Abstract
Resistance to chimaeric antigen receptor (CAR) T cell therapy develops through multiple mechanisms, most notably antigen loss and tumour-induced immune suppression. It has been suggested that T cells expressing multiple CARs may overcome the resistance of tumours and that T cells expressing receptors that switch inhibitory immune-checkpoint signals into costimulatory signals may enhance the activity of the T cells in the tumour microenvironment. However, engineering multiple features into a single T cell product is difficult because of the transgene-packaging constraints of current gene-delivery vectors. Here we describe a cell-sorting method that leverages leucine zippers for the selective single-step immunomagnetic purification of cells co-transduced with two vectors. Such ‘Zip sorting’ facilitated the generation of T cells simultaneously expressing up to four CARs and coexpressing up to three ‘switch’ receptors. In syngeneic mouse models, T cells with multiple CARs and multiple switch receptors eliminated antigenically heterogeneous populations of leukaemia cells coexpressing multiple inhibitory ligands. By combining diverse therapeutic strategies, Zip-sorted multi-CAR multi-switch-receptor T cells can overcome multiple mechanisms of CAR T cell resistance.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
118,99 € per year
only 9,92 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The genomic integration study dataset is available on the Sequence Read Archive database via the accession number PRJNA1052637. The scRNAseq data are available on the GEO database via the accession number GSE253563. The main data supporting the findings of the study are available within the article and its Supplementary Information. The raw data generated during the study are available from the corresponding authors on reasonable request. Source data are provided with this paper.
Code availability
Jupyter notebook and Conda environment files are available at https://github.com/sj1233/James_et_al_Zip-sort.
References
June, C. H. & Sadelain, M. Chimeric antigen receptor therapy. N. Engl. J. Med. 379, 64–73 (2018).
Mikkilineni, L. & Kochenderfer, J. N. CAR T cell therapies for patients with multiple myeloma. Nat. Rev. Clin. Oncol. 18, 71–84 (2021).
Berger, T. R. & Maus, M. V. Mechanisms of response and resistance to CAR T cell therapies. Curr. Opin. Immunol. 69, 56–64 (2021).
Majzner, R. G. & Mackall, C. L. Tumor antigen escape from CAR T-cell therapy. Cancer Discov. 8, 1219–1226 (2018).
Labanieh, L. & Mackall, C. L. CAR immune cells: design principles, resistance and the next generation. Nature 614, 635–648 (2023).
Chen, N., Li, X., Chintala, N. K., Tano, Z. E. & Adusumilli, P. S. Driving CARs on the uneven road of antigen heterogeneity in solid tumors. Curr. Opin. Immunol. 51, 103–110 (2018).
Fucà, G., Reppel, L., Landoni, E., Savoldo, B. & Dotti, G. Enhancing chimeric antigen receptor T-cell efficacy in solid tumors. Clin. Cancer Res. 26, 2444–2451 (2020).
Perna, F. et al. Integrating proteomics and transcriptomics for systematic combinatorial chimeric antigen receptor therapy of AML. Cancer Cell 32, 506–519 (2017).
Hamieh, M. et al. CAR T cell trogocytosis and cooperative killing regulate tumour antigen escape. Nature 568, 112–116 (2019).
Ruella, M. et al. Dual CD19 and CD123 targeting prevents antigen-loss relapses after CD19-directed immunotherapies. J. Clin. Invest. 126, 3814–3826 (2016).
Zah, E., Lin, M.-Y., Silva-Benedict, A., Jensen, M. C. & Chen, Y. Y. T cells expressing CD19/CD20 bispecific chimeric antigen receptors prevent antigen escape by malignant B cells. Cancer Immunol. Res. 4, 498–508 (2016).
Larson, S. M. et al. CD19/CD20 bispecific chimeric antigen receptor (CAR) in naive/memory T cells for the treatment of relapsed or refractory non-Hodgkin lymphoma. Cancer Discov. 13, 580–597 (2023).
Spiegel, J. Y. et al. CAR T cells with dual targeting of CD19 and CD22 in adult patients with recurrent or refractory B cell malignancies: a phase 1 trial. Nat. Med. 27, 1419–1431 (2021).
Shalabi, H. et al. Sequential loss of tumor surface antigens following chimeric antigen receptor T-cell therapies in diffuse large B-cell lymphoma. Haematologica 103, e215–e218 (2018).
Cordoba, S. et al. CAR T cells with dual targeting of CD19 and CD22 in pediatric and young adult patients with relapsed or refractory B cell acute lymphoblastic leukemia: a phase 1 trial. Nat. Med. 27, 1797–1805 (2021).
Pan, J. et al. Sequential CD19 and CD22 chimeric antigen receptor T-cell therapy for childhood refractory or relapsed B-cell acute lymphocytic leukaemia: a single-arm, phase 2 study. Lancet Oncol. 24, 1229–1241 (2023).
Chen, J. et al. NR4A transcription factors limit CAR T cell function in solid tumours. Nature 567, 530–534 (2019).
Seo, H. et al. TOX and TOX2 transcription factors cooperate with NR4A transcription factors to impose CD8+ T cell exhaustion. Proc. Natl Acad. Sci. USA 116, 12410–12415 (2019).
Jackson, Z. et al. Sequential single-cell transcriptional and protein marker profiling reveals TIGIT as a marker of CD19 CAR-T cell dysfunction in patients with non-Hodgkin lymphoma. Cancer Discov. 12, 1886–1903 (2022).
Ajina, A. & Maher, J. Strategies to address chimeric antigen receptor tonic signaling. Mol. Cancer Ther. 17, 1795–1815 (2018).
Ghorashian, S. et al. CD19/CD22 targeting with co-transduced CAR T-cells to prevent antigen negative relapse after CAR T-cell therapy of B-ALL. Blood 143, 118–123 (2023).
Cherkassky, L. et al. Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. J. Clin. Invest. 126, 3130–3144 (2016).
Oda, S. K. et al. A CD200R–CD28 fusion protein appropriates an inhibitory signal to enhance T-cell function and therapy of murine leukemia. Blood 130, 2410–2419 (2017).
Yamamoto, T. N. et al. T cells genetically engineered to overcome death signaling enhance adoptive cancer immunotherapy. J. Clin. Invest. 129, 1551–1565 (2019).
Oda, S. K. et al. A Fas-4–1BB fusion protein converts a death to a pro-survival signal and enhances T cell therapy. J. Exp. Med. 217, e20191166 (2020).
Weber, E. W. et al. Transient rest restores functionality in exhausted CAR-T cells through epigenetic remodeling. Science 372, eaba1786 (2021).
Eyquem, J. et al. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature 543, 113–117 (2017).
Feucht, J. et al. Calibration of CAR activation potential directs alternative T cell fates and therapeutic potency. Nat. Med. 25, 82–88 (2019).
Long, A. H. et al. 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat. Med. 21, 581–590 (2015).
Ghosh, S., Brown, A. M., Jenkins, C. & Campbell, K. Viral vector systems for gene therapy: a comprehensive literature review of progress and biosafety challenges. Appl. Biosaf. 25, 7–18 (2020).
Li, H.-S. et al. Multidimensional control of therapeutic human cell function with synthetic gene circuits. Science 378, 1227–1234 (2022).
Li, Y. et al. KIR-based inhibitory CARs overcome CAR-NK cell trogocytosis-mediated fratricide and tumor escape. Nat. Med. 28, 2133–2144 (2022).
Tousley, A. M. et al. Co-opting signalling molecules enables logic-gated control of CAR T cells. Nature 615, 507–516 (2023).
Hyrenius-Wittsten, A. et al. SynNotch CAR circuits enhance solid tumor recognition and promote persistent antitumor activity in mouse models. Sci. Transl. Med. 13, eabd8836 (2021).
Qin, H. et al. Preclinical development of bivalent chimeric antigen receptors targeting both CD19 and CD22. Mol. Ther. Oncolytics 11, 127–137 (2018).
Fernández de Larrea, C. et al. Defining an optimal dual-targeted CAR T-cell therapy approach simultaneously targeting BCMA and GPRC5D to prevent BCMA escape–driven relapse in multiple myeloma. Blood Cancer Discov. 1, 146–154 (2020).
Hegde, M. et al. Tandem CAR T cells targeting HER2 and IL13Rα2 mitigate tumor antigen escape. J. Clin. Invest. 126, 3036–3052 (2016).
Punwani, D. et al. Lentivirus mediated correction of artemis-deficient severe combined immunodeficiency. Hum. Gene Ther. 28, 112–124 (2017).
Montini, E. et al. The genotoxic potential of retroviral vectors is strongly modulated by vector design and integration site selection in a mouse model of HSC gene therapy. J. Clin. Invest. 119, 964–975 (2009).
Bacon, K., Lavoie, A., Rao, B. M., Daniele, M. & Menegatti, S. Past, present, and future of affinity-based cell separation technologies. Acta Biomater. 112, 29–51 (2020).
Moll, J. R., Ruvinov, S. B., Pastan, I. & Vinson, C. Designed heterodimerizing leucine zippers with a ranger of pIs and stabilities up to 10−15 M. Protein Sci. 10, 649–655 (2001).
Cho, J. H., Collins, J. J. & Wong, W. W. Universal chimeric antigen receptors for multiplexed and logical control of T cell responses. Cell 173, 1426–1438 (2018).
Flugel, C. L. et al. Overcoming on-target, off-tumour toxicity of CAR T cell therapy for solid tumours. Nat. Rev. Clin. Oncol. 20, 49–62 (2023).
Stephan, M. T. et al. T cell-encoded CD80 and 4-1BBL induce auto- and transcostimulation, resulting in potent tumor rejection. Nat. Med. 13, 1440–1449 (2007).
Wang, X. et al. A transgene-encoded cell surface polypeptide for selection, in vivo tracking, and ablation of engineered cells. Blood 118, 1255–1263 (2011).
Philip, B. et al. A highly compact epitope-based marker/suicide gene for easier and safer T-cell therapy. Blood 124, 1277–1287 (2014).
Cho, J. H. et al. Engineering advanced logic and distributed computing in human CAR immune cells. Nat. Commun. 12, 792 (2021).
Sherman, E. et al. INSPIIRED: a pipeline for quantitative analysis of sites of new DNA integration in cellular genomes. Mol. Ther. Methods Clin. Dev. 4, 39–49 (2017).
Berry, C. C. et al. INSPIIRED: quantification and visualization tools for analyzing integration site distributions. Mol. Ther. Methods Clin. Dev. 4, 17–26 (2017).
Kvaratskhelia, M., Sharma, A., Larue, R. C., Serrao, E. & Engelman, A. Molecular mechanisms of retroviral integration site selection. Nucleic Acids Res. 42, 10209–10225 (2014).
Vita, R. et al. The Immune Epitope Database (IEDB): 2018 update. Nucleic Acids Res. 47, D339–D343 (2019).
Straathof, K. C. et al. An inducible caspase 9 safety switch for T-cell therapy. Blood 105, 4247–4254 (2005).
James, S. E. et al. Antibody-mediated B-cell depletion before adoptive immunotherapy with T cells expressing CD20-specific chimeric T-cell receptors facilitates eradication of leukemia in immunocompetent mice. Blood 114, 5454–5463 (2009).
Stripecke, R. et al. Immune response to Philadelphia chromosome-positive acute lymphoblastic leukemia induced by expression of CD80, interleukin 2, and granulocyte-macrophage colony-stimulating factor. Hum. Gene Ther. 9, 2049–2062 (1998).
Gattinoni, L. et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J. Exp. Med. 202, 907–912 (2005).
Fraietta, J. A. et al. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat. Med. 24, 563–571 (2018).
Vardhana, S. A. et al. Impaired mitochondrial oxidative phosphorylation limits the self-renewal of T cells exposed to persistent antigen. Nat. Immunol. 21, 1022–1033 (2020).
Scheffel, M. J. et al. Efficacy of adoptive T-cell therapy is improved by treatment with the antioxidant N-acetyl cysteine, which limits activation-induced T-cell death. Cancer Res. 76, 6006–6016 (2016).
Scott, A. C. et al. TOX is a critical regulator of tumour-specific T cell differentiation. Nature 571, 270–274 (2019).
Guedan, S. et al. Single residue in CD28-costimulated CAR-T cells limits long-term persistence and antitumor durability. J. Clin. Invest. 130, 3087–3097 (2020).
Kofler, D. M. et al. CD28 costimulation impairs the efficacy of a redirected T-cell antitumor attack in the presence of regulatory T cells which can be overcome by preventing lck activation. Mol. Ther. 19, 760–767 (2011).
Guedan, S. et al. Enhancing CAR T cell persistence through ICOS and 4-1BB costimulation. JCI Insight 3, e96976 (2018).
Lee, Y. G. et al. Modulation of BCL-2 in both T cells and tumor cells to enhance chimeric antigen receptor T-cell immunotherapy against cancer. Cancer Discov. 12, 2372–2391 (2022).
Schumm, M. et al. Isolation of highly purified autologous and allogeneic peripheral CD34+ cells using the CliniMACS device. J. Hematother. 8, 209–218 (1999).
Guerrero, A. D., Welschhans, R. L., Chen, M. & Wang, J. Cleavage of anti-apoptotic Bcl-2 family members after TCR stimulation contributes to the decision between T cell activation and apoptosis. J. Immunol. 190, 168–173 (2013).
Ward-Kavanagh, L. K., Lin, W. W., Šedý, J. R. & Ware, C. F. The TNF receptor superfamily in co-stimulating and co-inhibitory responses. Immunity 44, 1005–1019 (2016).
Zhao, Z. et al. Structural design of engineered costimulation determines tumor rejection kinetics and persistence of CAR T cells. Cancer Cell 28, 415–428 (2015).
Kawalekar, O. U. et al. Distinct signaling of coreceptors regulates specific metabolism pathways and impacts memory development in CAR T cells. Immunity 44, 380–390 (2016).
Liu, X. et al. A chimeric switch-receptor targeting PD1 augments the efficacy of second-generation CAR T cells in advanced solid tumors. Cancer Res. 76, 1578–1590 (2016).
Diskin, B. et al. PD-L1 engagement on T cells promotes self-tolerance and suppression of neighboring macrophages and effector T cells in cancer. Nat. Immunol. 21, 442–454 (2020).
Lee, S.-J. et al. 4-1BB and OX40 dual costimulation synergistically stimulate primary specific CD8 T cells for robust effector function. J. Immunol. 173, 3002–3012 (2004).
Buchan, S. et al. OX40- and CD27-mediated costimulation synergizes with anti-PD-L1 blockade by forcing exhausted CD8+ T cells to exit quiescence. J. Immunol. 194, 125–133 (2015).
Soroosh, P., Ine, S., Sugamura, K. & Ishii, N. OX40-OX40 ligand interaction through T cell–T cell contact contributes to CD4 T cell longevity. J. Immunol. 176, 5975–5987 (2006).
Gomes-Silva, D. et al. Tonic 4-1BB costimulation in chimeric antigen receptors impedes T cell survival and is vector-dependent. Cell Rep. 21, 17–26 (2017).
Legut, M. et al. A genome-scale screen for synthetic drivers of T cell proliferation. Nature 603, 728–735 (2022).
Crawford, A. et al. Molecular and transcriptional basis of CD4+ T cell dysfunction during chronic infection. Immunity 40, 289–302 (2014).
Tillé, L. et al. Activation of the transcription factor NFAT5 in the tumor microenvironment enforces CD8+ T cell exhaustion. Nat. Immunol. 24, 1645–1653 (2023).
Guan, X. et al. Androgen receptor activity in T cells limits checkpoint blockade efficacy. Nature 606, 791–796 (2022).
Singer, M. et al. A distinct gene module for dysfunction uncoupled from activation in tumor-infiltrating T cells. Cell 166, 1500–1511 (2016).
Globig, A.-M. et al. The β1-adrenergic receptor links sympathetic nerves to T cell exhaustion. Nature 622, 383–392 (2023).
Chen, W. et al. Chronic type I interferon signaling promotes lipid-peroxidation-driven terminal CD8+ T cell exhaustion and curtails anti-PD-1 efficacy. Cell Rep. 41, 111647 (2022).
Mazet, J. M. et al. IFNγ signaling in cytotoxic T cells restricts anti-tumor responses by inhibiting the maintenance and diversity of intra-tumoral stem-like T cells. Nat. Commun. 14, 321 (2023).
Li, H. et al. Dysfunctional CD8 T cells form a proliferative, dynamically regulated compartment within human melanoma. Cell 176, 775–789 (2019).
Good, C. R. et al. An NK-like CAR T cell transition in CAR T cell dysfunction. Cell 184, 6081–6100 (2021).
Nakajima, S. & Kitamura, M. Bidirectional regulation of NF-κB by reactive oxygen species: a role of unfolded protein response. Free Radic. Biol. Med. 65, 162–174 (2013).
Song, M. et al. IRE1α–XBP1 controls T cell function in ovarian cancer by regulating mitochondrial activity. Nature 562, 423–428 (2018).
Qin, H. et al. CAR T cells targeting BAFF-R can overcome CD19 antigen loss in B cell malignancies. Sci. Transl. Med. 11, eaaw9414 (2019).
Ormhøj, M. et al. Chimeric antigen receptor T cells targeting CD79b show efficacy in lymphoma with or without cotargeting CD19. Clin. Cancer Res. 25, 7046–7057 (2019).
Roth, T. L. et al. Reprogramming human T cell function and specificity with non-viral genome targeting. Nature 559, 405–409 (2018).
Coles, S. J. et al. The immunosuppressive ligands PD-L1 and CD200 are linked in AML T-cell immunosuppression: identification of a new immunotherapeutic synapse. Leukemia 29, 1952–1954 (2015).
Buzyn, A. et al. Membrane-bound Fas (Apo-1/CD95) ligand on leukemic cells: a mechanism of tumor immune escape in leukemia patients. Blood 94, 3135–3140 (1999).
Ghorashian, S. et al. Enhanced CAR T cell expansion and prolonged persistence in pediatric patients with ALL treated with a low-affinity CD19 CAR. Nat. Med. 25, 1408–1414 (2019).
Zhou, X. et al. CTLA-4 tail fusion enhances CAR-T antitumor immunity. Nat. Immunol. 24, 1499–1510 (2023).
McKenzie, C. et al. Novel Fas-TNFR chimeras that prevent Fas ligand-mediated kill and signal synergistically to enhance CAR T cell efficacy. Mol. Ther. Nucleic Acids 32, 603–621 (2023).
Li, G. et al. 4-1BB enhancement of CAR T function requires NF-κB and TRAFs. JCI Insight 3, e121322 (2018).
Hirabayashi, K. et al. Dual-targeting CAR-T cells with optimal co-stimulation and metabolic fitness enhance antitumor activity and prevent escape in solid tumors. Nat. Cancer 2, 904–918 (2021).
Schambach, A. et al. Overcoming promoter competition in packaging cells improves production of self-inactivating retroviral vectors. Gene Ther. 13, 1524–1533 (2006).
Engels, B. et al. Retroviral vectors for high-level transgene expression in T lymphocytes. Hum. Gene Ther. 14, 1155–1168 (2003).
Hooijberg, E., Bakker, A. Q., Ruizendaal, J. J. & Spits, H. NFAT-controlled expression of GFP permits visualization and isolation of antigen-stimulated primary human T cells. Blood 96, 459–466 (2000).
Rivière, I., Brose, K. & Mulligan, R. C. Effects of retroviral vector design on expression of human adenosine deaminase in murine bone marrow transplant recipients engrafted with genetically modified cells. Proc. Natl Acad. Sci. USA 92, 6733–6737 (1995).
Yusa, K., Zhou, L., Li, M. A., Bradley, A. & Craig, N. L. A hyperactive piggyBac transposase for mammalian applications. Proc. Natl Acad. Sci. USA 108, 1531–1536 (2011).
Wang, Y., Bergelson, S. & Feschenko, M. Determination of lentiviral infectious titer by a novel droplet digital PCR method. Hum. Gene Ther. Methods 29, 96–103 (2018).
Berry, C., Hannenhalli, S., Leipzig, J. & Bushman, F. D. Selection of target sites for mobile DNA integration in the human genome. PLoS Comput. Biol. 2, e157 (2006).
Rosenbaum, E. et al. HLA genotyping in synovial sarcoma: identifying HLA-A*02 and its association with clinical outcome. Clin. Cancer Res. 26, 5448–5455 (2020).
Kousa, A. I. & Lemarquis, A. L. The shunPykeR’s guide to single cell analysis (version 1.0.0). Zenodo (2023); https://doi.org/10.5281/zenodo.7510613
Wolf, F. A., Angerer, P. & Theis, F. J. SCANPY: large-scale single-cell gene expression data analysis. Genome Biol. 19, 15 (2018).
Traag, V. A., Waltman, L. & Van Eck, N. J. From Louvain to Leiden: guaranteeing well-connected communities. Sci. Rep. 9, 5233 (2019).
Wolock, S. L., Lopez, R. & Klein, A. M. Scrublet: computational identification of cell doublets in single-cell transcriptomic data. Cell Syst. 8, 281–291 (2019).
Ntranos, V., Yi, L., Melsted, P. & Pachter, L. A discriminative learning approach to differential expression analysis for single-cell RNA-seq. Nat. Methods 16, 163–166 (2019).
Van Dijk, D. et al. Recovering gene interactions from single-cell data using data diffusion. Cell 174, 716–729 (2018).
Fang, Z., Liu, X. & Peltz, G. GSEApy: a comprehensive package for performing gene set enrichment analysis in Python. Bioinformatics 39, btac757 (2023).
Vardi, Y., Ying, Z. & Zhang, C. Two‐sample tests for growth curves under dependent right censoring. Biometrika 88, 949–960 (2001).
Seshan, V. E. clinfun: Clinical trial design and data analysis functions. R package version 1.1.0. (2022); https://CRAN.R-project.org/package=clinfun
Kassambara, A., Kosinski, M. & Biecek, P. survminer: Drawing survival curves using ‘ggplot2’. R package version 0.4.9. https://CRAN.R-project.org/package=survminer (2021).
Szklarczyk, D. et al. STRING v10: protein–protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 43, D447–D452 (2015).
Acknowledgements
This research was supported by the National Cancer Institute (NCI) award P30-CA008748 MSK Cancer Center Support Grant/Core Grant and National Heart, Lung, and Blood Institute (NHLBI) award number R01-HL147584. Additional funding was received from Comedy vs Cancer, the Parker Institute for Cancer Immunotherapy as well as the Paula and Rodger Riney Multiple Myeloma Research Initiative. S.E.J. is supported by a K08 career development award from the NCI (award number K08-CA252157). S.E.J. was also supported by a Young Investigator award from the American Society for Clinical Oncology, an Amy Program Award from the Be the Match Foundation and a Bridge Scholar award from the Parker Institute for Cancer Immunotherapy. S.C. was supported by a Research Fellowship from the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG). A.L.L. was supported by the Sweden America foundation, SSMF and DKMS. S.D. is supported by the MSK Leukaemia SPORE Career Enhancement Program and the Gerstner Physician Scholar program. J.U.P. is supported by an NHLBI NIH award (K08-HL143189) and the MSKCC Cancer Center Core Grant NCI P30-CA008748. S.A.V. is supported by a K08 career development award (NCI K08-CA237731) and the Parker Institute for Cancer Immunotherapy. C.A.K. was supported in part by NIH R37-CA259177, R01-CA286507, P50-CA217694 and P30-CA008748; Mr. William H. Goodwin and Mrs. Alice Goodwin and the Commonwealth Foundation for Cancer Research; The Center for Experimental Therapeutics at MSKCC; The MSK Technology Development Fund; The Parker Institute for Cancer Immunotherapy; The Sarcoma Center at MSKCC; The Damon Runyon Cancer Research Foundation CI-96-18; the Tow Center for Developmental Oncology and Cycle for Survival. We acknowledge the use of the Integrated Genomics Operation Core, funded by the NCI Cancer Center Support Grant (CCSG; P30 CA08748), Cycle for Survival and the Marie-Josée and Henry R. Kravis Center for Molecular Oncology.
Author information
Authors and Affiliations
Contributions
S.E.J., L.J. and M.R.M.v.d.B. conceived the study and designed experiments. S.E.J., L.J., S.A.V., C.A.K. and M.R.M.v.d.B. developed methodologies. S.E.J., S.C., B.D.N., J.S.F., L.J., A.P.B., A.R., H.K.E., A.M., D.M., K.B.N., A.L., N.L., A.M.R. and A.P. performed experiments. S.E.J., S.C., B.D.N., A.G.M., J.K.E., F.D.B., A.I.K., A.L.L., T.F., S.D. and J.U.P. analysed data. S.E.J. and S.C. wrote the manuscript with assistance from all authors. M.R.M.v.d.B. supervised the study.
Corresponding authors
Ethics declarations
Competing interests
S.E.J., L.J., and M.R.M.v.d.B. are co-inventors on patent applications related to this manuscript (‘Leucine zipper-based compositions and methods of use’, nos. US20210171601A1 (USA), EP3836944A4 (Europe), WO2020037178A1 (WIPO), CA3109630A1 (Canada) and CN112930186A (China); ‘Cell sorting systems and methods of use’, nos. US20210179686A1 (USA), EP3837287A4 (Europe), WO2020037181A2 (WIPO), CA3109635A1 (Canada) and CN112996819A (China)). A.P.B. has consulted for Bristol Myers Squibb and Cancer Study Group, LLC, and has received honoraria from OncLive. F.D.B. is a founder of Biocept and has intellectual property licensed to Novartis. J.U.P. reports research funding, intellectual property fees and travel reimbursement from Seres Therapeutics as well as consulting fees from Da Volterra, CSL Behring and MaaT Pharma. He serves on an Advisory board of, and holds equity in, Postbiotics Plus Research. He has filed intellectual property applications related to the microbiome. S.A.V. has received funding from Bristol-Meyers Squibb and has received consulting fees from Koch Disruptive Technologies and Generate Biomedicine. C.A.K. has previously filed intellectual property applications related to the FasDNR featured in this manuscript. C.A.K. is a scientific co-founder and holds equity in Affini-T Therapeutics. C.A.K. has previously consulted for or is on the scientific and/or clinical advisory boards of: Achilles Therapeutics, Affini-T Therapeutics, Aleta BioTherapeutics, Bellicum Pharmaceuticals, Bristol Myers Squibb, Catamaran Bio, Cell Design Labs, Decheng Capital, G1 Therapeutics, Klus Pharma, Obsidian Therapeutics, PACT Pharma, Roche/Genentech, Royalty Pharma and T-knife. M.R.M.v.d.B. has received research support and stock options from Seres Therapeutics and stock options from Notch Therapeutics and Pluto Therapeutics; has received royalties from Wolters Kluwer; has consulted, received honoraria from or participated in advisory boards for Seres Therapeutics, Vor Biopharma, Rheos Medicines, Frazier Healthcare Partners, Nektar Therapeutics, Notch Therapeutics, Ceramedix, Lygenesis, Pluto Therapeutics, GlaxoSmithKline, Da Volterra, Thymofox, Garuda, Novartis (spouse), Synthekine (spouse), Beigene (spouse), Kite (spouse); has intellectual property licensing with Seres Therapeutics and Juno Therapeutics; and holds a fiduciary role on the Foundation Board of DKMS (a non-profit organization). MSKCC has institutional financial interests relative to Seres Therapeutics.
Peer review
Peer review information
Nature Biomedical Engineering thanks Stephen Gottschalk and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Covalently linked blocking-zipper inhibits extracellular leucine zipper pairing.
a, Zip-sorting system vector maps. b, Vector map and diagram for EE-Thy1.1-P2A–BFP vector. c, Flow cytometry analysis of C1498 cells co-transduced with FLAG–RR-CBR–GFP and EE-Thy1.1-P2A–BFP vectors. Arrows depict combined intracellular + extracellular pairing (orange) and extracellular pairing (blue). d, Comparison of FLAG–zipper staining on dual-transduced (EGFP+ BFP+) and capture-zipper single-transduced (EGFP− BFP+) C1498 cells from four independent biological replicate co-transductions with FLAG–RR-CBR–GFP and EE-Thy1.1-P2A–BFP vectors as in panel c. Data are mean ± SEM of replicate samples. e, Maps of non-blocked EE-Thy1.1-P2A–BFP and blocked RR-EE-Thy1.1-P2A–BFP capture-zipper vectors. f, Diagrams and flow cytometry analysis of co-culture of single-transduced FLAG–zipper-secreting FLAG–RR-CBR–GFP C1498 with C1498 cells single-transduced with either (top) non-blocked EE-Thy1.1-P2A–BFP or (bottom) blocked RR-EE-Thy1.1-P2A–BFP capture-zipper vectors at depicted cell ratios for 48 h. FLAG staining represents extracellular pairing of FLAG–RR zippers on single-transduced capture-zipper+ C1498 cells, which capture FLAG–RR zippers secreted into the media by single-transduced FLAG–RR-secreting C1498 cells. g, FLAG–RR zipper surface expression on C1498 cells expressing blocked or non-blocked capture zippers depicted in panel f. n = 1 transduction for each cell line and n = 3 co-culture experiments. Data are mean ± SEM of triplicate samples. Error bars were too small to depict. h, Flow cytometry contour plots and histograms of unsorted, mixed populations of C1498 cells co-transduced with FLAG–RR-CBR–GFP and RR-EE-Thy1.1-P2A–BFP vector variants with capture zippers containing different repulsive mutations in blocking-zippers (See Supplementary Table 1). “EE” blocking-zipper is engineered to be fully repulsive against EE capture-zipper and maximally attractive towards the FLAG–RR zipper. Remaining mutants contain varying numbers of repulsive mutations “2 or 3” in the N-terminal “N” or middle “M” regions of the blocking-zipper. Representative of n = 3 separate transductions. i, Diagram depicting predicted effect of repulsive amino acid substitution on zipper binding affinity. j, Maps for non-blocked and blocked EGFRt-based capture-zipper vectors. k, Flow cytometry analysis of C1498 cells dual-transduced with FLAG–RR-CBR–GFP and either (top) non-blocked EE-EGFRt-P2A–BFP or (bottom) blocked capture-zipper RR-EE-EGFRt-BFP. l, Zip-sort of FLAG–RR-CBR–GFP/RR-EE-EGFRt-P2A–BFP C1498 cells. Representative of n = 2 transductions and Zip-sorts.
Extended Data Fig. 2 Zip-sorted dual-CAR T cells demonstrate CAR-dose-dependent upregulation of ROS and inhibitory receptor expression.
a, Flow cytometry analysis of BM185-ffluc-Thy1.1-Neo cell lines expressing combinations of CD19 and CD20. b, Schematic depicting BM185 syngeneic mouse model of antigen-loss escape in CAR T cell immunotherapy for acute lymphoblastic leukaemia. c–e. Sublethally irradiated BALB/c mice were injected with 1:1 mixture of BM185-CD19/BM185-CD20 at 1×105 BM185/mouse (50x increased dose vs. Figure 3g) and treated with Zip-sorted BALB/c CAR T cells on day 2. c, Leukaemia BLI (ffluc) from two combined experiments. Log-transformed BLI values were compared using a Vardi test to compare AUC values with FDR correction for multiple comparisons. d, Day 10 BLI images from representative experiment. e, Survival, compared via log-rank test. f, PD-1 expression on resting Zip-sorted BALB/c CAR T cells. g, Linear regression analysis of unstimulated T cell PD-1 expression vs. number of signalling-competent CARs expressed, n = 3 donors. h, Immunophenotype analysis of unstimulated Zip-sorted BALB/c CAR T cells; mean ± SEM from n = 3 donors. i, Total cellular ROS (CM-H2DCFDA) analysis of resting Zip-sorted CAR T cells. j, Z-score normalized mean CM-H2DCFDA MFI ± SEM results of n = 3 biological replicates. k, Mitochondrial superoxide (Mitosox Red) analysis of Zip-sorted CAR T cells. l, Z-score normalized mean Mitosox Red MFI ± SEM results of n = 3 biological replicates. Statistical differences for ROS and mitochondrial superoxide were compared using one-way ANOVA, with Tukey’s test.
Extended Data Fig. 3 CAR T cell culture with NAC or dasatinib and ITAM attenuation enhances the antileukaemia activity of dual-CAR T cells.
a,b. BM185-CD19/BM185-CD20 antigen-loss escape model. BALB/c mice were treated with Zip-sorted dual-CAR BALB/c T cells cultured with 1 μM dasatinib (2 days), 10 mM NAC (3 days), or DMSO (3 days). a, Leukaemia BLI from two combined experiments. Log-transformed BLI AUC values were compared using a Vardi test with FDR correction. b, Survival. c, NFAT, AP-1, or NFκB EGFP reporter analysis of unstimulated BALB/c T cells gated on dual-CAR positive or CAR-negative population following 24 h culture with 1 μM dasatinib, 10 mM NAC, or DMSO. Representative of n = 2 donor experiments, with mean ± SEM of triplicate wells. Statistical comparison via two-way ANOVA, with Tukey’s test. d,e, PD-1 and TOX expression in Zip-sorted dual-CAR or delta/delta BALB/c T cells following 24 h co-culture with BM185-CD19 or no targets. Mean ± SEM of triplicate samples were compared with a two-way ANOVA, with Tukey’s test. f, Intracellular flow cytometry analysis of TCF1 and TOX expression in Zip-sorted dual-CAR or delta/delta BALB/c T cells cultured for 2 days with 1 μM dasatinib or DMSO. Representative experiments from n = 2 donors. g, Diagram depicting ITAM mutations in 1XX CAR. h, Survival of BALB/c mice injected with BM185-CD19/BM185-CD20 (1:1) and treated with Zip-sorted dual-CAR WT CD3ζ or 1XX ITAM mutant dual-CAR T cells, from three combined experiments. i, Survival of BALB/c mice injected with dual-target-antigen expressing BM185-CD19-CD20 leukaemia and treated with WT CD3ζ or 1XX dual-CAR T cells, from three combined experiments. Survival curves were compared via log-rank tests or pairwise log-rank test, with FDR correction.
Extended Data Fig. 4 Coexpression of BCL-2 with dominant-negative receptors or combination with a single switch receptor transiently enhances antileukaemia activity of dual-CAR T cells.
a, (Left) Diagram and maps for vectors encoding combinations of the Zip-sorting system, CD19 and CD20 dual-CAR, BCL-2 D34A caspase-cleavage resistant mutant, iC9, 3N-mutant blocked zipper-tagged PD-1-DNR (DNR; dominant-negative receptor), and FasDNR. 3N mutant blocking-zipper was used to increase affinity-tagged zipper surface expression (See Extended Data Fig. 1h). Tandem hCD20 mimotopes were added as expression tag and as a target for cell Rituximab-mediated depletion. (Right) Flow cytometry analysis of Zip-sorted BALB/c T cells dual-transduced with vectors as depicted in legend. Numbers in flow plots refer to post Zip-sort purity percentage for each marker. b, Maps for vector set encoding Q2-RR-iC9 and 3N zipper-tagged PD-1-CAR. c, Expression of PD-1-CAR and surface bound Q2-RR zipper. d, PD-L1 expression on BM185-CD20-PD-L1 clone. e, Luciferase-based 24 h target lysis assay using Zip-sorted Q2-RR-iC9/3N-EE-PD-1-CAR BALB/c T cells or non-transduced T cells. Data are mean ± SEM of triplicate wells for a representative experiment from n = 2 donor experiments. f–i, Sublethally irradiated BALB/c mice were injected with a 1:1 mixture of BM185-CD19 and BM185-CD20 and treated with Zip-sorted CD45.1+ congenic BALB/c T cells. f, Leukaemia BLI (ffluc) from single experiment. g, Flow cytometry analysis of CAR T cells in peripheral blood on day 9. h, Linear regression analysis of leukaemia BLI signal vs. blood CD45.1+ T cell concentration. i, Survival. j–l, Experimental setup as in panel f, but mice were treated with dual-CAR T cells incorporating FasBB switch receptor instead of FasDNR. j, Leukaemia BLI from three combined experiments. k, Flow cytometry analysis of CAR T cells in peripheral blood on day 10, combined from two experiments. l, Survival from three combined experiments. Log-transformed BLI AUC values were compared using a Vardi test with FDR correction. Blood T cell counts were compared with a two-tailed t-test. Survival was compared with log-rank or pairwise log-rank comparison with FDR correction.
Extended Data Fig. 5 Multi-Switch receptor arrays enhance T cell activity in response to inhibitory ligands.
a,b, Flow cytometry analysis of Zip-sorted dual-CAR BALB/c T cells ± FasBB switch receptor co-cultured for 24 h with BM185-CD19 (E:T = 1:1) or left unstimulated. Data are mean ± SEM (biological replicates) of triplicate wells from n = 3 donor experiments. c, Vector maps for dual-CAR and switch receptor configurations as depicted in the table. d–g, Flow cytometry analysis of Zip-sorted dual-CAR, dual-CAR FasBB, and dual-CAR multi-Switch BALB/c T cells stimulated for 24 h with BM185-CD19 (E:T = 1:2) or left unstimulated. Data are mean ± SEM (biological replicates) of triplicate wells from n = 4 donor experiments. Statistical comparisons were calculated using two-way ANOVA, with Tukey’s test. h, Flow cytometry analysis of BM185-ffluc-Thy1.1-Neo cell lines engineered to express FasL, PD-L1, or CD200. i–k. Sublethally irradiated BALB/c mice were injected with 1:1:1 mixture of BM185-CD19-FasL, BM185-CD20-PD-L1, and BM185-CD20-CD200 and treated with Zip-sorted dual-CAR BALB/c T cells, pre-cultured for 2 days with 1 μM dasatinib. i, Leukaemia BLI, and j, mouse survival, respectively, from one experiment. Survival differences were compared via log-rank test. k, Flow cytometry analysis of inhibitory ligand expression of single-ligand-positive BM185 lines harvested from bone marrow of mice reaching humane endpoints. PD-L1 expression difference was compared via a two-tailed t-test. Data are mean ± SEM (biological replicates). l, Live-cell microscopy (Incucyte) analysis of EGFP-labelled dual-CAR and dual-CAR triple-Switch Zip-sorted BALB/c T cells co-cultured with iRFP713+ BM185 target cell lines as depicted (E:T = 1:1), with repetitive target addition on days 0, 1, and 2. Representative of n = 3 donor experiments. Data are mean ± SEM of triplicate samples. m, Live-cell microscopy (Incucyte) analysis of NFκB-EGFP-reporter labelled dual-CAR and dual-CAR triple-Switch Zip-sorted BALB/c T cells co-cultured with iRFP713+ BM185 target cell lines as depicted at E:T = 1:9. Representative of n = 2 donor experiments. Values represent mean ± SEM of triplicate samples.
Extended Data Fig. 6 Coexpression of switch receptors attenuates inhibitory receptor upregulation, ROS production, and endoplasmic reticulum stress response activation.
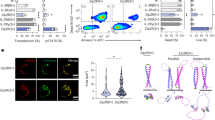
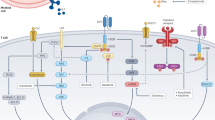
a, MAGIC110 imputed gene expression violin plots of inhibitory receptor and transcription-factor genes for dual-CAR ± multi-Switch T cells related to Fig. 5. Differentially expressed genes were compared via Wilcoxon test prior to imputation. b, Flow cytometry analysis of dual-CAR ± multi-Switch T cells 24 h following stimulation with BM185-CD19. Representative of n = 2 donor experiments. c, Dual-CAR ± multi-Switch T cells were stained with CM-H2DCFDA to measure total ROS two days following the last TCR stimulation. Left, representative flow cytometry plots from one of two donors. Right, bar graphs depicting mean CM-H2DCFDA values from triplicate wells from n = 2 donors. Replicate values were compared via one-way ANOVA, with Tukey’s test. Data are mean ± SEM. d, ER stress score gene set (GOBP_RESPONSE_TO_ENDOPLASMIC_RETICULUM_STRESS) comparison for dual-CAR ± multi-Switch T cells related to Fig. 5. Single-cell ER stress score values were compared via one-way ANOVA, with Tukey’s test. e, STRING115 analysis combining functional and physical protein interaction networks corresponding to the union of GO:0034976 ER stress response genes significantly upregulated (adjusted P < 0.05) in CD4+ or CD8+ dual-CAR T cells compared with dual-Switch and triple-Switch T cells. Gene nodes without connecting edges were excluded. Thickness of edges indicates strength of data support. f, Heat map depicting gene scaled expression of selected ER stress response genes in the IRE1α and ATF6 ER stress pathways. g, Full GSEA pathway illustrations for Fig. 5g.
Extended Data Fig. 7 Dual-CAR T cells eliminate cognate targets and promote antigen-negative escape of leukaemia mixture with high antigen heterogeneity.
a, C1498 acute myeloid leukaemia cell line was modified to singly express murine CD19, CD20, CD79bΔ (CD79b extracellular ___domain fused to CD28TM and CD3ζΔ; to promote surface expression without requiring CD79a coexpression), and BAFF-R. C1498 also was modified to express CBR, hCD8, and puroR. b–e, Albino B6 mice were sublethally irradiated and injected with either 1:1 mixture of C1498-CD19 and C1498-CD20 or a 1:1:1:1 mixture of C1498-CD19, C1498-CD20, C1498-CD79b, and C1498-BAFF-R and treated with dasatinib-cultured Zip-sorted dual-CAR 1XX T cells or left untreated. b, Leukaemia BLI from single experiment. Log-transformed BLI AUC values were compared using a Vardi test with FDR correction. c, Survival. Differences in survival were compared with a log-rank test. d, Target antigen expression of representative C1498 leukaemia harvested from bone marrow at time of euthanasia for leukaemia progression. Gated on hCD8+ C1498. e, Target antigen expression on bone marrow leukaemia obtained from mice reaching humane endpoints. Percentage CD20+ C1498 fraction of total bone marrow C1498 was compared with two-tailed t-test. Data are mean ± SEM of biological replicates.
Extended Data Fig. 8 Multi-Switch receptor arrays enhance the activity of triple-CAR T cells.
a, Diagrams and vector maps for triple-CAR ± dual-Switch receptor arrays. b, Flow cytometry analysis of Zip-sorted triple-CAR and triple-CAR dual-Switch albino B6 T cells. Numbers in flow plots represent post-sort purity percentage for each construct. c, 24 h luciferase-based target lysis assay with Zip-sorted BAFF-R/CD79b/CD20 triple-CAR BALB/c T cells with targets as depicted. Data are mean ± SEM of triplicate wells. d–f, Sublethally irradiated albino B6 mice were injected with 1:1:1 ratio of C1498-CD20, C1498-CD79b, C1498-BAFF-R and treated with dasatinib-cultured triple-CAR ± dual-Switch T cells co-transduced with a membrane-bound Gaussia luciferase (gLuc) vector: gLuc-PD-1H-CD24-GPI-P2A-EGFP for T cell BLI. d, C1498 BLI (CBR). e, Survival; from a single experiment. f, T cell BLI (Gaussia). Data are mean ± SEM of biological replicates. g,h, Experimental setup as in panels d–f, but with stress test 0.4 × 106 T cell dose and T cell BLI not performed. g, C1498 BLI from two combined experiments (left) and images from representative experiment (right). h, Survival. Log-transformed BLI AUC values were compared using a Vardi test with FDR correction. Survival differences were compared via pairwise log-rank test, with FDR correction.
Extended Data Fig. 9 Quad-CAR T cells including a single-___domain antibody-based CD19-CAR demonstrate cognate target lysis, but exhibit limited antileukaemia activity in vivo.
a, Diagram and vector maps for quad-CAR receptor array. b, Flow cytometry analysis of CAR expression. c, 24 h luciferase-based target lysis assay with quad-CAR B6 T cells and targets as listed. Data are mean ± SEM of triplicate wells for representative experiments from n = 2 donors. d–g, Sublethally irradiated albino B6 mice were injected with 1:1:1:1 ratio of C1498 singly expressing (hCD19, CD20, CD79bΔ, BAFF-R) and treated with dasatinib-cultured quad-CAR albino B6 T cells co-transduced with Gaussia luciferase vector gLuc-PD-1H-CD24-GPI-P2A-EGFP. d, C1498 BLI from three combined experiments with different BLI imaging timing. BLI AUC values were compared using a Vardi test with FDR correction. e, Survival. Differences in survival were compared with a log-rank test. f, Representative day 20 T cell BLI (Gaussia) and day 21 C1498 BLI (CBR) images. g, Antigen expression analysis from C1498 harvested from bone marrow of mice reaching humane endpoints. Percentage hCD19+ C1498 was compared with two-tailed t-test. Data are mean ± SEM of biological replicates.
Extended Data Fig. 10 Immunophenotype profiling of quad-CAR and quad-CAR triple-Switch T cells.
a–n, Resting and BM185-CD20-stimulated quad-CAR and quad-CAR triple-Switch T cells (as depicted in Fig. 6), were analysed by multiparameter spectral flow cytometry. Cells were stimulated at 1:1 E:T ratio with BM185-CD20 for 24 h or left unstimulated without IL-2. a, t-Distributed Stochastic Neighbour Embedding (t-SNE) projection with flowSOM metaclusters depicting combined stimulated and unstimulated CD4+ quad-CAR and quad-CAR triple-Switch T cells (n = 3 concatenated technical replicates from triplicate wells). b, tSNE projections illustrating flowSOM metaclusters separated by stimulated and unstimulated quad-CAR and quad-CAR triple-Switch T cell populations. c, tSNE projections depicting endogenous PD-1 and PD-1-OX40 switch receptor expression. d, Population distributions of stimulated (top) and unstimulated (bottom) T cell products in metaclusters. e, tSNE projections of selected protein expression for combined stimulated and unstimulated quad-CAR and quad-CAR triple-Switch T cells. f, Heat map depicting Z-score-transformed median fluorescence intensity expression values depicted for proteins in stimulated and unstimulated quad-CAR and quad-CAR triple-Switch T cells. g, Histograms depicting protein expression for selected markers. h–n, Concurrent analysis of CD8+ T cell populations as in panels a–g. Data are representative of n = 2 donor experiments with triplicate wells. d,k, Mean of technical replicates and individual data replicates are depicted.
Supplementary information
Supplementary Information
Supplementary figures.
Supplementary Table 1
Mutant RR12EE345L zipper sequences.
Supplementary Table 2
Zip-sort clonal abundance of most frequent chromosomal integrations.
Supplementary Table 3
Vector integration sites.
Supplementary Table 4
Construct sequences.
Supplementary Table 5
Element sequences.
Supplementary Table 6
Antibodies.
Supplementary Table 7
Vector integration study primer sequences.
Supplementary Data 1
Source data for Supplementary Figures 1–4.
Supplementary Video 1
CAR T cell cluster formation and target elimination.
Source data
Source data for Figs. 1–7 and Extended Fig. 1–10
Source data and statistics.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
James, S.E., Chen, S., Ng, B.D. et al. Leucine zipper-based immunomagnetic purification of CAR T cells displaying multiple receptors. Nat. Biomed. Eng 8, 1592–1614 (2024). https://doi.org/10.1038/s41551-024-01287-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-024-01287-3