phuket/Yam. Hemagglutination inhibition (HI) assays demonstrated that wild-type Phuket/Yam reacts poorly with serum to Wash/Vic and vice versa (Supplementary Data 1). Forty-four mutant viruses were antigenically Phuket/Yam-like, defined by an HI titer ≥80 against anti-Phuket/Yam serum and an HI titer ≤40 against anti-Wash/Vic serum. Seventy-one mutants were Wash/Vic-like, defined by an HI titer ≥80 against anti-Wash/Vic serum and an HI titer ≤40 against anti-Phuket/Yam serum. Sixty-nine mutants displayed HI titers of ≤40 against both ferret sera. Importantly, we also identified 33 mutants with HI titers ≥80 to both sera (Supplementary Data 1). These mutants retained reactivity with serum raised against the homologous parent virus and gained reactivity with serum raised against the heterologous parent virus; thus, these mutants may be antigenically located between the two influenza B virus lineages.
Antigenic cartography of influenza B viruses
Antigenic cartography21 is widely used to assess the antigenic properties of influenza viruses. To generate an antigenic map for influenza B viruses, we downloaded all available influenza B virus HA sequences from GISAID (>52,000) and generated a phylogenetic tree of the >8000 unique amino acid sequences by using RaxML. Using this tree, we classified all known influenza B virus HA sequences as either ‘Ancestral’, ‘Victoria’ or ‘Yamagata’. Based on the phylogenetic tree, we selected 36 viruses (4 ancestral, 18 Yamagata-, and 14 Victoria-lineage viruses), spanning several decades and including major sublineages. For the selected viruses, ferret sera were already available in our group or were generated by immunizing ferrets with live virus, followed by a boost with inactivated virus administered with adjuvant. Next, we determined the HI titers of the ferret sera against wild-type viruses and against the 33 influenza B mutants that reacted with ferret sera raised against Wash/Vic and Phuket/Yam (Supplementary Data 2). Since the mutant viruses differ in their HA2 and NA sequences (see Table 2), we also included recombinant control viruses in which wild-type Wash/Vic or Phuket/Yam HA1 sequences were combined with wild-type Wash/Vic or Phuket/Yam HA2 and/or NA sequences (Supplementary Data 2). Based on these HI titers, an antigenic map for wild-type and mutant influenza B viruses was generated (Fig. 2).
Viruses are depicted by circles; sera are depicted by squares. Sera generated against mutant viruses are depicted by small squares. Red, ancestral viruses; green, Victoria-lineage viruses; blue, Yamagata-lineage viruses. Mutant viruses are shown in light green (Victoria-lineage mutants) or light blue (Yamagata-lineage mutants), respectively. The two mutants selected for vaccination and challenge studies are indicated by red circles. Anti-sera generated against the two mutants are indicated by orange squares. The parent Wash/Vic and Phuket/Yam viruses are labeled and indicated by large circles in dark green or purple, respectively.
The antigenic map (Fig. 2) shows the antigenic separation of influenza B viruses into ancestral viruses (red circles; circulating before the split into the Yamagata- and Victoria-lineages), Yamagata-lineage viruses (blue circles), and Victoria-lineage viruses (green circles). The antigenically most advanced viruses are the Wash/Vic and Phuket/Yam vaccine viruses (shown in large blue and green circles, respectively, in Fig. 2) that were used as parent viruses in our study. The Wash/Vic and Phuket/Yam control viruses that differ in their HA2 and/or NA sequences are located close to each other in the antigenic map, confirming that the antigenic properties are primarily determined by amino acids in HA1 (Fig. 2).
Most mutant Wash/Vic viruses (Fig. 2, small light green circles) are antigenically different from the parent Wash/Vic viruses (Fig. 2, large dark green circles); compared with the wild-type Washington-lineage viruses (large- and mid-sized green circles), they are located closer to the Yamagata-lineage viruses (Fig. 2, blue circles). Similarly, most mutant Phuket/Yam viruses (Fig. 2, small light blue circles) are antigenically different from the parent Phuket/Yam viruses (Fig. 2, large blue circles) and compared to the wild-type Yamagata-lineage viruses, they located closer to the Wash/Vic viruses. Most of the mutant viruses are located between the two influenza B virus lineages.
Based on the HI titers and the ___location in the antigenic map, we selected seven Wash/Vic and seven Phuket/Yam mutants to generate ferret sera, which were then tested against wild-type viruses and selected mutant viruses (Supplementary Data 2). These data were integrated into the antigenic map (Fig. 2, small squares). Several of the sera raised against the mutant influenza B viruses displayed robust HI titers of 40 or higher against influenza B viruses from the homologous lineages but, importantly, also against influenza B viruses from the heterologous lineage.
Ferret immunization and challenge study
Based on the HI titers against wild-type and mutant viruses from both lineages (Supplementary Data 2) and their position in the antigenic map (Fig. 2), two mutants, namely Phuket/YamHA1-Phuket/YamHA2-Wash/VicNA-76 [Phuket/Yam-Mut, whose HA1, HA2, and NA are derived from Phuket/Yam (HA1 and HA2) and Wash/Vic (NA)] and Wash/VicHA1-Phuket/YamHA2-Phuket/YamNA-29 [Wash/Vic-Mut, whose HA1, HA2, and NA are derived from Wash/Vic (HA1) and Phuket/Yam (HA2 and NA)] (Fig. 2) were selected for immunization and challenge studies in ferrets. Ferrets (four per group) were immunized with recombinant HAs (used to avoid the contribution of other viral proteins to the immune responses) for Phuket/Yam-Mut, Wash/Vic-Mut, Phuket/Yam, or Wash/Vic (Fig. 3a). The ferrets were immunized with 15 µg of recombinant HA (with Alhydrogel® adjuvant 2%). Three weeks later, they received a booster with the same dose, followed by a second booster with the same dose eight weeks after the initial immunization. Additional animals were mock-vaccinated as controls. Sera collected five weeks after the second booster were tested for their reactivity against the four viruses tested here. Sera from ferrets immunized with recombinant wild-type Wash/Vic HA reacted with the parental viruses, but not with the HA of the other viruses (Fig. 3b). Immunization with recombinant wild-type Phuket/Yam HA elicited antibodies against the homologous virus, but also against the Phuket/Yam-Mut virus, indicating cross-reactivity. Likewise, sera from ferrets immunized with recombinant mutant Phuket/Yam-Mut HA reacted with Phuket/Yam virus. Moreover, immunization with recombinant mutant Phuket/Yam-Mut HA elicited low levels of cross-reactive antibodies against Wash/Vic-Mut virus, and vice versa (Fig. 3b).
a Immunization and challenge scheme. Ferrets were immunized three times with the indicated recombinant HA protein. Five weeks after the third immunization, ferrets were challenged with 106 pfu of wild-type Phuket/Yam or Wash/Vic virus. (Created in BioRender, Neumann, G (2024) https://BioRender.com/e49u244) (b) Ferret sera were collected 3 weeks after the third immunization (i.e., two weeks before challenge), and tested against Phuket/Yam, Wash/Vic, Phuket/Yam-Mut, and Wash/Vic-Mut viruses. c Virus titers in nasal swabs after challenge with wild-type Wash/Vic virus. d Virus titers in nasal swabs after challenge with wild-type Phuket/Yam virus. The values presented are the averages ±SD. P values were calculated by using a two-way ANOVA with multiple comparisons. Black asterisks indicate statistically significant differences between the PBS-treated control group and immunized animals. Blue asterisks (c) indicate statistically significant differences between the Phuket/Yam rHA-immunized group and any of the other immunized groups. Red asterisks (d) indicate statistically significant differences between the Wash/Vic rHA-immunized group and any of the other immunized groups. *P < 0.1; **P < 0.01; ***P < 0.001; ****P < 0.0001.
All immunized ferrets were challenged with 106 pfu of wild-type Wash/Vic or Phuket/Yam virus five weeks after the second boost (Fig. 3a). Nasal swab samples were collected each day for seven days post-challenge to assess virus replication (Fig. 3c, d). Additionally, the body temperature of infected ferrets was monitored daily for seven days; however, no significant differences were detected (Supplementary Fig. 1).
In mock-vaccinated animals, wild-type Wash/Vic and Phuket/Yam viruses replicated efficiently on Days 1 and 2 post-infection, whereas titers were lower on subsequent days and the infection was cleared by Day 7 post-infection (Fig. 3c, d). Virus titers after challenge were lowest in animals challenged with homologous wild-type virus, that is, in ferrets immunized with recombinant wild-type Wash/Vic HA and then challenged with wild-type Wash/Vic virus, or in ferrets immunized with recombinant wild-type Phuket/Yam HA and then challenged with wild-type Phuket/Yam virus. Immunization with recombinant wild-type Phuket/Yam HA followed by challenge with heterologous wild-type Wash/Vic virus reduced virus titers by 0.5–1.8 log units compared to mock-vaccinated animals, indicating low levels of cross-protection between the two influenza B virus lineages. Likewise, immunization with recombinant wild-type Wash/Vic HA followed by challenge with heterologous wild-type Phuket/Yam virus reduced virus titers by 0.9–1.5 log units compared to mock-vaccinated ferrets.
Importantly, immunization with recombinant mutant Phuket/Yam-Mut HA provided some protection against viruses from both lineages. Specifically, ferrets immunized with recombinant mutant Phuket/Yam-Mut HA were better protected against wild-type Wash/Vic challenge than ferrets immunized with recombinant wild-type Phuket/Yam HA (Fig. 3c, compare light and dark blue lines). Likewise, ferrets immunized with recombinant mutant Wash/Vic-HA were better protected against wild-type Phuket/Yam challenge than ferrets immunized with recombinant wild-type Wash/-Vic HA (Fig. 3d, compare light and dark red lines).
Bayesian inference of HA position effects
The Phuket/Yam and Wash/Vic mutants tested here differ by up to 39 positions in their HA1 amino acid sequences. We used a bespoke Bayesian model to identify HA positions with the greatest effect on HI titers (Fig. 4a, b). Briefly, effects were assigned to each individual HA position and for all pairs of amino acids. An HI titer between a virus and serum was then modeled as the sum of each position effect multiplied by the amino acid pair effect given the peptide sequences of the virus and virus used to generate the serum. Positive values in Fig. 4a, b indicate a positive effect on HI titers. The strongest effects were detected for amino acid positions 203, 133, and 149 (Fig. 4c).
a HA site effect sizes for all amino acid positions. Points show the posterior median effect size. Lines show the 95% highest density interval. b Top 10 effect sizes in (a). c Position effects colored on an HA monomer (PDB ID 4FQK3). Amino acid positions shown in gray were not variable in the dataset and therefore had no effect size estimated. The three positions with the highest effect sizes (133, 149, and 203) are labeled. The receptor-binding site is shown as a light red patch.
Discussion
Here, we devised a strategy involving reciprocal amino acid substitutions at sites at which Victoria- and Yamagata-lineage viruses differ to generate “hybrid viruses” Subsequently, two mutants, positioned antigenically between the two virus lineages, were tested as vaccine virus candidates in a ferret vaccination-challenge study. Recombinant HA proteins of both candidates demonstrated the ability to reduce virus titers and protect against challenges from both parental viruses when compared to mock-immunized animals. Importantly, the mutants tested here conferred stronger protection against heterologous challenge compared to the respective wild-type antigen (e.g., the mutant Phuket/Yam-Mut antigen provided better protection against Wash/Vic virus challenge than the wild-type Phuket/Yam antigen). Here, we used recombinant HA protein, but the mutants could also be presented in the context of live or inactivated influenza vaccine to stimulate potentially cross-protective immune responses against other influenza viral proteins. Our study was conducted with Alhydrogel®; because adjuvants affect the type and magnitude of immune responses; it will be important to evaluate other adjuvants for protective efficacy in combination with our vaccine candidates.
Recently, Fouchier and colleagues22 studied the genetic and antigenic relationships of influenza B viruses. They found that the Yamagata lineage emerged from early Victoria-lineage viruses, rather than from early (ancestral) influenza B viruses22. Consequently, some viruses previously classified as Yamagata-lineage viruses (based on phylogenetic trees) exhibited Victoria-lineage antigenic properties. This study also found that the amino acids at positions 149 and 203 of HA determine the influenza B virus lineage. Specifically, a Yamagata-lineage virus possessing HA-R149G and -N203K substitutions exhibited Victoria-lineage-like antigenic properties22. Interestingly, our modeling also identified HA amino acid positions 149 and 203 as having the greatest effect on HI titers, together with amino acid position 133 (see Fig. 4). Both mutants selected for our vaccination/challenge studies in ferrets encode the Wash/Vic amino acids at positions 149 and 203 but are located ‘between’ the lineages.
Kiseleva et al. 23 demonstrated that vaccines based on Victoria-lineage viruses protected ferrets better against challenge with Yamagata-lineage viruses than vice versa, suggesting that Victoria-lineage viruses may stimulate broader immune responses than Yamagata-lineage viruses. In our study, Phuket/Yam-Mut rHA (possessing 21 Victoria-like amino acids) provided slightly better protection against homologous and heterologous virus challenges than Wash/Vic-Mut rHA (possessing 19 Yamagata-like amino acids). However, more mutants would have to be tested to determine whether Yamagata-based HA with Victoria-like amino acid substitutions and Victoria-based HA with Yamagata-like amino acids differ in the breadth of immune responses they induce.
Several strategies for a universal vaccine for influenza B virus have been tested. For example, Ross and colleagues designed a computationally optimized broadly reactive antigen (COBRA) for influenza B viruses by analyzing sequences from 318 influenza B viruses isolated from 1940 to 201117. The COBRA antigen elicited HI and neutralizing antibodies against viruses from both influenza B lineages. In a vaccination and challenge study, none of the vaccinated ferrets (including those vaccinated with wild-type Victoria- or Yamagata-lineage viruses) exhibited virus replication after homologous or heterologous virus challenge17, so the protective efficacy of the COBRA antigen against virus replication could not be assessed. However, COBRA-designed vaccines have been shown to protect against strains that postdate the date-range of the input sequences, suggesting that this approach could provide protection against antigenically drifted viruses of the same subtype24,25,26,27.Two other strategies have been tested to redirect immune responses from the frequently mutating immunodominant HA head epitopes towards more conserved, immune-subdominant epitopes in HA. Krammer and colleagues16 tested chimeric HAs consisting of the HA stalk ___domain from an influenza B virus and HA globular head domains from influenza A subtypes, to which humans are not typically exposed. In a similar approach, the major antigenic epitopes of influenza B virus HA were replaced with sequences from ‘exotic’ influenza A viruses28,29, resulting in so-called ‘mosaic’ HAs. Both vaccines provided protection in the mouse model, which was conferred through Fc effector functions16,28,29. Although these methods can redirect the immune response towards the more conserved stalk of the HA protein, escape mutants can arise under immune pressure, identifying a potential barrier for this strategy as a universal influenza vaccine30. Pre-existing immunity plays an important role in shaping antibody responses in humans. Although it is difficult to capture the complexity of the immune history in humans, animal models with pre-existing immunity have been utilized (for example31,32,). Future studies could assess our hybrid recombinant proteins in a pre-immune animal model to determine if there are any differences in the breadth of protection provided compared to the naïve animal model.
Since March 2020, no Yamagata-lineage virus has been detected. However, Yamagata-lineage viruses could continue to circulate in remote areas and reemerge in the future. Importantly, it is also worth noting that our approach is not limited to cross-lineage vaccines and could be extended to develop broadly protective vaccines against novel Victoria-lineage viruses.
Methods
Viruses and cells
293 T human embryonic kidney cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal bovine serum. Humanized MDCK (hCK) cells (which express higher levels of α2,6- and lower levels of α2,3-linked sialic acids compared to parental MDCK cells33) were grown in minimal essential medium (MEM) containing 5% newborn calf serum in the presence of 2 μg/ml puromycin and 10 μg/ml blasticidin, and maintained in MEM containing 0.3% BSA and 1 μg/ml TPCK-trypsin after infection with influenza viruses. To generate recombinant B/Phuket/3073/2013 (Phuket/Yam, Yamagata-lineage) and B/Washington/02/2019 (Wash/Vic, Victoria-lineage) viruses, we employed reverse genetics19 to combine the viruses’ HA and NA viral RNA segments with the remaining viral RNA segments of a high-yield version of B/Yamagata/1/7320.
Construction of plasmid libraries
The HA1 proteins of Phuket/Yam and Wash/Vic differ by 39 amino acids (including two amino acid deletions in the Wash/Vic HA). HA mutagenesis was achieved by synthesizing two DNA fragment libraries (Synbio Technologies, Monmouth Junction, NJ, USA) based on Wash/Vic and Phuket/Yam that span HA amino acid positions 40–312 (numbers refer to the amino acid positions of mature Phuket/Yam), thus covering the 39 sites at which the two proteins differ. No mutations were introduced at amino acid positions 162 and 163, where Wash/Vic HA possesses a two-amino acid deletion compared to Phuket/Yam HA. At each of the remaining 37 sites, we designed codons to encode both amino acids present in Wash/Vic and Phuket/Yam. Thus, the synthesized DNA fragments theoretically encoded 237 different amino acid combinations. The two synthesized fragment libraries were amplified by PCR using high-fidelity DNA polymerase (PrimeSTAR Max DNA polymerase, Takara Bio, USA). We then constructed plasmid libraries by replacing the corresponding region of the RNA polymerase I-based plasmids19 of Wash/Vic and Phuket/Yam HAs with the PCR products of the synthesized fragments. To account for potential incompatibilities between HA2 and mutant HA1 proteins, we constructed four plasmid libraries in which Wash/Vic or Phuket/Yam HA2 proteins were combined with mutant Wash/Vic or Phuket/Yam HA1 proteins.
Generation of virus libraries
We used established reverse genetics approaches19 to combine each of the HA plasmid libraries with the NA viral RNA segments of Wash/Vic or Phuket/Yam, respectively, and the remaining viral RNA segments of high-yield B/Yamagata/1/73 virus20, resulting in eight virus libraries. To generate the virus libraries, the following plasmids for the transcription of viral RNA were mixed: 0.5 µg of mutant HA plasmid, 0.5 µg of NA plasmid, and 0.2 µg of the plasmids for each of the internal genes. In addition, four plasmids expressing the viral replication complex components (i.e., PB2, PB1, PA, and NP) and one plasmid expressing human airway trypsin-like protease34 were included in the mixture. These plasmids were transfected into 293 T cells by using the TransIT®-LT1 transfection reagent (Mirus, USA), following the manufacturer’s protocol. The 293 T cell supernatant was harvested 48 h post-transfection. All eight virus libraries were subjected to plaque assays in MDCK cells: 547 individual virus plaques were picked and their HA genes were Sanger-sequenced. In total, we identified 217 unique genotypes.
Virus purification for antiserum generation
The supernatant from virus-infected cells was harvested and centrifuged at 3000 rpm. It was then transferred to a new tube and ultracentrifuged at 18,500 rpm at 4 °C for 1.5 h. The pellet was reconstituted with 200 μl of phosphate-buffered saline (PBS) at 4 °C overnight. The reconstituted pellet was layered onto a sucrose density gradient (composed of sucrose concentrations of 50%, 45%, 40%, 35%, 30%, and 20%) within an ultracentrifuge tube. This setup was ultracentrifuged at 25,000 rpm at 4 °C for 2 h. The distinct virus bands were collected and transferred to a fresh ultracentrifuge tube containing PBS, followed by a secondary ultracentrifugation step at 25,000 rpm at 4 °C for 2 h. The resulting pellet was then reconstituted in PBS and stored at −80 °C for future use.
Phylogenetics
We added sequences of in-house virus isolates to a large HA phylogeny of influenza B viruses. The phylogeny was generated by downloading all influenza B virus HA sequences available on GISAID as of May 26, 2020. An alignment of unique sequences was generated using MAFFT35 and curated by hand to remove gaps introduced that were supported by single strains. A maximum likelihood tree search was conducted on amino acid sequences using the FLU + F + I model implemented in RAxML-NG36. The phylogeny was rooted using B/Lee/1940 as an outgroup. HA sequences of in-house virus isolates were subsequently added to the phylogeny using the default configuration of UShER37. Amino acid sequences (rather than nucleotide sequences as commonly used in phylogenetic trees) were used because this study focused on the effect of HA amino acid substitutions on antigenicity.
Generation of ferret antisera
Ferret antisera were generated by using six- to eight-month-old male ferrets (Triple F Farms, Gillett, PA, USA) that were serologically negative for circulating seasonal influenza A and B viruses. For each virus, one ferret was anesthetized with a mixture of Ketamine (4 mg/kg) and dexmedetomidine (20 µg) injected into the arm muscles, and once unconscious the ferret was intranasally inoculated with 105 or 106 plaque-forming units (pfu) of the desired virus in a volume of 500 μl (250 µl per nostril). Animals were injected with AntiSedan (200 µg) for recovery and monitored for convalescence. Three weeks later, ferrets with a hemagglutination inhibition titer of 320 or less against homologous virus were boosted with 100 μg of purified virus adjuvanted with an equal volume of TiterMax Gold Adjuvant (Sigma, St. Louis, MO, USA). The sera were collected two weeks after the boost by first anesthetizing ferrets with a mixture of Ketamine (6 mg/kg) and dexmedetomidine (50 µg) followed by euthanasia with Fatal-Plus (200 µl/kg) via intracardiac injection. Blood for sera collection was collected via the jugular vein.
Serum treatment
Ferret serum was treated with a three-fold volume of receptor-destroying enzyme (RDE; Denka Seiken Co.) at 37 °C for 18 h. The serum–RDE mixture was incubated at 56 °C for 1 h to denature the RDE. A six-fold volume of PBS and one-fold volume of pelleted turkey red blood cells (TRBCs) were added, and the mixture was incubated at room temperature for 1 h on a rotating mixer. After centrifugation at 3000 rpm at 4 °C for 5 min, the supernatants were aliquoted and stored at −80 °C for later use in HI assays.
Hemagglutination (HA) and hemagglutination inhibition (HI) assays
Hemagglutination assays were performed in 96-well V-bottom microtiter plates. Viruses were serially diluted two-fold with PBS and then mixed with 50 μl of 0.5% TRBCs. The plates were incubated at room temperature for 30 min. The HA titer is the highest dilution that agglutinates TRBCs. HI assays were conducted by evaluating the highest dilution of serum that prevents hemagglutination. RDE-treated sera were serially diluted two-fold with 25 μl of PBS in microtiter plates and mixed with 25 μl of virus containing 4 HA units, followed by incubation for 30 min at room temperature. After 50 μl of 0.5% TRBCs was added, the mixtures were incubated for 1 h at room temperature. The HI titers are the highest serum dilution that blocked TRBC agglutination. The limit of detection was 10; serum samples with titers below this limit were assigned a value of 5 for statistical analysis.
Antigenic cartography of selected mutants
The antigenic properties of mutant viruses were analyzed by using antigenic cartography as described previously21. Briefly, target antigenic distances between viruses and sera were calculated as Dij = b - log2(hij / 10), where hij is the titer of antigen i against serum j, and b is the log2 (titer/10) of the maximum titer against serum j. Antigens and sera were then positioned in a Euclidean space (a ‘map’) and positions were optimized using the error function (Dij – dij)2 summed over all pairs, where dij is the map distance. For further details see ref. 21. All maps were made based on 5000 optimization runs. Dimensionality testing was performed to determine the number of dimensions that best fit the titer data.
Recombinant protein expression and purification
Recombinant HAs (rHA) were constructed, comprising the signal peptide and ectodomain (amino acid residues 1–535 of full-length HA; Phuket/Yam numbering) of the HA protein. Stabilizing mutations (T35C and N412C) were introduced to facilitate the formation of disulfide bonds. Subsequently, a T4 foldon trimerization ___domain and a hexa-histidine tag were incorporated at the C-terminus38. These constructs were inserted into the pCAGGS protein expression plasmid39. The proteins were then expressed in Expi293F cells (Thermo Fisher Scientific) and purified by using TALON metal affinity resin (TaKaRa Bio USA).
Ferret vaccination and challenge study
In each group, four 4–6-month-old female ferrets were initially vaccinated with 15 µg of rHA of either Wash/Vic, Phuket/Yam, two mutant viruses, or PBS adjuvanted with Alhydrogel® adjuvant 2% (InvivoGen, San Diego, CA, USA). Three and eight weeks after the initial immunization, the ferrets were boosted with the same amount of rHA or PBS. Five weeks after the second boost, the ferrets were challenged under anesthesia with 106 pfu of recombinant Wash/Vic or Phuket/Yam virus, respectively. Nasal swabs were collected for seven days post-challenge under anesthesia as previously described. Virus titers in the nasal swabs were determined by performing plaque assays in hCK cells. All ferret experiments were performed by following the Animal Care and Use Committee guidelines of the University of Wisconsin—Madison (protocol number V6426).
Statistics and reproducibility
Differences in immunization groups
GraphPad Prism 9.3.1 was used to process and analyze the data. For the ferret immunization-challenge experiment, the values presented are the averages ± SD and the two-way ANOVA with multiple comparisons test was used to evaluate the statistical significance between different immunization group (*P < 0.1; **P < 0.01; ***P < 0.001; ****P < 0.0001).
Bayesian inference of HA position effects
We developed a Bayesian model to estimate the titer between a serum and antigen based on the sequence of the antigen and the sequence of the antigen used to raise the serum. The model decomposes a titer as a linear sum of sequence site effects crossed with amino acid pair effects. Amino acid pairs that match at a site for the serum and antigen are expected to increase titers and pairs that do not match are expected to decrease titers, although these expectations are not imposed as model constraints. For simplicity, amino acid pairs are treated symmetrically (i.e., a single term is used to represent the amino acid pair ‘AB’ as well as ‘BA’). Here, we applied this model to HA1 sequence positions 46–290. Threshold titers are handled appropriately as censored observations. As is typical in many datasets, titrations between some antigen-serum pairs were repeated. Importantly however, the model does not aggregate repeat titrations into a single titer. Instead, every raw titration is used, which allows different confidence depending on the number of times a titration was repeated.
8000 samples of the posterior distribution were sampled in eight chains using NUTS implemented in PyMC40. There were no issues sampling the posterior distribution: the mean R-hat of all parameters was 1.0001 (4 d.p.) and the maximum of any parameter was 1.0100 (4 d.p.); the mean bulk and tail effective sample size of all parameters were 7828 and 5351, respectively (0 d.p.), and the minimum values for any parameter were 915 and 991 (0 d.p.), respectively.
Data availability
All source data underlying animal and hemagglutination inhibition experiments described herein will be made available upon request to the corresponding author.
Code availability
Code used to generate Fig. 4 of this study is available upon request.
References
Iuliano, A. D. et al. Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet 391, 1285–1300 (2018).
Neumann G., Treanor J., Kawaoka Y. Orthomyxoviruses. in Fields Virology, vol. 1 (eds Knipe D. M., Howley P. M., Whelan S. P. J., Cohen J. I., Damania B., Enquist L., Freed E. O.) (Wolters Kluwer, 2021).
Dhanasekaran, V. et al. Human seasonal influenza under COVID-19 and the potential consequences of influenza lineage elimination. Nat. Commun. 13, 1721 (2022).
Belshe, R. B., Coelingh, K., Ambrose, C. S., Woo, J. C. & Wu, X. Efficacy of live attenuated influenza vaccine in children against influenza B viruses by lineage and antigenic similarity. Vaccine 28, 2149–2156 (2010).
Jennings, L. et al. Literature review of the epidemiology of influenza B disease in 15 countries in the Asia-Pacific region. Influenza Respir. Viruses 12, 383–411 (2018).
Tan, J., Asthagiri Arunkumar, G. & Krammer, F. Universal influenza virus vaccines and therapeutics: where do we stand with influenza B virus? Curr. Opin. Immunol. 53, 45–50 (2018).
WHO. 2024. Recommended composition of influenza virus vaccines for use in the 2024 southern hemisphere influenza season. https://www.who.int/publications/m/item/recommended-composition-of-influenza-virus-vaccines-for-use-in-the-2024-southern-hemisphere-influenza-season#:~:text=For%20quadrivalent%20egg%2D%20or%20cell,the%20B%2FYamagata%20lineage%20component%3A&text=a%20B%2FPhuket%2F3073%2F,Yamagata%20lineage)%2Dlike%20virus.
Fukuyama, H., Shinnakasu, R. & Kurosaki, T. Influenza vaccination strategies targeting the hemagglutinin stem region. Immunol. Rev. 296, 132–141 (2020).
Broecker, F. et al. A mosaic hemagglutinin-based influenza virus vaccine candidate protects mice from challenge with divergent H3N2 strains. NPJ Vaccines 4, 31 (2019).
Hai, R. et al. Influenza viruses expressing chimeric hemagglutinins: globular head and stalk domains derived from different subtypes. J. Virol. 86, 5774–5781 (2012).
Krammer, F. et al. H3 stalk-based chimeric hemagglutinin influenza virus constructs protect mice from H7N9 challenge. J. Virol. 88, 2340–2343 (2014).
Krammer, F. & Palese, P. Influenza virus hemagglutinin stalk-based antibodies and vaccines. Curr. Opin. Virol. 3, 521–530 (2013).
Krammer, F., Pica, N., Hai, R., Tan, G. S. & Palese, P. Hemagglutinin stalk-reactive antibodies are boosted following sequential infection with seasonal and pandemic H1N1 influenza virus in mice. J. Virol. 86, 10302–10307 (2012).
Margine, I. et al. Hemagglutinin stalk-based universal vaccine constructs protect against group 2 influenza A viruses. J. Virol. https://doi.org/10.1128/JVI.01715-13 (2013).
Nachbagauer, R. et al. A universal influenza virus vaccine candidate confers protection against pandemic H1N1 infection in preclinical ferret studies. NPJ Vaccines 2, 26 (2017).
Ermler, M. E. et al. Chimeric hemagglutinin constructs induce broad protection against influenza B virus challenge in the mouse model. J. Virol. 91, e00286–00217 (2017).
Carlock, M. A. & Ross, T. M. A computationally optimized broadly reactive hemagglutinin vaccine elicits neutralizing antibodies against influenza B viruses from both lineages. Sci. Rep. 13, 15911 (2023).
Chiba, S., Kong, H., Neumann, G. & Kawaoka, Y. Influenza H3 hemagglutinin vaccine with scrambled immunodominant epitopes elicits antibodies directed toward immunosubdominant head epitopes. mBio 14, e0062223 (2023).
Neumann, G. et al. Generation of influenza A viruses entirely from cloned cDNAs. Proc. Natl Acad. Sci. USA 96, 9345–9350 (1999).
Ping, J., Lopes, T. J., Neumann, G. & Kawaoka, Y. Development of high-yield influenza B virus vaccine viruses. Proc. Natl Acad. Sci. USA 113, E8296–E8305 (2016).
Smith, D. J. et al. Mapping the antigenic and genetic evolution of influenza virus. Science 305, 371–376 (2004).
Rosu, M. E. et al. Substitutions near the HA receptor binding site explain the origin and major antigenic change of the B/Victoria and B/Yamagata lineages. Proc. Natl Acad. Sci. USA 119, e2211616119 (2022).
Kiseleva, I. et al. Cross-protective efficacy of monovalent live influenza B vaccines against genetically different Lineages of B/Victoria and B/Yamagata in Ferrets. Biomed. Res. Int. 2018, 9695628 (2018).
Allen, J. D. & Ross, T. M. mRNA vaccines encoding computationally optimized hemagglutinin elicit protective antibodies against future antigenically drifted H1N1 and H3N2 influenza viruses isolated between 2018–2020. Front. Immunol. 15, 1334670 (2024).
Shi, H. et al. Inactivated influenza virus vaccines expressing COBRA hemagglutinin elicited broadly reactive, long-lived protective antibodies. Hum. Vaccin Immunother. 20, 2356269 (2024).
Ross, T. M. et al. A computationally designed H5 antigen shows immunological breadth of coverage and protects against drifting avian strains. Vaccine 37, 2369–2376 (2019).
Sautto, G. A., Ecker, J. W. & Ross, T. M. An H1N1 computationally optimized broadly reactive antigen elicits a neutralizing antibody response against an emerging human-infecting Eurasian Avian-Like Swine Influenza Virus. J. Virol. 95, e0242120 (2021).
Sun, W. et al. Development of influenza B universal vaccine candidates using the “Mosaic” Hemagglutinin approach. J. Virol. 93, e00333–19 (2019).
Liu, Y. et al. Mosaic hemagglutinin-based whole inactivated virus vaccines induce broad protection against influenza B virus challenge in mice. Front. Immunol. 12, 746447 (2021).
Park, J. K. et al. Pre-existing immunity to influenza virus hemagglutinin stalk might drive selection for antibody-escape mutant viruses in a human challenge model. Nat. Med. 26, 1240–1246 (2020).
Allen, J. D., Jang, H., DiNapoli, J., Kleanthous, H. & Ross, T. M. Elicitation of protective antibodies against 20 years of future H3N2 cocirculating influenza virus variants in ferrets preimmune to historical H3N2 influenza viruses. J. Virol. 93, e00946–18 (2019).
Chiba, S. et al. Ferret model to mimic the sequential exposure of humans to historical H3N2 influenza viruses. Vaccine 41, 590–597 (2023).
Takada, K. et al. A humanized MDCK cell line for the efficient isolation and propagation of human influenza viruses. Nat. Microbiol. https://doi.org/10.1038/s41564-019-0433-6 (2019).
Bottcher, E. et al. Proteolytic activation of influenza viruses by serine proteases TMPRSS2 and HAT from human airway epithelium. J. Virol. 80, 9896–9898 (2006).
Katoh, K. & Standley, D. M. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 30, 772–780 (2013).
Kozlov, A. M., Darriba, D., Flouri, T., Morel, B. & Stamatakis, A. RAxML-NG: a fast, scalable and user-friendly tool for maximum likelihood phylogenetic inference. Bioinformatics 35, 4453–4455 (2019).
Turakhia, Y. et al. Ultrafast Sample placement on Existing tRees (UShER) enables real-time phylogenetics for the SARS-CoV-2 pandemic. Nat. Genet. 53, 809–816 (2021).
Lee, P. S., Zhu, X., Yu, W. & Wilson, I. A. Design and structure of an engineered disulfide-stabilized influenza virus hemagglutinin trimer. J. Virol. 89, 7417–7420 (2015).
Niwa, H., Yamamura, K. & Miyazaki, J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108, 193–199 (1991).
Abril-Pla, O. et al. PyMC: a modern, and comprehensive probabilistic programming framework in Python. PeerJ Comput. Sci. 9, e1516 (2023).
Acknowledgements
We thank Susan Watson for scientific editing. This work was supported by NIAID grant 4R33AI159937-03. Ferret illustrations used in Figure 3a were created with BioRender.com.
Author information
Authors and Affiliations
Contributions
C.G., G.N., and Y.K. designed the study. C.G., S.C., P.J., and T.M. performed the experiments. L.B. and D.P. performed antigenic cartography. D.P. performed Bayesian inference. C.G. and G.N. wrote the manuscript. All authors reviewed and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
Y.K. has received grant support from Daiichi Sankyo Pharmaceutical, Toyama Chemical, Tauns Laboratories, Inc., Otsuka Pharmaceutical Co., Ltd., Shionogi & Co., Ltd., Otsuka Pharmaceutical, KM Biologics, Kyoritsu Seiyaku, Shinya Corporation, and Fuji Rebio. Y.K. and G.N. are co-founders of FluGen. The other authors have no conflicts of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Gu, C., Babujee, L., Pattinson, D. et al. Development of broadly protective influenza B vaccines. npj Vaccines 10, 2 (2025). https://doi.org/10.1038/s41541-024-01058-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41541-024-01058-w
This article is cited by
-
Nobel Prize in Chemistry 2024
Resonance (2025)