Abstract
In magnetic resonance imaging of the brain, an imaging-preprocessing step removes the skull and other non-brain tissue from the images. But methods for such a skull-stripping process often struggle with large data heterogeneity across medical sites and with dynamic changes in tissue contrast across lifespans. Here we report a skull-stripping model for magnetic resonance images that generalizes across lifespans by leveraging personalized priors from brain atlases. The model consists of a brain extraction module that provides an initial estimation of the brain tissue on an image, and a registration module that derives a personalized prior from an age-specific atlas. The model is substantially more accurate than state-of-the-art skull-stripping methods, as we show with a large and diverse dataset of 21,334 lifespans acquired from 18 sites with various imaging protocols and scanners, and it generates naturally consistent and seamless lifespan changes in brain volume, faithfully charting the underlying biological processes of brain development and ageing.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
118,99 € per year
only 9,92 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The large-scale images (N = 21,334) used in this study come from the following publicly available datasets: dHCP (https://biomedia.github.io/dHCP-release-notes/), MAP (https://circlelab.unc.edu/studies/completed-data-collection/multi-visit-advanced-pediatric-brain-imaging-map/), BCP (https://nda.nih.gov/edit_collection.html?id=2848), NDAR (https://nda.nih.gov/edit_collection.html?id=19), HBN (http://fcon_1000.projects.nitrc.org/indi/cmi_healthy_brain_network/), ABIDE (https://fcon_1000.projects.nitrc.org/indi/abide/), IXI (https://brain-development.org/ixi-dataset/), CCNP (https://ccnp.scidb.cn/en/detail?dataSetId=826407529641672704&version=V3&code=o00133), ICBM (https://ida.loni.usc.edu/login.jsp), HCP (https://www.humanconnectome.org/study/hcp-young-adult), SLIM (http://fcon_1000.projects.nitrc.org/indi/retro/southwestuni_qiu_index.html), SALD (http://fcon_1000.projects.nitrc.org/indi/retro/sald.html), DLBS (https://fcon_1000.projects.nitrc.org/indi/retro/dlbs.html), Chinese Adult Brain (https://www.nitrc.org/projects/adultatlas), ABVIB (https://ida.loni.usc.edu/login.jsp), AIBL (https://ida.loni.usc.edu/login.jsp), OASIS3 (https://www.oasis-brains.org) and ADNI (https://ida.loni.usc.edu). Additional images are available at the following links: https://brainlife.io/pub/60ddea776f0f540a79ca53d8 (EMEDEA-PED), https://surfer.nmr.mgh.harvard.edu/docs/synthstrip/ (SynthStrip dataset) and https://fcon_1000.projects.nitrc.org/indi/indiPRIME.html (An Open Resource for Non-human Primate Imaging). Source data are provided with this paper.
Code availability
The source code, training data and trained model are available on GitHub at https://github.com/DBC-Lab/Atlases-empowered_Lifespan_Skull_Stripping. The model is also available for use in iBEAT V2.0 Cloud/Docker (http://www.ibeat.cloud). The source codes of competing methods are also available; BET, https://ftp.nmr.mgh.harvard.edu/pub/dist/freesurfer/tutorial_packages/centos6/fsl_507/doc/wiki/BET(2f)UserGuide.html; 3DSS, https://afni.nimh.nih.gov/pub/dist/doc/program_help/3dSkullStrip.html; ROBEX, https://www.nitrc.org/projects/robex; FreeSurfer, https://surfer.nmr.mgh.harvard.edu/fswiki, version 7.2.0; SynthStrip, https://surfer.nmr.mgh.harvard.edu/docs/synthstrip/#tool; HD-BET, https://github.com/MIC-DKFZ/HD-BET. To assess the significance of the results quantitatively, we conducted statistical analyses P value using one-way ANOVA with repeated measures followed by Dunnett’s multiple comparisons tests. P values were denoted by asterisks: * for P < 0.05, ** for P < 0.01 and *** for P < 0.001. Besides, we used Cohen’s d score as a pivotal metric to convey and quantify the magnitude of the observed effect within the results, using Effect Size Calculators (https://lbecker.uccs.edu).
References
Giedd, J. N. Structural magnetic resonance imaging of the adolescent brain. Ann. N. Y. Acad. Sci. 1021, 77–85 (2004).
Hahn, H. K. & Peitgen, H.-O. The skull stripping problem in MRI solved by a single 3D watershed transform. In Medical Image Computing and Computer-Assisted Intervention—MICCAI 2000 (eds Delp, S. L., DiGoia, A. M. & Jaramaz, B.) 134–143 (Springer, 2000).
Tustison, N. J. et al. N4ITK: improved N3 bias correction. IEEE Trans. Med. Imaging 29, 1310–1320 (2010).
Fatima, A., Shahid, A. R., Raza, B., Madni, T. M. & Janjua, U. I. State-of-the-art traditional to the machine-and deep-learning-based skull stripping techniques, models, and algorithms. J. Digit. Imaging 33, 1443–1464 (2020).
Gibou, F., Fedkiw, R. & Osher, S. A review of level-set methods and some recent applications. J. Comput. Phys. 353, 82–109 (2018).
Li, C., Xu, C., Gui, C. & Fox, M. D. Distance regularized level set evolution and its application to image segmentation. IEEE Trans. Image Process. 19, 3243–3254 (2010).
Ziou, D. & Tabbone, S. Edge detection techniques—an overview. Pattern Recognit. Image Anal. 8, 537–559 (1998).
Yi, F. & Moon, I. Image segmentation: a survey of graph-cut methods. In 2012 International Conference on Systems and Informatics (ICSAI2012) https://doi.org/10.1109/ICSAI19873.2012 (IEEE, 2012).
Jenkinson, M. BET2: MR-based estimation of brain, skull and scalp surfaces. In Eleventh Annual Meeting of the Organization for Human Brain Mapping (2005).
Smith, S. M. Fast robust automated brain extraction. Hum. Brain Mapp. 17, 143–155 (2002).
Zeng, X., Staib, L. H., Schultz, R. T. & Duncan, J. S. Segmentation and measurement of the cortex from 3-D MR images using coupled-surfaces propagation. IEEE Trans. Med. Imaging 18, 927–937 (1999).
Shattuck, D. W., Sandor-Leahy, S. R., Schaper, K. A., Rottenberg, D. A. & Leahy, R. M. Magnetic resonance image tissue classification using a partial volume model. NeuroImage 13, 856–876 (2001).
Shanthi, K. & Kumar, M. S. Skull stripping and automatic segmentation of brain MRI using seed growth and threshold techniques. In 2007 International Conference on Intelligent and Advanced Systems https://doi.org/10.1109/ICIAS12643.2007 (IEEE, 2007).
Mohsin, S., Sajjad, S., Malik, Z. & Abdullah, A. H. Efficient way of skull stripping in MRI to detect brain tumor by applying morphological operations, after detection of false background. Int. J. Inform. Educ. Technol. 2, 335–337 (2012).
Fein, G. et al. Statistical parametric mapping of brain morphology: sensitivity is dramatically increased by using brain-extracted images as inputs. NeuroImage 30, 1187–1195 (2006).
Somasundaram, K. & Kalavathi, P. Brain segmentation in magnetic resonance human head scans using multi-seeded region growing. Imaging Sci. J. 62, 273–284 (2014).
Eskildsen, S. F. et al. BEaST: brain extraction based on nonlocal segmentation technique. NeuroImage 59, 2362–2373 (2012).
Huang, A., Abugharbieh, R., Tam, R. & Traboulsee, A. MRI brain extraction with combined expectation maximization and geodesic active contours. In 2006 IEEE International Symposium on Signal Processing and Information Technology https://doi.org/10.1109/ISSPIT11677.2006 (IEEE, 2006).
Rehm, K. et al. Putting our heads together: a consensus approach to brain/non-brain segmentation in T1-weighted MR volumes. NeuroImage 22, 1262–1270 (2004).
Somasundaram, K. & Shankar, R. S. Skull stripping of MRI using clustering and 2D region growing method. In Image Processing NCIMP (eds Somasundaram, K.) 1–12 (Allied Publishers Pvt, 2010).
Somasundaram, K. & Shankar, R. S. Automated skull stripping method using clustering and histogram analysis for MRI human head scans. Int. J. Adv. Res. Comput. Sci. Technol. 2, 117–122 (2014).
Rigatti, S. J. Random forest. J. Insur. Med. 47, 31–39 (2017).
Cox, R. W. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 29, 162–173 (1996).
Fischl, B. FreeSurfer. NeuroImage 62, 774–781 (2012).
Iglesias, J. E., Liu, C.-Y., Thompson, P. M. & Tu, Z. Robust brain extraction across datasets and comparison with publicly available methods. IEEE Trans. Med. Imaging 30, 1617–1634 (2011).
Pei, L. et al. A general skull stripping of multiparametric brain MRIs using 3D convolutional neural network. Sci. Rep. 12, 10826 (2022).
Lucena, O., Souza, R., Rittner, L., Frayne, R. & Lotufo, R. Convolutional neural networks for skull-stripping in brain MR imaging using silver standard masks. Artif. Intell. Med. 98, 48–58 (2019).
Kleesiek, J. et al. Deep MRI brain extraction: a 3D convolutional neural network for skull stripping. NeuroImage 129, 460–469 (2016).
Hwang, H., Rehman, H. Z. U. & Lee, S. 3D U-Net for skull stripping in brain MRI. Appl. Sci. 9, 569–584 (2019).
Isensee, F. et al. Automated brain extraction of multisequence MRI using artificial neural networks. Hum. Brain Mapp. 40, 4952–4964 (2019).
Hoopes, A., Mora, J. S., Dalca, A. V., Fischl, B. & Hoffmann, M. SynthStrip: skull-stripping for any brain image. NeuroImage 260, 119474 (2022).
Sun, Y. et al. Multi-site infant brain segmentation algorithms: the iSeg-2019 challenge. IEEE Trans. Med. Imaging 40, 1363–1376 (2021).
Fischl, B. et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33, 341–355 (2002).
Avants, B. B., Tustison, N. J., Wu, J., Cook, P. A. & Gee, J. C. An open source multivariate framework for n-tissue segmentation with evaluation on public data. Neuroinformatics 9, 381–400 (2011).
Cabezas, M., Oliver, A., Lladó, X., Freixenet, J. & Cuadra, M. B. A review of atlas-based segmentation for magnetic resonance brain images. Comput. Methods Programs Biomed. 104, 158–177 (2011).
Sabuncu, M. R., Yeo, B. T., Van Leemput, K., Fischl, B. & Golland, P. A generative model for image segmentation based on label fusion. IEEE Trans. Med. Imaging 29, 1714–1729 (2010).
Puonti, O., Iglesias, J. E. & Van Leemput, K. Fast and sequence-adaptive whole-brain segmentation using parametric Bayesian modeling. NeuroImage 143, 235–249 (2016).
Chen, L. et al. A 4D infant brain volumetric atlas based on the UNC/UMN baby connectome project (BCP) cohort. NeuroImage 253, 119097 (2022).
Wu, Y. et al. High resolution 0.5 mm isotropic T1-weighted and diffusion tensor templates of the brain of non-demented older adults in a common space for the MIITRA atlas. NeuroImage 282, 120387 (2023).
Hughes, E. J. et al. A dedicated neonatal brain imaging system. Magn. Reson. Med. 78, 794–804 (2017).
Cordero-Grande, L. et al. Sensitivity encoding for aligned multishot magnetic resonance reconstruction. IEEE Trans. Comput. Imaging 2, 266–280 (2016).
Hazlett, H. C. et al. Brain volume findings in 6-month-old infants at high familial risk for autism. Am. J. Psychiatry 169, 601–608 (2012).
Di Martino, A. et al. Enhancing studies of the connectome in autism using the autism brain imaging data exchange II. Sci. Data 4, 1–15 (2017).
Gao, P. et al. A Chinese multi-modal neuroimaging data release for increasing diversity of human brain mapping. Sci. Data 9, 286–296 (2022).
Liu, S. et al. Chinese Color Nest Project: an accelerated longitudinal brain-mind cohort. Dev. Cogn. Neurosci. 52, 101020 (2021).
Weiner, M. W. et al. The Alzheimer’s disease neuroimaging initiative 3: continued innovation for clinical trial improvement. Alzheimer’s Dement. 13, 561–571 (2017).
Jenkinson, M., Beckmann, C. F., Behrens, T. E., Woolrich, M. W. & Smith, S. M. FSL. NeuroImage 62, 782–790 (2012).
Howell, B. R. et al. The UNC/UMN Baby Connectome Project (BCP): an overview of the study design and protocol development. NeuroImage 185, 891–905 (2019).
Woodburn, M. A. Trajectories of Group and Individual-Level Structural Brain Network Organization from Birth to Childhood and Their Cognitive Relevance. MA Thesis (The University of North Carolina at Chapel Hill, 2020).
Bethlehem, R. A. et al. Brain charts for the human lifespan. Nature 604, 525–533 (2022).
Lai, M. M. et al. Relationship of established cardiovascular risk factors and peripheral biomarkers on cognitive function in adults at risk of cognitive deterioration. J. Alzheimer’s Dis. 74, 163–171 (2020).
Alexander, L. M. et al. An open resource for transdiagnostic research in pediatric mental health and learning disorders. Sci. Data 4, 1–26 (2017).
Van Essen, D. C. & Glasser, M. F. The human connectome project: progress and prospects. In Cerebrum: The Dana Forum on Brain Science https://pmc.ncbi.nlm.nih.gov/articles/PMC5198757 (Dana Foundation, 2016).
LaMontagne, P. J. et al. OASIS-3: longitudinal neuroimaging, clinical, and cognitive dataset for normal aging and Alzheimer disease. Preprint at medRxiv https://doi.org/10.1101/2019.12.13.19014902 (2019).
Zöllei, L., Iglesias, J. E., Ou, Y., Grant, P. E. & Fischl, B. Infant FreeSurfer: an automated segmentation and surface extraction pipeline for T1-weighted neuroimaging data of infants 0–2 years. NeuroImage 218, 116946 (2020).
Amorosino, G. et al. DBB-A distorted brain benchmark for automatic tissue segmentation in paediatric patients. NeuroImage 260, 119486 (2022).
Rohlfing, T., Zahr, N. M., Sullivan, E. V. & Pfefferbaum, A. The SRI24 multichannel atlas of normal adult human brain structure. Hum. Brain Mapp. 31, 798–819 (2010).
Milham, M. P. et al. An open resource for non-human primate imaging. Neuron 100, 61–7462 (2018).
Nerland, S. et al. A comparison of intracranial volume estimation methods and their cross-sectional and longitudinal associations with age. Hum. Brain Mapp. 43, 4620–4639 (2022).
Sgouros, S., Goldin, J. H., Hockley, A. D., Wake, M. J. & Natarajan, K. Intracranial volume change in childhood. J. Neurosurg. 91, 610–616 (1999).
Kamdar, M. R., Gomez, R. A. & Ascherman, J. A. Intracranial volumes in a large series of healthy children. Plast. Reconstr. Surg. 124, 2072–2075 (2009).
Hill, C. A. et al. Intracranial volume and whole brain volume in infants with unicoronal craniosynostosis. Cleft Palate-Craniofacial J. 48, 394–398 (2011).
Royle, N. A. et al. Estimated maximal and current brain volume predict cognitive ability in old age. Neurobiol. Aging 34, 2726–2733 (2013).
Dimitrova, R. et al. Phenotyping the preterm brain: characterizing individual deviations from normative volumetric development in two large infant cohorts. Cereb. Cortex 31, 3665–3677 (2021).
Zacharia, A. et al. Early assessment of brain maturation by MR imaging segmentation in neonates and premature infants. Am. J. Neuroradiol. 27, 972–977 (2006).
Blatter, D. D. et al. Quantitative volumetric analysis of brain MR: normative database spanning 5 decades of life. Am. J. Neuroradiol. 16, 241–251 (1995).
Reite, M. et al. Brain size and brain/intracranial volume ratio in major mental illness. BMC Psychiatry 10, 1–9 (2010).
Tanskanen, P. et al. Volumes of brain, grey and white matter and cerebrospinal fluid in schizophrenia in the Northern Finland 1966 Birth Cohort: an epidemiological approach to analysis. Psychiatry Res. Neuroimaging 174, 116–120 (2009).
Narr, K. L. et al. Increases in regional subarachnoid CSF without apparent cortical gray matter deficits in schizophrenia: modulating effects of sex and age. Am. J. Psychiatry 160, 2169–2180 (2003).
Arango, C. et al. Patterns of cranial, brain and sulcal CSF volumes in male and female deficit and nondeficit patients with schizophrenia. Psychiatry Res. Neuroimaging 162, 91–100 (2008).
Matsumae, M. et al. Age-related changes in intracranial compartment volumes in normal adults assessed by magnetic resonance imaging. J. Neurosurg. 84, 982–991 (1996).
Jenkins, R., Fox, N. C., Rossor, A. M., Harvey, R. J. & Rossor, M. N. Intracranial volume and Alzheimer disease: evidence against the cerebral reserve hypothesis. Archives Neurol. 57, 220–224 (2000).
Edland, S. et al. Total intracranial volume: normative values and lack of association with Alzheimer’s disease. Neurology 59, 272–274 (2002).
Lenroot, R. K. et al. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. NeuroImage 36, 1065–1073 (2007).
Lenroot, R. K. & Giedd, J. N. Sex differences in the adolescent brain. Brain Cogn. 72, 46–55 (2010).
Kempton, M. J. et al. A comprehensive testing protocol for MRI neuroanatomical segmentation techniques: evaluation of a novel lateral ventricle segmentation method. NeuroImage 58, 1051–1059 (2011).
Gulban, O. F., Schneider, M., Marquardt, I., Haast, R. A. M. & De Martino, F. A scalable method to improve gray matter segmentation at ultra high field MRI. PLoS ONE 13, 1–31 (2018).
Hoffmann, M., Hoopes, A., Fischl, B. & Dalca, A. V. Anatomy-specific acquisition-agnostic affine registration learned from fictitious images. In Proc. SPIE 12464, Medical Imaging 2023: Image Processing, 1246402 (ed Olivier, C.) https://doi.org/10.1117/12.2653251 (SPIE, 2023).
Hoffmann, M. et al. SynthMorph: learning contrast-invariant registration without acquired images. IEEE Trans. Med. Imaging 41, 543–558 (2021).
Caspi, Y. et al. Changes in the intracranial volume from early adulthood to the sixth decade of life: a longitudinal study. NeuroImage 220, 116842 (2020).
Dosovitskiy, A. et al. An image is worth 16 × 16 words: transformers for image recognition at scale. In International Conference on Learning Representations 2021 https://openreview.net/pdf?id=YicbFdNTTy (ICLR, 2021).
Wang, W. et al. Pyramid vision transformer: a versatile backbone for dense prediction without convolutions. In Proc. IEEE/CVF International Conference on Computer Vision https://doi.org/10.1109/ICCV48922.2021 (IEEE, 2021).
Vaswani, A. Attention is all you need. In 31st Conference on Neural Information Processing Systems (eds Luxburg, U. et al.) https://papers.nips.cc/paper_files/paper/2017/file/3f5ee243547dee91fbd053c1c4a845aa-Paper.pdf (NIPS, 2017).
Xu, J., Li, Z., Du, B., Zhang, M. & Liu, J. Reluplex made more practical: Leaky ReLU. In 2020 IEEE Symposium on Computers and Communications (ISCC) https://doi.org/10.1109/ISCC50000.2020 (IEEE Computer Society, 2020).
Li, J., Wang, Y., Wang, S., Zhang, K. & Li, G. Landmark-guided rigid registration for temporomandibular joint MRI-CBCT images with large field-of-view difference. In Machine Learning in Medical Imaging: 12th International Workshop (eds Lian, C. et al.) 527–536 (Springer, 2021).
Balakrishnan, G., Zhao, A., Sabuncu, M. R., Guttag, J. & Dalca, A. V. VoxelMorph: a learning framework for deformable medical image registration. IEEE Trans. Med. Imaging 38, 1788–1800 (2019).
Jaderberg, M. et al. Spatial transformer networks. In Advances in Neural Information Processing Systems (eds Cortes, C. et al.) 28 (Curran Associates Inc, 2015).
Fonov, V. et al. Unbiased average age-appropriate atlases for pediatric studies. NeuroImage 54, 313–327 (2011).
Mazziotta, J. et al. A probabilistic atlas and reference system for the human brain: International Consortium for Brain Mapping (ICBM). Phil. Trans. R. Soc. B 356, 1293–1322 (2001).
Liu, W. et al. Longitudinal test–retest neuroimaging data from healthy young adults in southwest China. Sci. Data 4, 1–9 (2017).
Wei, D. et al. Structural and functional brain scans from the cross-sectional Southwest University Adult Lifespan Dataset. Sci. Data 5, 1–10 (2018).
Zhu, J. & Qiu, A. Chinese adult brain atlas with functional and white matter parcellation. Sci. Data 9, 352–362 (2022).
Acknowledgements
Limei Wang, Y.S. and Li Wang were supported by the National Institute of Mental Health under award numbers MH133845, MH117943, MH123202 and MH116225. G.L. was supported by the National Institutes of Health under award numbers MH133845, MH117943, MH123202, MH116225, AG075582 and NS128534. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work also utilizes approaches developed by NIH grants (U01MH110274 and R01MH104324) and the efforts of the UNC/UMN Baby Connectome Project Consortium.
Author information
Authors and Affiliations
Contributions
Limei Wang: methodology, implementation, validation, writing and editing; Y.S.: validation, review and editing; J.S., R.A.I.B., A.A.-B. and L.D.: editing and providing brain volumes by FreeSurfer/Infant FreeSurfer and so on; G.L.: review; J.T.E.: resources; W.L.: resources; Li Wang: methodology, validation, supervision, review, editing, resources, project administration and funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Biomedical Engineering thanks Guray Erus, Philipp Vollmuth, Xi-Nian Zuo and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Comparison of lifespan brain volume by the LifespanStrip method and by previous work.
Lifespan charts in terms of total brain volume (mm3) by previous work50 (left) and by the LifespanStrip framework (right).
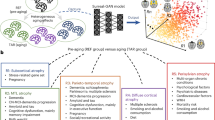
Extended Data Fig. 2 Flowchart of the atlases-powered lifespan skull-stripping (LifespanStrip) framework.
a, The LifespanStrip framework consists of a brain-extraction module and a registration module. The brain-extraction module is designed to generate an initial estimation of brain intracranial cavity. The registration module aligns an atlas with the estimated brain to offer personalized prior knowledge. Subsequently, the deformation field is applied to the atlas, resulting in the final brain mask. b, The brain-extraction network integrates dual convolution (DC) and dual transformer (DT), detailed in the bottom right corner. c, The registration module includes both rigid registration and deformable registration components.
Supplementary information
Source data
Source Data Fig. 4
Source data.
Source Data Fig. 6
Source data.
Source Data Fig. 7
Source data.
Source Data Fig. 8
Source data.
Source Data Extended Data Fig. 1
Source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, L., Sun, Y., Seidlitz, J. et al. A lifespan-generalizable skull-stripping model for magnetic resonance images that leverages prior knowledge from brain atlases. Nat. Biomed. Eng 9, 700–715 (2025). https://doi.org/10.1038/s41551-024-01337-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-024-01337-w