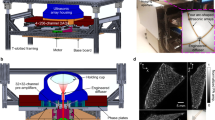
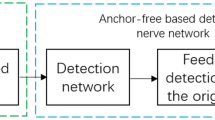
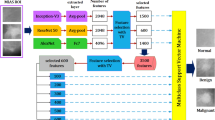
Abstract
Breast cancer diagnosis is crucial due to the high prevalence and mortality rate associated with the disease. However, mammography involves ionizing radiation and has compromised sensitivity in radiographically dense breasts, ultrasonography lacks specificity and has operator-dependent image quality, and magnetic resonance imaging faces high cost and patient exclusion. Photoacoustic computed tomography (PACT) offers a promising solution by combining light and ultrasound for high-resolution imaging that detects tumour-related vasculature changes. Here we introduce a workflow using panoramic PACT for breast lesion characterization, offering detailed visualization of vasculature irrespective of breast density. Analysing PACT features of 78 breasts in 39 patients, we develop learning-based classifiers to distinguish between normal and suspicious tissue, achieving a maximum area under the receiver operating characteristic curve of 0.89, which is comparable with that of conventional imaging standards. We further differentiate malignant and benign lesions using 13 features. Finally, we developed a learning-based model to segment breast lesions. Our study identifies PACT as a non-invasive and sensitive imaging tool for breast lesion evaluation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
118,99 € per year
only 9,92 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The calculated features for all patients and the classification results are available on Figshare at https://doi.org/10.6084/m9.figshare.28675031 (ref. 81). The rest of the main data supporting the results in this study is available within the article and its Supplementary Information. The PA data are available for research purposes from the corresponding author on reasonable request.
Code availability
The code for data analysis is available on Figshare at https://doi.org/10.6084/m9.figshare.28675031 (ref. 81). The original code for MedSAM is available on GitHub82. We applied this code to our dataset with the customized settings described in Methods. We have opted not to make reconstruction and post-processing codes (described in detail in Methods and ref. 74) publicly available because the code is proprietary and used for other projects.
References
Feuer, E. J. et al. The lifetime risk of developing breast cancer. J. Natl Cancer Inst. 85, 892–897 (1993).
Hockenberger, S. J. Fibrocystic breast disease: every woman is at risk. Plast. Aesthet. Nurs. 13, 37–40 (1993).
Siegel, R. L., Miller, K. D., Fuchs, H. E. & Jemal, A. Cancer statistics, 2021. CA: Cancer J. Clin. 71, 7–33 (2021).
Spak, D. A., Plaxco, J. S., Santiago, L., Dryden, M. J. & Dogan, B. E. BI-RADS fifth edition: a summary of changes. Diagn. Interv. Imaging 98, 179–190 (2017).
Pesapane, F. et al. Will traditional biopsy be substituted by radiomics and liquid biopsy for breast cancer diagnosis and characterisation? Med. Oncol. 37, 29 (2020).
Popli, M. B., Teotia, R., Narang, M. & Krishna, H. Breast positioning during mammography: mistakes to be avoided. Breast Cancer 8, 119–124 (2014).
Kolb, T. M., Lichy, J. & Newhouse, J. H. Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: an analysis of 27,825 patient evaluations. Radiology 225, 165–175 (2002).
Berg, W. A. et al. Diagnostic accuracy of mammography, clinical examination, US, and MR imaging in preoperative assessment of breast cancer. Radiology 233, 830–849 (2004).
von Euler-Chelpin, M., Lillholm, M., Vejborg, I., Nielsen, M. & Lynge, E. Sensitivity of screening mammography by density and texture: a cohort study from a population-based screening program in Denmark. Breast Cancer Res. 21, 111 (2019).
Brem, R. F., Lenihan, M. J., Lieberman, J. & Torrente, J. Screening breast ultrasound: past, present, and future. Am. J. Roentgenol. 204, 234–240 (2015).
Lehman, C. D. et al. Cancer yield of mammography, MR, and US in high-risk women: prospective multi-institution breast cancer screening study. Radiology 244, 381–388 (2007).
Corsetti, V. et al. Breast screening with ultrasound in women with mammography-negative dense breasts: evidence on incremental cancer detection and false positives, and associated cost. Eur. J. Cancer 44, 539–544 (2008).
Raza, S., Chikarmane, S. A., Neilsen, S. S., Zorn, L. M. & Birdwell, R. L. BI-RADS 3, 4, and 5 lesions: value of US in management—follow-up and outcome. Radiology 248, 773–781 (2008).
Errico, C. et al. Ultrafast ultrasound localization microscopy for deep super-resolution vascular imaging. Nature 527, 499–502 (2015).
Sigrist, R. M. S., Liau, J., Kaffas, A. E., Chammas, M. C. & Willmann, J. K. Ultrasound elastography: review of techniques and clinical applications. Theranostics 7, 1303–1329 (2017).
Perazella, M. A. Gadolinium-contrast toxicity in patients with kidney disease: nephrotoxicity and nephrogenic systemic fibrosis. Curr. Drug Saf. 3, 67–75 (2008).
Eshed, I., Althoff, C. E., Hamm, B. & Hermann, K.-G. A. Claustrophobia and premature termination of magnetic resonance imaging examinations. J. Magn. Reson. Imaging 26, 401–404 (2007).
Faris, O. P. & Shein, M. J. Government viewpoint: US Food & Drug Administration: pacemakers, ICDs and MRI. Pacing Clin. Electrophysiol. 28, 268–269 (2005).
Leff, D. R. et al. Diffuse optical imaging of the healthy and diseased breast: a systematic review. Breast Cancer Res. Treat. 108, 9–22 (2008).
Lin, L. & Wang, L. V. The emerging role of photoacoustic imaging in clinical oncology. Nat. Rev. Clin. Oncol. 19, 365–384 (2022).
Wang, L. V. Multiscale photoacoustic microscopy and computed tomography. Nat. Photonics 3, 503–509 (2009).
Lin, L. et al. Single-breath-hold photoacoustic computed tomography of the breast. Nat. Commun. 9, 2352 (2018).
Lin, L. et al. Photoacoustic computed tomography of breast cancer in response to neoadjuvant chemotherapy. Adv. Sci. https://doi.org/10.1002/advs.202003396 (2021).
Lin, L. et al. High-speed three-dimensional photoacoustic computed tomography for preclinical research and clinical translation. Nat. Commun. 12, 882 (2021).
Han, S., Lee, H., Kim, C. & Kim, J. Review on multispectral photoacoustic analysis of cancer: thyroid and breast. Metabolites 12, 382 (2022).
Dantuma, M. et al. Fully three-dimensional sound speed-corrected multi-wavelength photoacoustic breast tomography. Preprint at https://arxiv.org/abs/2308.06754 (2023).
Weidner, N., Semple, J. P., Welch, W. R. & Folkman, J. Tumor angiogenesis and metastasis—correlation in invasive breast carcinoma. N. Engl. J. Med. 324, 1–8 (1991).
Schneider, B. P. & Miller, K. D. Angiogenesis of breast cancer. J. Clin. Oncol. 23, 1782–1790 (2005).
Reynolds, A. R. et al. Stimulation of tumor growth and angiogenesis by low concentrations of RGD-mimetic integrin inhibitors. Nat. Med. 15, 392–400 (2009).
Vaupel, P., Mayer, A., Briest, S. & Höckel, M. in Oxygen Transport to Tissue XXVI (eds. Okunieff, P. et al.) 333–342 (Springer, 2005); https://doi.org/10.1007/0-387-26206-7_44
Gilkes, D. M. & Semenza, G. L. Role of hypoxia-inducible factors in breast cancer metastasis. Future Oncol. 9, 1623–1636 (2013).
Folkman, J. Role of angiogenesis in tumor growth and metastasis. Semin. Oncol. 29, 15–18 (2002).
Wang, L. V. & Hu, S. Photoacoustic tomography: in vivo imaging from organelles to organs. Science 335, 1458–1462 (2012).
Ermilov, S. A. et al. Laser optoacoustic imaging system for detection of breast cancer. J. Biomed. Opt. 14, 024007 (2009).
Li, X., Heldermon, C. D., Yao, L., Xi, L. & Jiang, H. High resolution functional photoacoustic tomography of breast cancer. Med. Phys. 42, 5321–5328 (2015).
Heijblom, M. et al. Photoacoustic image patterns of breast carcinoma and comparisons with magnetic resonance imaging and vascular stained histopathology. Sci. Rep. 5, 11778 (2015).
Schoustra, S. M. et al. Imaging breast malignancies with the Twente Photoacoustic Mammoscope 2. PLoS ONE 18, e0281434 (2023).
Menezes, G. L. G. et al. Downgrading of breast masses suspicious for cancer by using optoacoustic breast imaging. Radiology 288, 355–365 (2018).
Neuschler, E. I. et al. A pivotal study of optoacoustic imaging to diagnose benign and malignant breast masses: a new evaluation tool for radiologists. Radiology 287, 398–412 (2018).
Dogan, B. E. et al. Optoacoustic imaging and gray-scale US features of breast cancers: correlation with molecular subtypes. Radiology 292, 564–572 (2019).
Nyayapathi, N. et al. Photoacoustic dual-scan mammoscope: results from 38 patients. Biomed. Opt. Express 12, 2054–2063 (2021).
Abeyakoon, O. et al. An optoacoustic imaging feature set to characterise blood vessels surrounding benign and malignant breast lesions. Photoacoustics https://doi.org/10.1016/j.pacs.2022.100383 (2022).
Zheng, W. et al. Deep learning enhanced volumetric photoacoustic imaging of vasculature in human. Adv. Sci. 10, 2301277 (2023).
Rodrigues, J. et al. Machine learning enabled photoacoustic spectroscopy for noninvasive assessment of breast tumor progression in vivo: a preclinical study. ACS Sens. 9, 589–601 (2024).
Li, G. et al. Deep learning combined with attention mechanisms to assist radiologists in enhancing breast cancer diagnosis: a study on photoacoustic imaging. Biomed. Opt. Express 15, 4689–4704 (2024).
Center for Devices and Radiological Health. Imagio Breast Imaging System—P200003 (FDA, 2021).
Pereira, R. et al. Evaluation of the accuracy of mammography, ultrasound and magnetic resonance imaging in suspect breast lesions. Clinics 75, e1805 (2020).
Fitzjohn, J., Zhou, C. & Chase, J. G. Critical assessment of mammography accuracy. IFAC-PapersOnLine 56, 5620–5625 (2023).
Park, A. Y. et al. An innovative ultrasound technique for evaluation of tumor vascularity in breast cancers: superb micro-vascular imaging. J. Breast Cancer 19, 210–213 (2016).
Chang, Y.-C., Huang, Y.-H., Huang, C.-S. & Chang, R.-F. Vascular morphology and tortuosity analysis of breast tumor inside and outside contour by 3-D power Doppler ultrasound. Ultrasound Med. Biol. 38, 1859–1869 (2012).
Park, A. Y. et al. A prospective study on the value of ultrasound microflow assessment to distinguish malignant from benign solid breast masses: association between ultrasound parameters and histologic microvessel densities. Korean J. Radiol. 20, 759–772 (2019).
Hintze, J. L. & Nelson, R. D. Violin plots: a box plot-density trace synergism. Am. Stat. 52, 181–184 (1998).
Good, P. Permutation Tests: A Practical Guide to Resampling Methods for Testing Hypotheses (Springer Science & Business Media, 2013).
Freund, Y. & Schapire, R. E. A decision-theoretic generalization of on-line learning and an application to boosting. J. Comput. Syst. Sci. 55, 119–139 (1997).
Chen, T. & Guestrin, C. XGBoost: a scalable tree boosting system. In Proc. 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining 785–794 (Association for Computing Machinery, 2016); https://doi.org/10.1145/2939672.2939785
van der Maaten, L. & Hinton, G. Visualizing data using t-SNE. J. Mach. Learn. Res. 9, 2579–2605 (2008).
Ma, J. et al. Segment anything in medical images. Nat. Commun. 15, 654 (2024).
Wu, C., Pineda, F., Hormuth, D. A. II, Karczmar, G. S. & Yankeelov, T. E. Quantitative analysis of vascular properties derived from ultrafast DCE-MRI to discriminate malignant and benign breast tumors. Magn. Reson. Med. 81, 2147–2160 (2019).
Weidner, N. Intratumor microvessel density as a prognostic factor in cancer. Am. J. Pathol. 147, 9–19 (1995).
Smith, A. M., Mancini, M. C. & Nie, S. Second window for in vivo imaging. Nat. Nanotechnol. 4, 710–711 (2009).
Jacques, S. L. Optical properties of biological tissues: a review. Phys. Med. Biol. 58, R37–R61 (2013).
Li, L. et al. Single-impulse panoramic photoacoustic computed tomography of small-animal whole-body dynamics at high spatiotemporal resolution. Nat. Biomed. Eng. 1, 0071 (2017).
Fenner, J. et al. Macroscopic stiffness of breast tumors predicts metastasis. Sci. Rep. 4, 5512 (2014).
Ramião, N. G. et al. Biomechanical properties of breast tissue, a state-of-the-art review. Biomech. Model. Mechanobiol. 15, 1307–1323 (2016).
Berg, W. A. et al. Combined screening with ultrasound and mammography vs mammography alone in women at elevated risk of breast cancer. JAMA 299, 2151–2163 (2008).
Sardanelli, F. et al. Multicenter surveillance of women at high genetic breast cancer risk using mammography, ultrasonography, and contrast-enhanced magnetic resonance imaging (the High Breast Cancer Risk Italian 1 Study): final results. Invest. Radiol. 46, 94–105 (2011).
Shen, S. et al. A multi-centre randomised trial comparing ultrasound vs mammography for screening breast cancer in high-risk Chinese women. Br. J. Cancer 112, 998–1004 (2015).
Guo, R., Lu, G., Qin, B. & Fei, B. Ultrasound imaging technologies for breast cancer detection and management: a review. Ultrasound Med. Biol. 44, 37–70 (2018).
Devolli-Disha, E., Manxhuka-Kërliu, S., Ymeri, H. & Kutllovci, A. Comparative accuracy of mammography and ultrasound in women with breast symptoms according to age and breast density. Biomol. Biomed. 9, 131–136 (2009).
Hanley, J. A. & McNeil, B. J. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 143, 29–36 (1982).
Qian, X. et al. Prospective assessment of breast cancer risk from multimodal multiview ultrasound images via clinically applicable deep learning. Nat. Biomed. Eng. 5, 522–532 (2021).
Witowski, J. et al. Improving breast cancer diagnostics with deep learning for MRI. Sci. Transl. Med. 14, eabo4802 (2022).
American National Standards Institute. ANSI Z136.1-2014—American National Standard for Safe Use of Lasers. (Laser Institute of America, 2014).
Xu, M. & Wang, L. V. Universal back-projection algorithm for photoacoustic computed tomography. Phys. Rev. E 71, 016706 (2005).
Frangi, A. F., Niessen, W. J., Vincken, K. L. & Viergever, M. A. Multiscale vessel enhancement filtering. In Proc. Medical Image Computing and Computer-Assisted Intervention (eds Wells, W. M. et al.) 130–137 (Springer, 1998).
Cho, S., Baik, J., Managuli, R. & Kim, C. 3D PHOVIS: 3D photoacoustic visualization studio. Photoacoustics 18, 100168 (2020).
Lam, L., Lee, S. W. & Suen, C. Y. Thinning methodologies—a comprehensive survey. IEEE Trans. Pattern Anal. Mach. Intell. 14, 869–885 (1992).
Tong, X. et al. Non-invasive 3D photoacoustic tomography of angiographic anatomy and hemodynamics of fatty livers in rats. Adv. Sci. 10, 2205759 (2023).
Hu, M.-K. Visual pattern recognition by moment invariants. IRE Trans. Inf. Theory 8, 179–187 (1962).
Thirion, J.-P. Image matching as a diffusion process: an analogy with Maxwell’s demons. Med. Image Anal. 2, 243–260 (1998).
Tong, X. & Liu, C. Panoramic photoacoustic computed tomography with learning-based classification and segmentation enhances breast lesion characterization. Datasets. figshare https://doi.org/10.6084/m9.figshare.28675031 (2025).
Ma, J. et al. MedSAM: segment anything model for medical image analysis. Source code. Github https://github.com/bowang-lab/MedSAM (2023).
Acknowledgements
We thank Y. Aborahama and G. Zhao for machine-learning-related discussion, and R. Nelson, D. Schmolze and L. Vora for their interpretation of the radiology findings. This project has been made possible in part by National Institutes of Health grants R35 CA220436 (Outstanding Investigator Award), R01 CA282505, U01 EB029823 and R01 EB028277. Y.Z. was sponsored by the National Institutes of Health grant K99 EB035645. C.Z.L. was sponsored by the National Institutes of Health grants NIGMS T32 GM008042 and NIGMS T32 GM152342.
Author information
Authors and Affiliations
Contributions
L.V.W., L.D.Y., L.L.L., L.L. and X.T. conceived and designed the study. X.T., L.L. and R.C. constructed the hardware system. Y.Z. constructed the control program. P.H. and X.T. developed the software system and the reconstruction algorithm. X.T., C.Z.L., Y.L. and L.L. performed the experiments. X.T., C.Z.L., Y.L., R.C. and J.Z. analysed the data. L.D.Y., L.L.L., M.I., J.D., S.D.S., J.T. and A.K. guided the patient study. L.V.W. supervised the project. All authors contributed to writing the paper.
Corresponding author
Ethics declarations
Competing interests
L.V.W. has a financial interest in Microphotoacoustics Inc., CalPACT LLC and Union Photoacoustic Technologies Ltd., which, however, did not support this work. The other authors declare no competing interests.
Peer review
Peer review information
Nature Biomedical Engineering thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–27 and Tables 1–7.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tong, X., Liu, C.Z., Luo, Y. et al. Panoramic photoacoustic computed tomography with learning-based classification enhances breast lesion characterization. Nat. Biomed. Eng (2025). https://doi.org/10.1038/s41551-025-01435-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41551-025-01435-3