Abstract
Multiple PCV2 vaccines originating from different antigens and formula are commercially available and have shown great effectiveness in protecting pigs from clinical disease. However, our understanding of the immune mechanisms underlying these vaccine-induced protection is fairly limited, except for antibody responses. Head-to-head comparisons of T-cell responses induced by these vaccines in pigs would provide valuable insights into the mechanisms of protective immunity against PCV2. Here, T-cell responses in peripheral blood of pigs after vaccination with four representative PCV2 vaccines, as well as local and systemic recall responses following challenge with a PCV2d strain were examined. All four PCV2 vaccines induce a rapid cellular immune response that could be detected as early as 7 days post-vaccination. Some vaccine-primed CD4 T cells exhibit multifunctionality, being capable of secreting double (IFNγ/TNFα) and even triple cytokines (IFNγ/TNFα/IL-2) simultaneously. In contrast, a weak CD8 T cell response was also detected in the vaccinated pigs but just IFNγ/TNFα double producer and lack of cytotoxicity. These vaccine-activated CD4 and CD8 T cells displayed phenotypes of effector memory or terminally-differentiated effector memory T cells, which rapidly expand to subsequent PCV2d challenges. Prior-vaccinated pigs exhibited a stronger T cell cytokine response post-challenge, being most evident in the spleen. Notably, the cellular immune response induced by different types of PCV2 vaccines exhibited high similarity in phenotypic and functional properties, while showing significant differences in kinetics and magnitude. These results advance our understanding of cell-mediated immune protection afforded by different PCV2 vaccines and unravel fundamental differences in cellular immune response induced by PCV2 vaccines utilizing diverse technologies.
Similar content being viewed by others
Introduction
Porcine circovirus type 2 (PCV2), a small, non-enveloped, single-stranded circular DNA virus belonging to the genus Circovirus within the family Circoviridae, is the primary causative agent of porcine circovirus-associated diseases (PCVAD)1. Pigs infected with PCV2 alone rarely develop clinical disease2. However, PCV2 infection causes immunosuppression in pigs, thereby leading to increased susceptibility to other pathogens and impaired immune response to other vaccinations3. Co-infection of PCV2 with other pathogens, such as porcine reproductive and respiratory syndrome virus (PRRSV), porcine parvovirus (PPV), Mycoplasma hyopneumoniae (Mhp), etc., occurs frequently in swine herds, and results in more severe disease and higher mortality rates4. Currently, PCV2 has become a ubiquitous pathogen with a global footprint, causing severe economic losses to the swine industry worldwide.
The prevention and control of PCVAD heavily relies on vaccination. Over the last few decades, many vaccines for PCV2 have been developed and widely applied in swine herds. At present, there are five commercial PCV2 vaccines in the international market and at least ten available in the Chinese market5. These vaccines are classified into three types based on selected antigens and formula: inactivated whole virus vaccines, inactivated chimeric virus vaccines, and recombinant capsid-based subunit vaccines6. All types of PCV2 vaccines have been shown to improve growth performance, alleviate clinical signs of PCVAD, and reduce the impact of PCV2 infection on swine health6. However, current PCV2 vaccines are considered “leaky vaccines”, as they do not eliminate viral replication or transmission6,7, which may be a factor for the persistence of the virus in swine herds. Additionally, PCV2 has undergone genotype shifts two times in the past two decades, changing from PCV2a to PCV2b and then to PCV2d8. To date, PCV2d has emerged as the predominant genotype in many countries, including China8,9,10, whereas existing PCV2 vaccines have been developed based on PCV2a or PCV2b subtypes5,7. Although numerous studies show that these vaccines could provide sufficient cross-protection against PCV2d under experimental conditions5,7,11, the outbreaks of PCV2d-caused PCVAD have been reported in some vaccinated swine herds12,13,14, indicating these vaccines may not be capable of fully controlling heterologous PCV2 infection. Thus, a side-by-side comparative study of immune responses induced by current PCV2 vaccines is likely to be informative for understanding protective immunity against PCV2, thereby guiding the development of more potent and broader-spectrum vaccines.
Humoral immune response against PCV2 has been shown to play a critical role in safeguarding from subsequent infections15,16,17,18,19. Reductions in viral load have been reported to coincide with the emergence of virus-neutralizing antibodies18,19. The very low incidence of PCVAD in piglets younger than 4 weeks of age is also attributed to the protective effect of maternally derived neutralizing antibodies20,21,22. However, PCV2 viremia is frequently observed in seropositive pigs23,24, and vaccines can confer protection against PCV2 infection even without inducing antibody response or only eliciting low levels of antibody25,26, indicating that the protection against PCV2 infection may not rely solely on humoral immunity. Cellular immunity is an equally important arm of acquired protective immunity as humoral immunity, but current knowledge on the cellular immune response to PCV2 is sparse. Previous research indicated that both CD4 and CD8 T cells are involved in antiviral immune responses27, and PCV2-specific IFNγ-secreting cells (IFNγ-SCs) potentially contributed to viral clearance17,28,29. Later on, PCV2-specific IFNγ/TNFα-co-producing CD4 T cells were identified during natural infection and vaccination and are speculated to be a potential correlate of protection26. Importantly, evidence that implied the presence of cellular memory responses to PCV2 has emerged in some studies28,30. Overall, the existing data support the crucial role of cellular immune responses in PCV2 protective immunity.
The present study aimed to elucidate the magnitude, kinetics, and characteristics of cellular immune responses elicited by various types of PCV2 vaccines, with a particular focus on memory responses following challenges with heterologous strains. Four PCV2 vaccines produced using different types of antigens and adjuvants were selected for evaluation, including two capsid-based subunit vaccines (Jinyu Biotechnology, China and Boehringer Ingelheim, Germany), an inactivated chimeric virus vaccine (Zoetis, USA), and an inactivated whole virus vaccine (Huizhong Biotech, China). Multiple indicators of cellular immune response, such as IFNγ-SC response, T cell cytokine response, CD8 T cell cytotoxicity, T cell memory subsets, and T-cell proliferative capacity, were examined in the blood and tissues. Furthermore, we performed virological tests to assess the cross-protection offered by these vaccines against PCV2d. To our knowledge, this is the most comprehensive evaluation of cellular immune response to PCV2 thus far and provides critical insights into the mechanisms of protective immunity and may have implications for understanding vaccine-induced protection against other swine pathogens.
Results
PCV2-ORF2-specific IFNγ-SC responses induced by different vaccines exhibit distinct kinetics
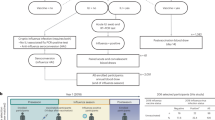
A range of immunological assays was performed at indicated time points to comprehensively evaluate the cellular immune response in blood and tissues (Fig. 1). Firstly, IFNγ-ELISPOT assays were conducted in peripheral blood mononuclear cells (PBMCs) upon restimulation with PCV2-ORF2 antigen to assess the overall magnitude and time course of the peripheral T-cell responses elicited by different vaccines (G1-G4). The results showed all four PCV2 vaccines induced rapid cellular immune responses in blood. Specifically, most vaccinated pigs (G1: 4/5, G2: 3/5, G3: 4/5, G4: 4/5) developed PCV2-ORF2-specific IFNγ-SC response at 7 dpv (Fig. 2a). Following vaccination, the frequency of PCV2-ORF2-specific IFNγ-SCs remained at basal level (lower than the LOD) in the Mock group, gradually increased over time in the G2 group, and peaked before declining in the G1, G3, and G4 groups (Fig. 2a, b). Consequently, the majority of pigs in the G2 group reached peaks of IFNγ-SC responses at 28 dpv, whereas most pigs in the G1, G3, and G4 groups peaked at 14 dpv (Fig. 2c). Consistent with the above observations, the frequency of PCV2-ORF2-specific IFNγ-SCs in the G1, G3, and G4 groups was significantly higher than that in the G2 group at 14 dpv (Fig. 2a, top panel).
Thiry SPF pigs were divided into six groups (5 pigs/group): Mock (tangerine), PCV2d (red), G1 (green), G2 (mustard), G3 (blue), and G4 (purple) group. The G1-G4 groups were vaccinated with different vaccines, while the PCV2d and Mock groups served as negative controls. At 28 dpv, the G1-G4 and PCV2d groups were challenged with PCV2d, while the Mock group remained unchallenged. Heparinized blood was collected weekly (0, 7, 14, 21, 28 dpv, and 7, 14 dpc) to prepare peripheral blood mononuclear cells (PBMCs) for different immunological assays at indicated time points. All pigs were euthanized at 42 dpv (14 dpc), and spleen and inguinal lymph node (ILN) were collected to isolate mononuclear cells (MNCs) for several immunological tests. This figure and all its elements were originally created by the authors using Adobe Illustrator and Microsoft PowerPoint without adaptation of copyrighted material.
PBMCs from different groups were prepared at indicated time points to enumerate the IFNγ-secreting cells (IFNγ-SC) following stimulation with PCV2-ORF2 antigen, with cRPMI-incubated cultures serving as blank controls. a Dynamic changes of the PCV2-ORF2-specific IFNγ-SC in PBMCs across all groups post-vaccination. Data was expressed as the number of IFNγ-SC/106 PBMCs after background subtraction. Individual pigs were shown as gray symbols with connecting lines. The dotted black line indicates the limit of detection (LOD) and the thick color-coded lines represent the mean at each time point post-vaccination. Statistical significance was analyzed using an unpaired two-tailed t-test with Welch’s correction when indicated. “Intergroup Comparison” on the top panel shows the difference between each time point in different groups, color-coded per comparison based on the group compared (Mock-tangerine, G1-green, G2-mustard, G3-blue, and G4-purple). P values < 0.1 are indicated in bold, and <0.05 are shown in underlined and bold. Responders on the top depict the number of pigs with positive responses at different time points. b Representative images of ELISPOT wells of each group at 0, 7, 14, 21, and 28 dpv. c Temporal patterns of peak IFNγ-SC responses across groups.
Taken together, these results indicated all four PCV2 vaccines effectively induce a peripheral T-cell response in pigs, while exhibiting distinct dynamic profiles.
PCV2-ORF2-specific multifunctional T-cell responses elicited by various vaccines share comparable features
In addition to the magnitude, the quality of T-cell response, defined as T cells that exert multiple effector functions simultaneously (multifunctionality), was shown to be very critical for vaccine-induced protection31. We therefore analyzed cytokine profiles of circulating CD4 (CD3+ TCRγδ− CD4+) and CD8 T cells (CD3+ TCRγδ− CD4− CD8α+) when stimulated with PCV2-ORF2 antigen by intracellular cytokine staining (ICS) at 14 and 28 dpv. The cytokine profile included seven cytokine-secreting subsets: single producers (IFNγ+ TNFα− IL-2−, IFNγ− TNFα− IL-2+, IFNγ− TNFα+ IL-2−), double producers (IFNγ+ TNFα+ IL-2−, IFNγ+ TNFα− IL-2+, IFNγ− TNFα+ IL-2+), and triple producer (IFNγ+ TNFα+ IL-2+).
PCV2-ORF2 specific CD4 T cell responses were detectable in more than half of pigs in each vaccinated group (Fig. 3b, d). Cytokine profiling identified IFNγ/TNFα double producer and IFNγ/TNFα/IL-2 triple producer as the most predominant cytokine-secreting subsets among PCV2-ORF2 specific CD4 T cells, with these cytokine secretion profiles remaining remarkably consistent across different vaccinated groups (Fig. 3a–d). Notably, the frequencies and responder numbers of double/triple cytokine-producing CD4 T cells decreased in the G1, G3, and G4 groups at 28 dpv compared to 14 dpv, whereas G2 group displayed a potential increase trend (Fig. 3b, d). These dynamic profiles paralleled the kinetics of PCV2-ORF2-specific IFNγ-SCs (Fig. 2a, c).
Intracellular cytokine staining of PBMCs isolated from 14 and 28 dpv was performed following restimulation with PCV2-ORF2 antigen, with cRPMI-incubated cultures as blank controls. Representative dot plots for IFNγ/TNFα double-positive and IFNγ/TNFα/IL-2 triple-positive CD4 T cells in PBMCs from different groups at 14 dpv (a) and 28 dpv (c). Comparison of single-, double-, or triple-positive cytokine responses in CD4 T cells at 14 dpv (b), and 28 dpv (d) among different groups. Each colored symbol represents background-subtracted data from one pig, with horizontal lines indicating mean ± standard deviation (SD). The limit of detection (LOD) is shown with dashed black lines. Responders on the top depict the number of positives within different groups. Statistical significance was analyzed using an unpaired two-tailed t-test with Welch’s correction when appropriate; ns non-significant, *P < 0.05, **P < 0.01.
CD8 T cell cytokine responses induced by these four vaccines were predominantly characterized by IFNγ/TNFα double producer, indicating a less differentiated state compared to CD4 T cells (Fig. 4a–c). The dynamics of CD8 T cell cytokine response (including the percentage and responders) were consistent with those of the CD4 T cell cytokine responses and IFNγ-SC responses in the G1, G2, and G3 groups, while an opposite trend was noted in the G4 group (Fig. 4b, c). Moreover, the cytotoxicity of CD8 T cells was also assessed at 14 and 28 dpv using a CD107a degranulation assay, and CD8 T cells from only three pigs (spread over three vaccine cohorts) at 28 dpv displayed cytotoxicity (Supplementary Fig. 1a, b).
At 14 and 28 dpv, intracellular cytokine staining of PBMCs was conducted after in vitro restimulation with PCV2-ORF2 antigen, with cRPMI-maintained cultures as blank controls. a Representative graphs for IFNγ/TNFα double-positive CD8 T cells in PBMCs from different groups at 14 and 28 dpv. Comparison of single-, double-, or triple-positive cytokine responses in CD8 T cells at 14 dpv (b), and 28 dpv (c) across distinct groups. Background-subtracted data are shown as scatterplots with individual samples (dots); the horizontal line represents the mean; error bars show standard deviations (SD); dashed black line denotes the limit of detection (LOD); Responders (top row) depicts the number of positives in diverse groups. P values were determined from an unpaired two-tailed t-test with Welch’s correction as appropriate.
These overall findings suggested that T cells elicited by four distinct PCV2 vaccines possess highly similar functional properties, and indicated that all four PCV2 vaccines induced multifunctional CD4 T cell responses and a comparatively weaker CD8 T cell response, albeit with variations in magnitudes and kinetics.
T cells evoked by distinct PCV2 vaccines display similar memory phenotypes
Having observed the presence of PCV2-ORF2-specific T cells in the PBMCs from vaccinated pigs, we set out to explore the memory phenotypes of these responding cells via multiparameter flow cytometry. Previous studies showed that porcine CD4 T cells can be sorted into three categories: naïve (CD4 TN cells, CD27+ CD8α−), central memory (CD4 TCM cells, CD27+ CD8α+), effector memory (CD4 TEM cells, CD27− CD8α+), and CD8 T cells can be divided into four subgroups: naïve (CD8 TN cells, CD27+ CD45RA+), central memory (CD8 TCM cells, CD27+ CD45RA−), effector memory (CD8 TEM cells, CD27− CD45RA−), terminally-differentiated effector memory (CD8 TEMRA cells, CD27− CD45RA+)32,33.
With the progression of time, a more pronounced decrease in the percentage of CD4 TN cells (Supplementary Fig. 2a, top panel) and a faster increase in the frequency of CD4 TEM cells (Fig. 5a, b, top panel) were found across four vaccinated groups compared to the Mock group. Although temporal fluctuations in CD4 TCM cells were observed (Supplementary Fig. 2b), intergroup comparisons revealed no significant differences (Supplementary Fig. 2b, top panel). Notably, the G2 group was the only vaccine cohort showing no significant difference in CD4 TEM cell levels from baseline at 14 dpv (p = 0.087; Fig. 5b), suggesting delayed differentiation of CD4 memory subsets in this cohort compared to other vaccine groups.
Prepared PBMCs were utilized to analyze the phenotypic changes in CD4 and CD8 T cells at 0, 14, and 28 dpv. (a) Representative dot plots of CD27 and CD8α expression on CD4 T cells in PBMCs from different groups at 0, 14, and 28 dpv. The values in the quadrants indicate the percentages of each CD4 T cell subset, with red-shaded areas depicting the percentage of CD4 TEM cells. Dynamic changes of CD4 TEM cells (b), CD8 TEM cells (c), and CD8 TEMRA cells (d) in PBMCs from different groups. Groups are color-coded: Mock in tangerine, G1 in green, G2 in mustard, G3 in blue, and G4 in purple. Gray symbols indicate individual samples, connected by gray lines. Color-coded bold lines represent the mean at each time point. Statistical significance of different groups at each time point was determined using an unpaired two-tailed t-test with Welch’s correction as appropriate and is shown as “Intergroup Comparison” on the top panel, color-coded based on the groups compared. 0.05 ≤ P values < 0.1 are shown as bold; P values < 0.05 are indicated by bold underline; P values < 0.001 are denoted as 0.000. Asterisks indicate significant intragroup differences between points in time (*P < 0.05, **P < 0.01, ***P < 0.001) as detected by paired t-tests.
Similarly, the percentage of CD8 T cells with TEM and TEMRA phenotypes increased quicker in the four vaccinated groups compared to the Mock group with increasing time (Fig. 5c, d, top panel), while the proportion of CD8 TN and TCM cells decreased more rapidly (Supplementary Fig. 2c–e, top panel). Notably, at 28 dpv, CD8 TEM cell frequencies in the G4 group demonstrated the least divergence from both the Mock group (p = 0.057; Fig. 5c, top panel) and its baseline (p = 0.055; Fig. 5c) across vaccine cohorts. Conversely, this group exhibited a higher percentage of CD8 TEMRA cells among four vaccine cohorts at this time point, showing significant differences from G2 and G3 groups (Fig. 5d, top panel), indicative of a greater propensity to the CD8 TEMRA phenotypes in the G4 group. Furthermore, at 14 dpv, intragroup comparisons revealed the G2 group exhibited the smallest divergence in CD8 TEM cell frequencies from baseline among vaccinated groups (Fig. 5c). Simultaneously observed TEMRA cell dynamics paralleled this pattern, with the G2 group maintaining proportionally smaller deviations from baseline despite two pigs showing elevated responses (Fig. 5d). These findings collectively suggest the G2 group exhibits slower kinetics in CD8 T cell memory subset alterations, comparable to those observed in CD4 T cell.
Overall, these findings suggested that CD4 TEM, CD8 TEM, and CD8 TEMRA cells, were all predominant among the T cell subsets induced by four different PCV2 vaccines.
PCV2 vaccines confer significant but incomplete cross-protection against PCV2d
Currently, PCV2d has displaced both PCV2a and PCV2b as the predominant genotype worldwide8,9,10. To evaluate the cross-protection of four vaccines, pigs in four vaccinated groups (G1-G4) and one unvaccinated group (PCV2d) were all challenged with CQ2302 PCV2d strain at 28 dpv, while the Mock group was used as the unvaccinated unchallenged control. After the challenge, none of the pigs exhibited clinical symptoms (data not shown) or rectal temperatures above 40 °C (Fig. 6a). Meanwhile, the weight gain of the PCV2d group was significantly lower than four vaccinated groups and the Mock group during 7–14 dpc (Fig. 6b), and PCV2d viremia and viral shedding (nasal and fecal) were detected only in PCV2d group (Fig. 6c–e), suggested that all four vaccines provide adequate protection from PCV2d infection. However, autopsies showed that the virus was still detectable in tissues (lung and ILN) from all vaccinated groups, indicating these vaccines did not completely clear the virus (Fig. 6f). These results revealed that all four PCV2 vaccines could provide sufficient cross-protection against PCV2d, but did not induce sterilizing immunity.
After the challenge, rectal temperature was measured daily, body weight was monitored at 0, 7, and 14 dpc, and viremia, viral shedding, and viral load of tissues were determined by detecting PCV2 DNA. Rectal temperature (a), average daily weight gain (b), viremia (c), fecal viral shedding (d), nasal viral shedding (e), and viral load of tissues (f) in distinct groups after the PCV2d challenge. Rectal temperatures are shown as means ± standard deviation (error bars), and above 40 °C (indicated by dashed line) are defined as fever. The average daily weight gain (ADWG) of individual animals is expressed as a color-coded symbol with midline and bars indicating means ± standard deviation. In (c–e), symbols represent the mean, the vertical bars indicate ± one standard deviation, and the numbers on the top of the error bar depict the percentage of positives in the PCV2d group at different time points. Data in (f) is expressed as per (b). Asterisks in (b, f) denote significant differences (*P < 0.05, ***P < 0.001) between the indicated groups, as determined by an unpaired two-tailed t-test with Welch’s correction as appropriate.
The proliferating T cell memory subsets following the challenge show high consistency across distinct vaccine cohorts
The recall response mediated by memory T cells is generally more rapid and vigorous than the primary response. Thus, we performed Ki-67 staining combined with memory subsets phenotyping to assess the magnitude of T-cell anamnestic response following the PCV2d challenge. At 7 dpc, proliferative responses in total CD4 T cells and distinct memory subsets showed no statistical differences between any challenged groups (G1-G4, PCV2d) and Mock group (Supplementary Fig. 3a–c), indicating a basal homeostatic proliferative state across all cohorts during this early phase. Divergence emerged by 14 dpc: the G1 group exhibited remarkably enhanced proliferation in total CD4 T cells compared to both Mock and PCV2d groups (Fig. 7b, Supplementary Fig. 3a). Phenotypic analysis identified CD4 TEM cells as the predominant proliferating populations among memory subsets, with their percentage significantly higher than that of the Mock group and the PCV2d group (Fig. 7a, c). Comparable trends were also noted in the G3 groups despite lacking statistical significance compared to the PCV2d group (Fig. 7b, c). Notably, only very few pigs in the G2/G4 groups displayed similar proliferative responses at this time point (Fig. 7b, c). These observations collectively indicate CD4 TEM cells dominate the secondary response of CD4 T cells while revealing recall response heterogeneity across vaccine groups.
PBMCs were isolated at 14 dpc to evaluate Ki-67 expression in different T cell subsets. a Representative dot plots of CD27 and CD8α expression on Ki-67+ CD4 T cells (left), and CD27 and CD45RA expression on Ki-67+ CD8 T cells (right) in PBMCs of each group at 14 dpc. Numbers in quadrants indicate the percentage of cells. Red-shaded and green-shaded areas depict the percentage of effector memory cells and terminally differentiated effector memory cells, respectively. b Comparison of the Ki-67 expression in CD4 T cells among groups at 14 dpc. c Comparison of the Ki-67 expression in CD4 TN cells, CD4 TCM cells, and CD4 TEM cells among groups at 14 dpc. d Comparison of the Ki-67 expression in CD8 T cells across groups at 14 dpc. e Comparison of the Ki-67 expression in CD8 TN cells, CD8 TCM cells, CD8 TEM cells, and CD8 TEMRA cells across groups at 14 dpc. Each colored symbol represents an individual animal; horizontal lines show the mean; error bars indicate standard deviation (SD). Asterisks denote statistically significant differences (*P < 0.05, **P < 0.01, ***P < 0.001) between the specified groups, as determined by an unpaired two-tailed t-test with Welch’s correction applied when appropriate.
Regarding the CD8 T cells, no significant differences in proliferation levels were detected between any vaccinated group and PCV2d group at examined time points (Fig. 7d, Supplementary Fig. 3d). However, further phenotypic analysis revealed different distribution patterns of memory subsets in proliferating CD8 T cell from vaccinated and PCV2d group (Fig. 7a, e). Specifically, at 14 dpc, compared to the Mock group, the proliferating CD8 T cell skewed memory phenotypes toward TEM in the PCV2d group but toward TEMRA in the four vaccinated groups (Fig. 7a, e). These differences in proliferating CD8 T cell subsets were further confirmed by comparing each vaccinated group with the PCV2d group (Fig. 7e), indicating the presence of memory CD8 T cell responses. Notably, the hierarchy of proliferating CD8 TEMRA cells across vaccine groups (Fig. 7e) was highly similar to those observed in proliferating CD4 TEM cells.
In conclusion, these findings suggested that prior vaccine-primed CD4 TEM and CD8 TEMRA cells mediate recall responses to the PCV2d challenge while revealing substantial heterogeneity in recall response magnitude across vaccine groups.
Vaccination may promote the functional enhancement of circulating T cells in response to the PCV2d challenge
To further evaluate the magnitude and quality of the secondary T cell response, a series of ex vivo immunological assays, including IFNγ-ELISPOT, ICS, and degranulation assay, were performed in PBMCs following restimulation with PCV2-ORF2 antigen. Pigs in the PCV2d group developed PCV2-ORF2-specific IFNγ-SC responses at 7 dpc that progressively increased over time, while secondary expansion of IFNγ-SCs was not evident in any vaccinated groups (Fig. 8a, b). In addition, the cytotoxicity of CD8 T cells was detected in two pigs from the PCV2d group but absent in all vaccinated pigs (Supplementary Fig. 1c, d). Of note, triple cytokine-secreting CD4 and CD8 T cells were only observed in vaccine cohorts, albeit limited to a few pigs (Fig. 8c, d). Importantly, the percentage or responder numbers of triple-producers showed a potential increase trend from 0 dpc (28 dpv) to 14 dpc (Figs. 3d, 4c, and 8c, d), being evident in the G1 and G3 groups which showed relatively superior proliferative response across vaccine cohorts.
The IFNγ-ELISPOT and intracellular cytokine staining assays were used to evaluate PCV2-ORF2-specific T-cell cytokine response in PBMCs after the PCV2d challenge. a Dynamic changes of the PCV2-ORF2-specific IFNγ-SC in PBMCs among all groups post-challenge. b Representative images of ELISPOT wells from each group at 7, 14 dpc. Comparison of single-, double-, or triple-positive cytokine responses in CD4 T cells (c) and CD8 T cells (d) at 14 dpc among groups. All data were background subtracted. The dotted black line indicates the limit of detection (LOD). Responders (top row) depict the number of pigs with positive responses. In (a), the thick color-coded lines show the group mean at each time point post-challenge and gray symbols represent individual animals with gray lines connecting identical animals. In (c, d), data are expressed as means ± SD with one symbol indicating one pig. Asterisks in (c, d) represent significant differences (*P < 0.05) between indicated groups, while “Intergroup Comparison” (bold: P < 0.01, bold and underlined: P < 0.05) on the top panel of (a) indicate the intergroup difference at different time points and are color-coded as follows: Mock (tangerine), PCV2d (red), G1 (green), G2 (mustard), G3 (blue), and G4 (purple). The statistical significance of data was analyzed using unpaired two-tailed t-tests with Welch’s correction when indicated.
Collectively, these findings indicated that prior vaccination did not enhance the cytokine-producing magnitude of peripheral T cells upon the PCV2d challenge. Instead, the enhancement of T cell quality observed in some pigs may be the hallmark of memory responses.
Vaccination enhances T-cell cytokine responses in secondary lymphoid tissues following the PCV2d challenge
Secondary lymphoid organs, including ILN and the spleen, are important sites for the activation of T cells, hence these tissues were collected during necropsy to analyze cellular immune responses. In the spleen, PCV2-ORF2-specific IFNγ-SCs were detected at similar frequencies between four vaccinated groups and PCV2d group (Fig. 9a, b). However, PCV2-ORF2-specific T cells displayed distinct cytokine expression profiles. Higher frequencies of triple-positive CD4 T cells were found in any vaccinated groups than in the PCV2d group, along with a greater number of responders, while the opposite was noted for single producers (Fig. 9c, d). IFNγ/TNFα double producer dominated among double cytokine-producing T cells, with no difference between the vaccinated groups and the PCV2d group (Fig. 9d). A comparable trend was also observed in CD8 T cells cytokine response (Fig. 9c, e), albeit less evident compared to CD4 T cells. In the ILN, PCV2-ORF2-specific IFNγ-SCs were also detected, with G1 and G3 groups showing higher frequency than others despite lacking statistical difference (Fig. 9a, b). Unfortunately, weak T-cell responses limited the analysis of cytokine-secreting profile data (Supplementary Fig. 4a, b). Additionally, the cytotoxicity of CD8 T cells was undetectable in both the spleen and ILN across all groups (Supplementary Fig. 1e).
The IFNγ-ELISPOT and intracellular cytokine staining assays were performed to assess PCV2-ORF2-specific T-cell cytokine response in the spleen and inguinal lymph node (ILN). a Representative images of ELISPOT wells in the spleen and ILN from each group. b PCV2-ORF2-specific IFNγ-SC responses in the spleen and ILN across groups. c Representative dot plots for IFNγ/TNFα/IL-2 triple-positive CD4 and CD8 T cells in the spleen from distinct groups. Comparison of single-, double-, or triple-positive cytokine responses in splenic CD4 T cells (d) and CD8 T cells (e) across groups. Colored dots represent background-subtracted data from individuals; horizontal lines display means; error bars indicate standard deviations. The dotted black line denotes the limit of detection (LOD). Responders (top row) show the number of pigs with positive responses. Asterisks denote significant differences (*P < 0.05, **P < 0.01) between the indicated groups as determined by an unpaired two-tailed t-test with Welch’s correction as appropriate.
Overall, the G1 group exhibited a relatively stronger cellular immune response in both the spleen and ILN, the G3 group displayed a slightly enhanced T cell response than the G4 group in the ILN, but a marginally weaker response in the spleen, while the G2 group showed the weakest cellular immune response in both tissues. Importantly, these results indicated that prior PCV2 vaccinations enhance T cell quality in the spleen to subsequent PCV2d challenge while exerting limited effects on cellular immune response in the ILN.
Discussion
PCV2 vaccines have achieved extraordinary success in protecting against infection and PCVAD, but several limitations persist, including incomplete viral clearance and insufficient cross-protection7,34,35. By evaluating a range of quantitative metrics of cellular immune responses, this study systematically elucidates the characteristics and differences of T-cell immunity induced by different types of PCV2 vaccines to heterologous PCV2d infection. As far as we know, this is the first study to conduct such a large-scale evaluation of T-cell immune responses to veterinary vaccines using such rich outcome indicators, and all samples collected from different vaccine cohorts were processed uniformly and analyzed concurrently on the same experimental platform to ensure the consistency and reliability of the data.
In the present study, mixtures (PCV2-ORF2 antigen) of capsid protein and peptide pools spanning capsid protein were used to identify antigen-specific T cells, as they amplify the signal of response, thereby improving detection sensitivity, compared to stimulation with either recombinant protein or peptide pools alone (data not shown). After a single vaccination, PCV2-ORF2-specific IFNγ-SCs can be detected as early as 7 dpv. However, this finding may be overestimated relative to the real field conditions since the data were obtained under strictly clean conditions using SPF pigs.
A prior report demonstrated that all pigs protected by vaccination developed antigen-specific IFNγ/TNFα co-secreting CD4 T cells, whereas less than half of pigs generated PCV2-specific antibodies, indicating that these T cell subsets may correlate with vaccine-mediated protection26. Regrettably, they neglected the detection of IL-2 production. Although IL‑2 has almost no direct effector functions, it promotes T cell proliferation and amplifies T‑cell responses, thereby is frequently combined with IFNγ and TNFα as a simple set of cytokines to assess T cell quality31. In this study, we found that PCV2 vaccine-induced CD4 T cells exhibited the capacity to concurrently secrete two and even three cytokines (IFNγ/TNFα/IL-2), with varying magnitude of multifunctional T cell responses across vaccines. These findings underscore both the functional diversity of CD4 T cells and their crucial role in PCV2 vaccine-induced immune protection. PCV2-specific CD8 T cells have been identified in PCV2 vaccinated/infected pigs using IFNγ-ELISPOT or T cell activation assay27,30, but detailed functional characterizations remained unknown. Our results showed that most vaccinated pigs developed antigen-specific CD8 T cells that co-produced two cytokines (IFNγ/TNFα) but rarely three cytokines (IFNγ/TNFα/IL-2), implying a relatively minor role of CD8 T cells compared to CD4 T cells. Unsurprisingly, cytotoxic CD8 T cells were scarcely detectable following vaccination, indicating the virus will be difficult to eradicate once infection is established. Considering the inherent limitations of inactivated/subunit vaccines in eliciting cellular immunity, the emergence of any CD8 T cell responses in this study is particularly noteworthy. The observed CD8 T cell activation is likely attributable to adjuvant-mediated cross-presentation enhancements, that was evidenced for squalene36 (adjuvant for G1 vaccine37) and carbomer38 (adjuvant for G2 vaccine37). Although the adjuvants in G3 and G4 vaccines remain undisclosed, their induction of CD8 T cell responses implies potential cross-presentation facilitation capabilities. Additionally, memory phenotype analysis revealed that all tested PCV2 vaccine-induced memory T cell subsets were characterized by CD27− T cells (TEM and TEMRA cells), contrasting with previous reports of a homogenous distribution of TEM and TCM cells within PCV2-specific CD4 T cells26. The reasons for this may be manifold, with the adoption of bivalent vaccines rather than monovalent ones and the porcine background potentially being key factors.
In this study, the G2 group exhibited a unique delayed cellular response in terms of IFNγ-SC response, multifunctional T cell response, and memory phenotype alterations. This dynamic divergence likely stems from adjuvant-specific effects, as the same capsid antigen formulated with different adjuvants (carbomer in G2 versus an undisclosed adjuvant in G4) elicited distinct response kinetics. The delayed response in the G2 group aligns with the retention and delayed release properties of carbomer39. Additionally, the G4 vaccine displayed distinctive immunodifferentiation capacity, manifested as a preferential propensity of CD8 T cell memory subsets towards TEMRA phenotypes at 28 dpv and enhanced CD8 T cell cytokine responses (despite being limited to some pigs) from 14 dpv to 28 dpv, which may also be attributable to adjuvant-specific effects.
To avoid potential underestimation of G2 vaccine efficacy due to its delayed kinetics, peak response comparison was carried out. The IFNγ-ELISPOT assay was prioritized for assessing the overall magnitude of T cell response due to its superior sensitivity in quantifying T cell response (including CD4 and CD8 T cells). Peak analysis revealed a ranked IFNγ-SC response across vaccine cohorts: G1 (410) > G3 (289) > G4 (214) ≈ G2 (191) at respective peak time points (14/28 dpv), demonstrating that the inactivated vaccines (G1/G3) induced moderately stronger cellular immunity compared to the subunit vaccines (G2/G4). This inactivated/subunit vaccine disparity aligns with prior findings37,40,41. The mechanisms underlying this disparity require further investigation, as concurrent variations in adjuvant and antigen content make it impossible to clearly attribute the difference solely to antigen types. Notably, the peak response rank of IFNγ-SC paralleled that of the CD4 T cell cytokine peak response (considering responder numbers and frequencies) across vaccine cohorts, but contrasted with CD8 T cell responses, suggesting limited contribution of CD8 T cell to IFNγ production in this study. Although triple-cytokine producer of CD8 T cells were identified in the G4 group, when integrating both overall response magnitude and positive responder numbers, the current data failed to find differences among these vaccines in inducing CD8 T cell responses. Additionally, the duration of T-cell responses remains undefined given the limited observation period, and the lack of ICS/cytotoxicity data at 7/21 dpv may have overlooked critical findings, necessitating extended longitudinal studies with increased sampling.
It is well known that PCV2 infection alone generally does not result in overt clinical disease2,26. Accordingly, no clinical signs of PCVAD were observed in either group after the challenge. Nevertheless, viremia, viral shedding, and reduced weight gain were evident in unvaccinated-challenged pigs, indicating the success of our experimental infection. Consistent with other studies11,40,42, our finding showed that PCV2 vaccines could confer adequate cross-protection against PCV2d but fail to induce sterilizing immunity.
Immunological memory is the foundation of nearly all vaccines. Several studies have hinted at the existence of the T-cell anamnestic response to PCV228,30, but its characteristics remain incompletely described. After the PCV2d challenge, the prior vaccine-primed CD4 TEM cells and CD8 TEMRA displayed vigorous proliferation, providing direct evidence for recall responses while indicating their potentially critical role in PCV2 vaccine-induced protection. The enhanced cytokine-producing T-cell response to secondary PCV2 infections is controversial across various reports26,28. Although the secondary expansion of PCV2-ORF2-specific IFNγ-SCs was not evident post-challenge in this study, triple-cytokine co-producing T cells showed a trend of progressive increase from 0 dpc (28 dpv) to 14 dpc in both frequencies and responder numbers. Notably, T cells from vaccine cohorts displayed higher multifunctionality in cytokine secretion than those from challenged-only groups. This trend was particularly evident in the spleen but weak in peripheral blood. These findings collectively demonstrate that PCV2 vaccine-primed T cells establish memory responses against PCV2d, and the observed enhancement in T cell multifunctionality may represent an early hallmark of the PCV2 memory response. However, given that the proliferative response did not occur until 14 dpc, our study may not have captured the peak of cellular memory responses.
In side-by-side comparisons of the cellular immune response following the PCV2d challenge, the G1 group consistently exhibited relatively superior responses across all parameters, including T-cell cytokine production and proliferation in peripheral blood, and T-cell cytokine production in the tissues. The G3 group demonstrated intermediate responses, whereas the G2 and G4 groups showed the weakest responses. These response hierarchies mirrored those observed in the primary responses. Notably, although substantial individual variability frequently leads to the lack of statistical difference between vaccinated groups, the consistent trends observed across both primary and secondary immune responses as well as multiple measurement indicators support the differences in cellular immune responses elicited by the four PCV2 vaccines.
Overall, our findings demonstrate that all four PCV2 vaccines provided adequate cross-protection against PCV2d, with T-cell responses induced by the distinct vaccines showing remarkably similarity in functional properties and memory phenotypes. Prior vaccination in pigs led to enhanced T-cell proliferation and cytokine responses following the PCV2d challenge. The observed characteristics in both primary and memory responses across various vaccines may represent a critical factor in vaccine-mediated protection against PCV2. The differences among these vaccines are reflected in the magnitude and kinetics of the immune response, which may be associated with both antigen types and adjuvants. These detailed immunological evaluations not only enhance the understanding of protective cellular immune response to PCV2 but also contribute to broader efforts in porcine vaccine development.
Materials and methods
Viruses and vaccines
Four commercial PCV2 and Mycoplasma hyopneumoniae (Mhp) bivalent vaccines were used in this study, including Fostera® Gold PCV MH (G1, Serial No: 626248B, Zoetis, USA), Ingelvac CircoFLEX®/Ingelvac MycoFLEX® (G2, Serial No: 3091661A/2730820A, Boehringer Ingelheim, Germany), Porcine Circovirus Type 2 and Mycoplasma Hyopneumoniae Vaccine, Inactivated (G3, Strain SH+Strain HN0613, Serial No: 230415, Huizhong Biotech, China), Porcine Circovirus Type 2 and Swine Mycoplasma Hyopneumoniae Vaccine, Inactivated (G4, Recombinant Baculovirus Strain DBN01 + Strain DJ-166, Serial No: 22510704, Jinyu Biotechnology, China).
PK-15 cells (free of PCV1) were cultured and maintained in Dulbecco’s minimum essential medium (DMEM) supplemented with 10% FBS (Gibco, USA) and 1% antibiotics (Coolaber, China), and CQ2302 PCV2d strain (GenBank accession no. PQ511330.1) were propagated and titrated in PK-15 cells following the standard protocol43.
Animal and experiment design
Thirty healthy 3-week-old specific-pathogen-free (SPF) Landrace pigs were purchased from Chundian Biotech Co., LTD (Qingdao, China), and housed in separate animal enclosure units with negative pressure ventilation in the experimental animal center of Zhongchong Sino Biotech Co., LTD (Taizhou, China). Upon arrival, piglets were acclimatized for 5 days, and then randomly assigned to six groups of five pigs. Pigs in the first 4 groups (G1–G4) were vaccinated with four commercial PCV2 and Mhp bivalent vaccines, respectively, via intramuscular injection following the doses recommended by manufacturers. Pigs in the fifth (PCV2d) and sixth (Mock) groups received an equivalent volume of sterile saline. At 28 days post-vaccination (dpv), pigs in the first 5 groups (G1–G4, and PCV2d) were intranasally (1 mL) and intramuscularly (1 mL) inoculated with CQ2302 PCV2d strain (105.0 TCID50/mL), while pigs in Mock group inoculated with PK-15 cell-culture supernatant in the same routes and volumes. After the challenge, all pigs were monitored daily for rectal temperature and clinical signs, and body weight was recorded at 0, 7, and 14 days post-challenge (dpc). At 14 dpc, in accordance with Laboratory Animal Guidelines for Euthanasia (T/CALAS 31-2017; Chinese Association for Laboratory Animal Sciences), all animals were humanely euthanized via intravenous injection of 20 mL saturated potassium chloride after being anesthetized with an intravenous injection of propofol (3 mg/kg). After confirmation of permanent cessation of circulation, lung and inguinal lymph nodes (ILN) were harvested for analysis of viral load and cellular immune responses. Additionally, blood samples were collected weekly after vaccination/challenge to assess T-cell immune responses, and serum, swabs (nasal and fecal) were collected at 0, 3, 7, 10, and 14 dpc to monitor viral load. A brief overview of our study design about T-cell immune responses is illustrated in Fig. 1.
Ethics statement
The animal experiment was approved by the Animal Welfare and Ethics Committee of Sino Technology with reference number SY2023018-Z, and all procedures were conducted strictly under the Guide for the Care and Use of Laboratory Animals.
Viral load and viral shedding measurement
The viral loads in serum, lung, ILN, and swab samples were quantified by a standard curve quantitative real-time PCR assay established in our previous study44. Briefly, the DNA was extracted from tissues (25 mg), serum (200 µL), and swab samples (200 µL) using the DNA Viral Genome Extraction Kit (Solarbio, China) according to the manufacturer’s instructions, and the real-time PCR was performed on a Light Cycler 480 instrument (Roche, Germany) with AceQ U+ Probe Master Mix (Vazyme, China), followed by calculation with the standard curve quantitation method.
Isolation of PBMCs and tissue mononuclear cells
The isolation of peripheral blood mononuclear cells (PBMCs) and tissue mononuclear cells (MNCs) was carried out as the method described in our previous reports45,46. Briefly, diluted heparin-anticoagulated blood (mixed with PBS at the ratio of 1:1) and tissue single-cell suspensions (recovered after passage through a 70 µm cell strainer) were gently layered over equal volumes of Ficoll-Paque (TBD science, China), followed by density gradient centrifugation at 500 × g for 30 min. The cloudy interphase layer containing MNCs was collected, pelleted, washed, and then resuspended in the cRPMI (RPMI-1640 containing 10% FBS and 1% antibiotics). The number of live MNCs was counted using a hemocytometer (Sigma, UK) with trypan blue (Solarbio, China), after which the cells were adjusted to a final concentration of 2 × 107 cells/mL.
IFNγ-ELISPOT assay
Porcine IFNγ-ELISPOT assays were performed as previously described with minor modifications47. In brief, MultiScreen-IP plates (Millipore, USA) were coated with anti‑porcine IFNγ mAb (10 µg/mL, pIFNγ-I, Mabtech, Sweden) overnight at 4 °C, and then blocked with the cRPMI for 2 hours at 37 °C. PBMCs or tissue MNCs (3 × 105 cells per well) were co-cultured in duplicates with either PCV2-ORF2 antigen or cRPMI alone (blank control). The PCV2-ORF2 antigen comprised two components: recombinant capsid protein (endotoxin-free, derived from E. coli, stock in PBS, 5 mg/mL) and overlapping peptide pools (21 peptides, 20-mer with 10-amino acid overlap, derived from cap protein, stock in DMSO/PBS mixture, 10 mg/mL per peptide). Both components were standardized to 2 µg/mL (protein/single peptide) in the final culture system, with total DMSO content maintained below 0.02% (v/v). After 24-h incubation at 37 °C, the plates were washed and biotinylated anti‑porcine IFNγ mAb (0.5 µg/mL, P2C11, Mabtech, Sweden) was added, followed by streptavidin‑HRP (1:1000, Mabtech, Sweden), and spots were visualized using TMB ELISpot substrate (Mabtech, Sweden). Plates were dried and spots were counted on a S6-Entry ImmunoSpot Analyzer (CTL, USA). Determinations from replicate tests were averaged, and ELISPOT data was adjusted to the number of IFNγ-secreting cells (IFNγ-SC) per million PBMCs/tissue MNCs after subtracting respective backgrounds (blank control). The limit of detection (LOD) for positive PCV2-ORF2-specific IFNγ-SC responses was calculated by mean + 3-fold SD of all mocks, and in this study, it was 30 IFNγ-SC/106 PBMCs or tissue MNCs.
Immunophenotypic analysis
Immunophenotyping of PBMCs was performed by staining for various cell surface markers as we previously reported45,46,47. PBMCs (2 × 106 cells) were stained with Fixable vital dye eFluor 780 (Thermo Fisher Scientific, USA), followed by incubation with the antibody cocktail (list of antibodies is provided in Supplementary Table 1) for 30 min. All stained cells were washed twice and resuspended in PBS for analysis by flow cytometry (FCM).
Intracellular cytokine staining
Cytokine secretion profile of antigen-specific T cells was assessed through intracellular cytokine staining (ICS) as described before45,47. 2 × 106 cells were seeded in 96-well U-bottom plates and stimulated with PCV2-ORF2 antigen (dose components see above) for 18 h, with the presence of Brefeldin A (5 μg/ml, Biolegend, USA) at the last 6 h of co-culture. Cells incubated with cRPMI alone were set as blank control. After incubation, cells were harvested, washed, and stained for surface markers using the aforementioned protocol, followed by fixed in 4% paraformaldehyde (PFA) for 10 min and permeabilized with 0.2% saponin for 20 min. Subsequently, cells were washed and incubated with fluorochrome-labeled intracellular antibodies for 30 min. Finally, cells were washed and resuspended in PBS for FCM analysis. A detailed description of mAbs used in this panel is summarized in the Supplementary Table 1.
Degranulation assay
To detect the degranulation of CD8 T cells (CTL), CD107a staining was performed following the procedure from our previous study47. Briefly, cells (2 × 106 cells/well) were stimulated with PCV2-ORF2 antigen (dose components see above) or mock stimulated (cRPMI-only, blank control) in the presence of anti-CD107a mAbs (clone 4E9/11, Bio-Rad, USA) for 18 h, with Brefeldin A (5 μg/ml, Biolegend, USA) and monensin (2 μM, Biolegend, USA) being added during the last 6 h. Afterward, cells were harvested for surface staining and FCM analysis by the procedures mentioned above. Details on the mAbs used for degranulation assay are presented in the Supplementary Table 1.
Proliferation analysis
Intranuclear staining of the proliferation marker Ki-67 was implemented to evaluate the proliferation of different T-cell subsets as previously above45,47. Simply put, after extracellular surface marker staining as the above protocol, cells were fixed and permeabilized using eBioscience Foxp3/Transcription Factor Staining Buffer Set (Thermo Fisher Scientific, USA) according to the manufacturer’s recommendation and then stained with anti-Ki-67 mAb (clone B56, BD, USA). Cells were washed and resuspended in PBS before FCM analysis. Detailed information about mAbs used in proliferation analysis is shown in the Supplementary Table 1.
FCM analysis
Flow cytometry measurements were performed on a CytoFLEX S flow cytometer (Beckman Coulter, USA). Compensation was adjusted using single-stained samples. At least 100,000 events within the live lymphocyte gate were collected for immunophenotypic analysis, and a minimum of 200,000 live lymphocytes were recorded for ICS, degranulation assay, or proliferation analysis. Data analysis was conducted using FlowJo software (Tree Star Inc., Ashland, OR), and a Boolean analysis was performed to calculate the percentage of antigen-specific CD4 and CD8 T cells producing only one, a combination of two, or all of three cytokines tested (IFNγ, TNFα, and IL-2). All gating strategies, based on fluorescence minus one (FMO) control, were adapted from our previous studies45,47. For ICS and degranulation assay, the frequency of antigen-specific T cells was calculated by subtracting background measured in the corresponding blank control, and antigen-specific T cell response was considered positive only if the value was higher than the LOD (mean plus 3-fold SD of all mock).
Statistical analysis
Statistical analyses were carried out using the SPSS statistics software (SPSS 23.0, IBM). Differences among groups were determined using two-tailed unpaired t-tests with Welch’s correction when indicated, and intragroup differences were assessed by paired t-tests. The statistical details of the experiments were provided in the respective figure legends. Results were considered statistically significant at P < 0.05 and displayed as *P < 0.05, **P < 0.01, ***P < 0.001. Details pertaining to significance were also noted in the respective figure legends.
Data availability
All the data generated or analyzed during the study are included in this published article. The datasets used and/or analyzed during the present research project are available from the corresponding authors upon reasonable request.
References
Meng, X.-J. Porcine circovirus type 2 (PCV2): pathogenesis and interaction with the immune system. Annu. Rev. Anim. Biosci. 1, 43–64 (2013).
Opriessnig, T. & Halbur, P. G. Concurrent infections are important for expression of porcine circovirus associated disease. Virus Res. 164, 20–32 (2012).
Segalés, J. Porcine circovirus type 2 (PCV2) infections: clinical signs, pathology and laboratory diagnosis. Virus Res. 164, 10–19 (2012).
Ouyang, T., Zhang, X., Liu, X. & Ren, L. Co-Infection of Swine with Porcine Circovirus Type 2 and Other Swine Viruses. Viruses 11, https://doi.org/10.3390/v11020185 (2019).
Guo, J. et al. Porcine Circovirus Type 2 Vaccines: Commercial Application and Research Advances. Viruses 14, https://doi.org/10.3390/v14092005 (2022).
Chae, C. Commercial porcine circovirus type 2 vaccines: efficacy and clinical application. Vet. J. (Lond., Engl. : 1997) 194, 151–157 (2012).
Franzo, G. & Segalés, J. Porcine Circovirus 2 Genotypes, Immunity and Vaccines: Multiple Genotypes but One Single Serotype. Pathogens 9, https://doi.org/10.3390/pathogens9121049 (2020).
Maity, H. K., Samanta, K., Deb, R. & Gupta, V. K. Revisiting Porcine Circovirus Infection: Recent Insights and Its Significance in the Piggery Sector. Vaccines 11, https://doi.org/10.3390/vaccines11081308 (2023).
Jia, Y. et al. Detection and genetic characteristics of porcine circovirus type 2 and 3 in Henan province of China. Mol. Cell. Probes 61, 101790 (2022).
Rudova, N. et al. Genetic Diversity of Porcine Circovirus 2 in Wild Boar and Domestic Pigs in Ukraine. Viruses 14, https://doi.org/10.3390/v14050924 (2022).
Park, K. H. et al. Evaluation of a porcine circovirus type 2a (PCV2a) vaccine efficacy against experimental PCV2a, PCV2b, and PCV2d challenge. Vet. Microbiol. 231, 87–92 (2019).
Opriessnig, T., Xiao, C.-T., Gerber, P. F. & Halbur, P. G. Emergence of a novel mutant PCV2b variant associated with clinical PCVAD in two vaccinated pig farms in the U.S. concurrently infected with PPV2. Vet. Microbiol. 163, 177–183 (2013).
Ramos, N., Mirazo, S., Castro, G. & Arbiza, J. First identification of Porcine Circovirus Type 2b mutant in pigs from Uruguay. Infect. Genet. Evol. 33, 320–323 (2015).
Seo, H. W. et al. Genetic and antigenic characterization of a newly emerging porcine circovirus type 2b mutant first isolated in cases of vaccine failure in Korea. Arch. Virol. 159, 3107–3111 (2014).
Shi, R., Hou, L. & Liu, J. Host immune response to infection with porcine circoviruses. Anim. Dis. 1, 23 (2021).
Trible, B. R. et al. Antibody recognition of porcine circovirus type 2 capsid protein epitopes after vaccination, infection, and disease. Clin. Vaccin. Immunol. 18, 749–757 (2011).
Meerts, P., Van Gucht, S., Cox, E., Vandebosch, A. & Nauwynck, H. J. Correlation between type of adaptive immune response against porcine circovirus type 2 and level of virus replication. Viral Immunol. 18, 333–341 (2005).
Meerts, P. et al. Correlation between the presence of neutralizing antibodies against porcine circovirus 2 (PCV2) and protection against replication of the virus and development of PCV2-associated disease. BMC Vet. Res. 2, 6 (2006).
Fort, M., Olvera, A., Sibila, M., Segalés, J. & Mateu, E. Detection of neutralizing antibodies in postweaning multisystemic wasting syndrome (PMWS)-affected and non-PMWS-affected pigs. Vet. Microbiol. 125, 244–255 (2007).
Ostanello, F. et al. Experimental infection of 3-week-old conventional colostrum-fed pigs with porcine circovirus type 2 and porcine parvovirus. Vet. Microbiol. 108, 179–186 (2005).
McKeown, N. E. et al. Effects of porcine circovirus type 2 (PCV2) maternal antibodies on experimental infection of piglets with PCV2. Clin. Diagn. Lab. Immunol. 12, 1347–1351 (2005).
Kekarainen, T. et al. Immune responses and vaccine-induced immunity against Porcine circovirus type 2. Vet. Immunol. Immunop 136, 185–193 (2010).
Rodríguez-Arrioja, G. M. et al. Dynamics of porcine circovirus type 2 infection in a herd of pigs with postweaning multisystemic wasting syndrome. Am. J. Vet. Res. 63, 354–357 (2002).
Sibila, M. et al. Use of a polymerase chain reaction assay and an ELISA to monitor porcine circovirus type 2 infection in pigs from farms with and without postweaning multisystemic wasting syndrome. Am. J. Vet. Res. 65, 88–92 (2004).
Fenaux, M., Opriessnig, T., Halbur, P. G., Elvinger, F. & Meng, X. J. A chimeric porcine circovirus (PCV) with the immunogenic capsid gene of the pathogenic PCV type 2 (PCV2) cloned into the genomic backbone of the nonpathogenic PCV1 induces protective immunity against PCV2 infection in pigs. J. Virol. 78, 6297–6303 (2004).
Koinig, H. C. et al. PCV2 vaccination induces IFN-γ/TNF-α co-producing T cells with a potential role in protection. Vet. Res. 46, 20 (2015).
Steiner, E., Balmelli, C., Gerber, H., Summerfield, A. & McCullough, K. Cellular adaptive immune response against porcine circovirus type 2 in subclinically infected pigs. BMC Vet. Res. 5, 45 (2009).
Martelli, P. et al. One dose of a porcine circovirus 2 subunit vaccine induces humoral and cell-mediated immunity and protects against porcine circovirus-associated disease under field conditions. Vet. Microbiol. 149, 339–351 (2011).
Fort, M. et al. Development of cell-mediated immunity to porcine circovirus type 2 (PCV2) in caesarean-derived, colostrum-deprived piglets. Vet. Immunol. Immunop. 129, 101–107 (2009).
Ferrari, L., Borghetti, P., De Angelis, E. & Martelli, P. Memory T cell proliferative responses and IFN-γ productivity sustain long-lasting efficacy of a Cap-based PCV2 vaccine upon PCV2 natural infection and associated disease. Vet. Res. 45, 44 (2014).
Seder, R. A., Darrah, P. A. & Roederer, M. T-cell quality in memory and protection: implications for vaccine design. Nat. Rev. Immunol. 8, 247–258 (2008).
Reutner, K. et al. CD27 expression discriminates porcine T helper cells with functionally distinct properties. Vet. Res. 44, 18 (2013).
Gerner, W., Mair, K. H. & Schmidt, S. Local and Systemic T Cell Immunity in Fighting Pig Viral and Bacterial Infections. Annu. Rev. Anim. Biosci. 10, 349–372 (2022).
Kekarainen, T. & Segalés, J. Porcine circovirus 2 immunology and viral evolution. Porcine Health Manag. 1, 17 (2015).
Karuppannan, A. K. & Opriessnig, T. Porcine Circovirus Type 2 (PCV2) Vaccines in the Context of Current Molecular Epidemiology. Viruses 9, https://doi.org/10.3390/v9050099 (2017).
Kim, E. H. et al. Squalene emulsion-based vaccine adjuvants stimulate CD8 T cell, but not antibody responses, through a RIPK3-dependent pathway. Elife 9, https://doi.org/10.7554/eLife.52687 (2020).
Seo, H. W., Lee, J., Han, K., Park, C. & Chae, C. Comparative analyses of humoral and cell-mediated immune responses upon vaccination with different commercially available single-dose porcine circovirus type 2 vaccines. Res. Vet. Sci. 97, 38–42 (2014).
Lee, W. & Suresh, M. Vaccine adjuvants to engage the cross-presentation pathway. Front. Immunol. 13, 940047 (2022).
Mishra, N. et al. in Smart Polymeric Nano-Constructs in Drug Delivery (eds Suresh P. Vyas, Udita Agrawal, & Rajeev Sharma) 151–186 (Academic Press, 2023).
Venegas-Vargas, C. et al. Cellular and humoral immunity following vaccination with two different PCV2 vaccines (containing PCV2a or PCV2a/PCV2b) and challenge with virulent PCV2d. Vaccine 39, 5615–5625 (2021).
Seo, H. W., Han, K., Park, C. & Chae, C. Clinical, virological, immunological and pathological evaluation of four porcine circovirus type 2 vaccines. Vet. J. 200, 65–70 (2014).
Opriessnig, T. et al. A commercial porcine circovirus (PCV) type 2a-based vaccine reduces PCV2d viremia and shedding and prevents PCV2d transmission to naïve pigs under experimental conditions. Vaccine 35, 248–254 (2017).
Tischer, I., Peters, D., Rasch, R. & Pociuli, S. Replication of porcine circovirus: induction by glucosamine and cell cycle dependence. Arch. Virol. 96, 39–57 (1987).
Chen, N. et al. A novel NADC30-like porcine reproductive and respiratory syndrome virus (PRRSV) plays a limited role in the pathogenicity of porcine circoviruses (PCV2 and PCV3) and PRRSV co-infection. Transbound. Emerg. Dis. 66, 28–34 (2019).
Li, S. et al. A potent CD8 T-cell response may be associated with partial cross-protection conferred by an attenuated Chinese HP-PRRSV vaccine against NADC30-like PRRSV challenge. J. Gen. Virol. 104, https://doi.org/10.1099/jgv.0.001850 (2023).
Tian, Y. et al. Immune cell early activation, apoptotic kinetic, and T-cell functional impairment in domestic pigs after ASFV CADC_HN09 strain infection. Front. Microbiol. 15, 1328177 (2024).
Mu, S. et al. Enhanced antigen-specific CD8 T cells contribute to early protection against FMDV through swine DC vaccination. J. Virol. 98, e0200223 (2024).
Acknowledgements
We would like to express appreciation for the efforts and contributions of Feng Li, DVM, Yabin Liu, DVM, and Chencai Hou, DVM from Zoetis, as well as Jing Cao, Juncheng Zhang, Zheng Liu, and Xiujie Jiang from our laboratory. They provide technical help in sacrifice, necropsy, and sampling of 30 pigs at the same time. This study was financially supported by the National Key Research and Development Program of China (2022YFD1800303), the Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX22_3544), and a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) and JSSCTD202224.
Author information
Authors and Affiliations
Contributions
S.L. and S. S. conceived, designed, and supervised this project; S.L., J.L., L.M., S.Y., H.W., J.Z., D.Y., H.H., G.Y. and X.J. performed the experiments; S.L., J.L., and L.M. collected and analyzed the data; S.L., H.W., D.Y., H.H., G.Y., X.J., X.H. and S.S. contributed reagents/materials/analysis tools; S.L. drafted the first version of the manuscript; S.S. revised the manuscript; All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, S., Liu, J., Meng, L. et al. Cellular immune signatures and differences of four porcine circovirus type 2 vaccines to heterologous PCV2d infection. npj Vaccines 10, 92 (2025). https://doi.org/10.1038/s41541-025-01138-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41541-025-01138-5