Abstract
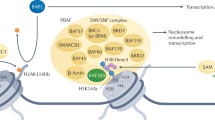
PBRM1 encodes an accessory subunit of the PBAF SWI/SNF chromatin remodeller, and the inactivation of PBRM1 is a frequent event in kidney cancer. However, the impact of PBRM1 loss on chromatin remodelling is not well examined. Here we show that, in VHL-deficient renal tumours, PBRM1 deficiency results in ectopic PBAF complexes that localize to de novo genomic loci, activating the pro-tumourigenic NF-κB pathway. PBRM1-deficient PBAF complexes retain the association between SMARCA4 and ARID2, but have loosely tethered BRD7. The PBAF complexes redistribute from promoter proximal regions to distal enhancers containing NF-κB motifs, heightening NF-κB activity in PBRM1-deficient models and clinical samples. The ATPase function of SMARCA4 maintains chromatin occupancy of pre-existing and newly acquired RELA specific to PBRM1 loss, activating downstream target gene expression. Proteasome inhibitor bortezomib abrogates RELA occupancy, suppresses NF-κB activation and delays growth of PBRM1-deficient tumours. In conclusion, PBRM1 safeguards the chromatin by repressing aberrant liberation of pro-tumourigenic NF-κB target genes by residual PBRM1-deficient PBAF complexes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
209,00 € per year
only 17,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
ChIP–seq, RNA-seq and ATAC-seq data that support the findings of this study have been deposited in the Gene Expression Omnibus (GEO) under accession code GSE152735 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE152735). Mass spectrometry data have been deposited in ProteomeXchange with the primary accession code PXD020553 and PXD020554 (http://proteomecentral.proteomexchange.org/cgi/GetDataset?ID=PXD020554; http://proteomecentral.proteomexchange.org/cgi/GetDataset?ID=PXD020553). Sequencing tags were mapped against the human reference genome (hg19) (http://hgdownload.cse.ucsc.edu/goldenpath/hg19/chromosomes/). RNA-seq reads were mapped against GENCODE annotation (gencode.v19.annotation.gtf) (https://ftp.ebi.ac.uk/pub/databases/gencode/Gencode_human/release_19/gencode.v19.annotation.gtf.gz). The human kidney renal cell carcinoma data were derived from the TCGA Research Network (http://cancergenome.nih.gov/). All other data supporting the findings of this study are available from the corresponding author on reasonable request. Source data are provided with this paper.
Code availability
Code is available at https://github.com/xiaosaiyao/PBRM1.
References
Varela, I. et al. Exome sequencing identifies frequent mutation of the SWI/SNF complex gene PBRM1 in renal carcinoma. Nature 469, 539–542 (2011).
Gerlinger, M. et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N. Engl. J. Med. 366, 883–892 (2012).
Gerlinger, M. et al. Genomic architecture and evolution of clear cell renal cell carcinomas defined by multiregion sequencing. Nat. Genet. 46, 225–233 (2014).
Turajlic, S. et al. Tracking cancer evolution reveals constrained routes to metastases: TRACERx Renal. Cell 173, 581–594.e512 (2018).
Nargund, A. M. et al. The SWI/SNF protein PBRM1 restrains VHL-loss-driven clear cell renal cell carcinoma. Cell Rep. 18, 2893–2906 (2017).
Gu, Y.-F. et al. Modeling renal cell carcinoma in mice: Bap1 and Pbrm1 inactivation drive tumor grade. Cancer Discov. 7, 900–917 (2017).
King, H. W. & Klose, R. J. The pioneer factor OCT4 requires the chromatin remodeller BRG1 to support gene regulatory element function in mouse embryonic stem cells. eLife 6, e22631 (2017).
Xu, G. et al. ARID1A determines luminal identity and therapeutic response in estrogen-receptor-positive breast cancer. Nat. Genet. 52, 198–207 (2020).
Slaughter, M. J. et al. PBRM1 bromodomains variably influence nucleosome interactions and cellular function. J. Biol. Chem. 293, 13592–13603 (2018).
Liao, L. et al. High affinity binding of H3K14ac through collaboration of bromodomains 2, 4 and 5 is critical for the molecular and tumor suppressor functions of PBRM 1. Mol. Oncol. 13, 811–828 (2019).
Xia, W. et al. BAF180 is a critical regulator of p21 induction and a tumor suppressor mutated in breast cancer. Cancer Res. 68, 1667–1674 (2008).
Liao, L. et al. Multiple tumor suppressors regulate a HIF-dependent negative feedback loop via ISGF3 in human clear cell renal cancer. eLife 7, e37925 (2018).
Brownlee, Peter et al. BAF180 promotes cohesion and prevents genome instability and aneuploidy. Cell Rep. 6, 973–981 (2014).
Espana-Agusti, J., Warren, A., Chew, S. K., Adams, D. J. & Matakidou, A. Loss of PBRM1 rescues VHL dependent replication stress to promote renal carcinogenesis. Nat. Commun. 8, 2026 (2017).
Porter, E. G. et al. PBRM1 regulates stress response in epithelial cells. iScience 15, 196–210 (2019).
Kakarougkas, A. et al. Requirement for PBAF in transcriptional repression and repair at DNA breaks in actively transcribed regions of chromatin. Mol. Cell 55, 723–732 (2014).
McDermott, D. F. et al. Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat. Med. 24, 749–757 (2018).
Motzer, R. J. et al. Molecular subsets in renal cancer determine outcome to checkpoint and angiogenesis blockade. Cancer Cell 38, 803–817.e804 (2020).
Miao, D. et al. Genomic correlates of response to immune checkpoint therapies in clear cell renal cell carcinoma. Science 359, 801–806 (2018).
Braun, D. A. et al. Clinical validation of PBRM1 alterations as a marker of immune checkpoint inhibitor response in renal cell carcinoma. JAMA Oncol. 5, 1631 (2019).
Hakimi, A. A. et al. A pan-cancer analysis of PBAF complex mutations and their association with immunotherapy response. Nat. Commun. 11, 4168 (2020).
Pan, D. et al. A major chromatin regulator determines resistance of tumor cells to T cell–mediated killing. Science 359, 770–775 (2018).
Liu, X.-D. et al. PBRM1 loss defines a nonimmunogenic tumor phenotype associated with checkpoint inhibitor resistance in renal carcinoma. Nat. Commun. 11, 2135 (2020).
Gao, W., Li, W., Xiao, T., Liu, X. S. & Kaelin, W. G. Inactivation of the PBRM1 tumor suppressor gene amplifies the HIF-response in VHL−/− clear cell renal carcinoma. Proc. Natl Acad. Sci. USA 114, 1027–1032 (2017).
Wang, X. et al. SMARCB1-mediated SWI/SNF complex function is essential for enhancer regulation. Nat. Genet. 49, 289–295 (2017).
Wang, S. et al. Target analysis by integration of transcriptome and ChIP–seq data with BETA. Nat. Protoc. 8, 2502–2515 (2013).
Taniguchi, K. & Karin, M. NF-κB, inflammation, immunity and cancer: coming of age. Nat. Rev. Immunol. 18, 309–324 (2018).
Iurlaro, M. et al. Mammalian SWI/SNF continuously restores local accessibility to chromatin. Nat. Genet. 53, 279–287 (2021).
Schick, S. et al. Acute BAF perturbation causes immediate changes in chromatin accessibility. Nat. Genet. 53, 269–278 (2021).
Schoenfeld, D. A. Characterizing the Mechanism of Tumor Suppression by PBRM1 in Clear Cell Renal Cell Carcinoma. PhD thesis, Columbia Univ. https://doi.org/10.7916/D8B56JJX (2015).
Juvekar, A. et al. Bortezomib induces nuclear translocation of IκBα resulting in gene-specific suppression of NF-κB–dependent transcription and induction of apoptosis in CTCL. Mol. Cancer Res. 9, 183–194 (2011).
Pierce, J. W. et al. Novel inhibitors of cytokine-induced IκBα phosphorylation and endothelial cell adhesion molecule expression show anti-inflammatory effects in vivo. J. Biol. Chem. 272, 21096–21103 (1997).
Yuan, J., Chen, K., Zhang, W. & Chen, Z. Structure of human chromatin-remodelling PBAF complex bound to a nucleosome. Nature 605, 166–171 (2022).
Limpert, A. S. et al. NF-κB forms a complex with the chromatin remodeler BRG1 to regulate Schwann cell differentiation. J. Neurosci. 33, 2388–2397 (2013).
Nishida, J. et al. Epigenetic remodelling shapes inflammatory renal cancer and neutrophil-dependent metastasis. Nat. Cell Biol. 22, 465–475 (2020).
Zhang, J. et al. VHL substrate transcription factor ZHX2 as an oncogenic driver in clear cell renal cell carcinoma. Science 361, 290–295 (2018).
Huang, Z. et al. Efficacy of therapy with bortezomib in solid tumors: a review based on 32 clinical trials. Future Oncol. 10, 1795–1807 (2014).
Kondagunta, G. V. et al. Phase II trial of bortezomib for patients with advanced renal cell carcinoma. J. Clin. Oncol. 22, 3720–3725 (2004).
Falchook, G. S. et al. Targeting hypoxia-inducible factor-1α (HIF-1α) in combination with antiangiogenic therapy: a phase I trial of bortezomib plus bevacizumab. Oncotarget 5, 10280–10292 (2014).
Yao, X. et al. VHL deficiency drives enhancer activation of oncogenes in clear cell renal cell carcinoma. Cancer Discov 7, 1284–1305 (2017).
Li, H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. Preprint at arXiv https://doi.org/10.48550/arXiv.1303.3997 (2013).
Zhang, Y. et al. Model-based analysis of ChIP-Seq (MACS). Genome Biol. 9, R137 (2008).
Shen, L., Shao, N., Liu, X. & Nestler, E. ngs.plot: quick mining and visualization of next-generation sequencing data by integrating genomic databases. BMC Genomics 15, 284 (2014).
Ramírez, F. et al. deepTools2: a next generation web server for deep-sequencing data analysis. Nucleic Acids Res. 44, W160–W165 (2016).
Ross-Innes, C. S. et al. Differential oestrogen receptor binding is associated with clinical outcome in breast cancer. Nature 481, 389–393 (2012).
Lun, A. T. L. & Smyth, G. K. csaw: a Bioconductor package for differential binding analysis of ChIP–seq data using sliding windows. Nucleic Acids Res. 44, e45–e45 (2016).
Yu, G., Wang, L.-G. & He, Q.-Y. ChIPseeker: an R/Bioconductor package for ChIP peak annotation, comparison and visualization. Bioinformatics 31, 2382–2383 (2015).
Welch, R. P. et al. ChIP-Enrich: gene set enrichment testing for ChIP–seq data. Nucleic Acids Res. 42, e105–e105 (2014).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Buenrostro, J. D., Wu, B., Chang, H. Y. & Greenleaf, W. J. ATAC‐seq: a method for assaying chromatin accessibility genome‐wide. Curr. Protoc. Mol. Biol. https://doi.org/10.1002/0471142727.mb2129s109 (2015).
Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 17, 10 (2011).
Schep, A. N. et al. Structured nucleosome fingerprints enable high-resolution mapping of chromatin architecture within regulatory regions. Genome Res. 25, 1757–1770 (2015).
Acknowledgements
We thank L. Kai Koh for his administrative assistance, J. Workman, D. Reddy, R. Banchereau, A. Lun and J. Raab for their discussion of this project and C. Chuan Shih for his help with file management. This work was supported by the A*STAR Computational Resources Centre through the use of its high-performance computing facilities. X. Yao and M.S.W.N. were supported by IMCB core funds. J.H.H. is supported by National Research Medical Council Singapore under its Young Individual Research Grant (MOH-000232/MOH-OFYIRG18nov-0006) and Khoo Postdoctoral Fellowship Award (Duke-NUS-KPFA/2019/0034). B.T.T. is supported by National Medical Research Council Singapore Translational Research Investigator Award (NMRC MOH-000248-00) and NCC Cancer Fund and Verdant Foundation. A.M.N. is supported by St. Baldrick’s Foundation Research Award. P.G. is supported by the National Research Foundation Singapore under its NRF-NSFC funding programme (NRF2016NRF-NSFC001-057). R.M.S., L.C.W. and K.-Y.T. are supported by A-STAR core funding and National Research Foundation infrastructure grant (NRF-SIS) Singapore. W.L.T. is supported by National Medical Research Council Singapore (OFIRG17may061 and OFIRG19nov-0106) and National Research Foundation Singapore (NRF-CRP22-2019-0003 and NRF-CRP23-2019-0004). The organizations that provided the grants had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
X. Yao, J.H.H. and A.M.N. designed the project and analysed most of the experiments. B.T.T. conceived the project and provided supervision, suggestions and guidance. M.S.W.N., X. Yao, J.H.H., H.L.H., M.S., P.L.C., X. Ye, X.Y.K., J.K., Y.-F.P., Y.S.O., J.Y.G., L.P., D.H., S.T.T., M.L. and X.H.Y. performed most of the experiments. Z.L. and S.R.N. performed the in vivo experiments. X. Yao, P.G. and W.F.O. performed bioinformatics analysis. L.C.W., K.-Y.T. and R.M.S. performed mass spectrometry experiments. J.Q. provided sequencing support. J.C.T.L. and S.Y.R. provided patient samples. J.P.S.Y., Z.W., A.L., W.L.T., S.L., E.G., A.F., J.T., W.H., R.Y., K.T.-E.C. and P.T. provided resources, suggestions and guidance. X. Yao wrote the manuscript with input from J.H. Hong and A. Nargund. All authors discussed the results and contributed to the manuscript.
Corresponding authors
Ethics declarations
Competing interests
X. Yao, X. Ye and R.Y. are employees of Genentech Inc. The remaining authors declare no conflicts of interest. A Singapore Provisional Patent with X. Yao, J.H.H., A.M.N. and M.S.W.N. and B.T.T. as co-inventors has been filed (10202251154K).
Peer review
Peer review information
Nature Cell Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Effects of PBRM1 loss on tumor growth.
a) Immunofluorescence staining validates the loss of PBRM1 by CRISPR mediated knockout in 786-O and HK2 cells (results representative of 2 biological replicates). b) Representative images of colony formation assay of isogenic pairs cultured in normal 10% FBS or low serum conditions (results representative of 3 biological replicates). c) PBRM1-deficient ccRCC cell lines were transduced with either empty vector (EV) control or wild-type PBRM1, cultured in soft agar for 3 weeks and assessed for colony formation potential. d) Shown are the photographs of 786-O and NCC432 tumors harvested at the end of the in vivo tumor formation studies. e) Tumor weights of NCC2112 xenograft tumors with and without PBRM1 were measured at the end of in vivo tumor formation studies (n = 6 mice per group). Data are presented as mean values + /- s.d. P-value was computed by two-tailed t-test.
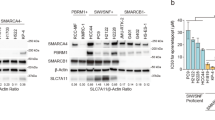
Extended Data Fig. 2 Effects of PBRM1 loss on PBAF integrity in NCC2112 cells.
a) Co-immunoprecipitation of PBRM1 in NCC2112 cells expressing empty vector control or wildtype PBRM1 protein. b) Co-immunoprecipitation of SMARCA4 in NCC2112 cells expressing empty vector control or wildtype PBRM1. c) Co-immunoprecipitation of BRD7 and ARID2 in NCC2112 cells expressing empty vector control or PBRM1. Results representative of 2 biological replicates in a-c.
Extended Data Fig. 3 Genomic localization of PBRM1 and other PBAF subunits.
a) PBRM1 ChIP-seq profiles of PBRM1-deficient NCC432 ccRCC cells expressing either empty vector (EV) or wild-type PBRM1. b) Genomic localization of PBRM1 binding sites in parental PBRM1-proficient cell lines (endogenous) and in PBRM1-deficient cell lines expressing wild-type PBRM1 cDNA (exogenous) annotated by ChIPseeker. c) Genomic localization of PBRM1, BRD7 and ARID2 ChIP-seq in 786-O cells shows promoter-centric occupancy of PBRM1 and BRD7 but equal distribution of ARID2 in both promoters and distal regions. d) While the majority of the PBRM1 binding sites are bound by all subunits of the PBAF subunits and SMARCA4 (canonical sites), there are also sites that are bound by PBRM1- or ARID2- deficient PBAF subcomplexes.
Extended Data Fig. 4 Effects of PBRM1 loss on the genomic occupancy of PBAF subunits.
a) Altered SMARCA4 peaks were linked to promoters of the nearest genes by ChIP-Enrich (defined as ± 2.5 kb of TSS) and the log 2 fold change (left) or difference in gene expression (right) after PBRM1 KO in 786-O cells (n = 1527 genes mapped to gained regions and n = 134 genes mapped to lost regions). P-values were computed by two-sided Wilcoxon test. Boxplots presented as median values + /- 25%. Lower whisker is the smallest observation greater than or equal to 25% quantile - 1.5 × interquantile range (IQR). Upper whisker represents the largest observation less than or equal to 75% + 1.5 × IQR. b) Peak regions of all the PBAF subunits were merged, and differences in occupancies of the various PBAF subunits between parental (WT) and PBRM1 knockout (KO) 786-O cells were plotted against the differences in PBRM1 occupancy for all regions. Many regions with large changes in SMARCA4 and ARID2 occupancy have no corresponding changes in PBRM1 occupancy, suggesting that these regions are located away from PBRM1 binding sites. Shown are the spearman correlation coefficients of the changes in the occupancy of PBAF subunits after PBRM1 KO. c) Spearman correlation of changes in chromatin occupancy between parental (WT) and PBRM1 knockout (KO) 786-O cells for all PBAF bound regions.
Extended Data Fig. 5 PBRM1 loss is associated with gain in NF-κB activity across various models.
a) BETA analysis with P-values calculated by Kolmogorov-Smirnov test. b) Gene expression changes of selected NF-κB targets after PBRM1 expression in 786-O PBRM1 KO cells. c-h) Enrichment of TNFa_signaling_via_NF-κB geneset in c) 786-O PBRM1 KO cells expressing empty vector or PBRM1, d) 769-P cells expressing sgRNA against non-targeting control or PBRM1), e) PBRM1-deficient NCC432 and f) PBRM1-deficient A704 cells expressing empty vector or PBRM1, g) genetic mouse model with VHL single knockout or double knockout of PBRM1 and VHL (DKO) and h) TCGA data (KIRC) of tumors deficient or proficient in PBRM1. P-values were calculated by Kolmogorov-Smirnov test and q value was adjusted for multiple hypotheses testing using gene permutation. i) Heatmap shows all the significantly upregulated genes of the TNFa_signaling_via_NF-κB geneset in the PBRM1-deficient TCGA KIRC samples relative to PBRM1-proficient samples. P-value computed by Wald test. j) Normalized gene expression of selected upregulated NF-κB targets in NCC432 expressing empty vector control or wildtype PBRM1 after TNFα stimulation for 6 hours measured by RT-qPCR (n = 3 technical replicates representative of 2 biological replicates). Data are presented as mean values + /- s.d. *p < 0.05, ** p < 0.01 (two-sided t-test). k-m) Enrichment of the Hallmark geneset inflammatory response in k) 786-O PBRM1 KO cells expressing empty vector or PBRM1, l) PBRM1-deficient NCC432 cells expressing empty vector or PBRM1 and m) genetic mouse model with VHL single knockout or double knockout of PBRM1 and VHL (DKO). P values were calculated by Kolmogorov-Smirnov test and q value was adjusted for multiple hypotheses testing using gene permutation. n) Gene expression changes of NF-κB target genes after shRNA-mediated knockdown of NF-κB subunits measured by RT-qPCR (n = 3 technical replicates). *p < 0.05, ** p < 0.01, *** p < 0.001, ****p < 0.0001 (two-sided t-test). Data are presented as mean values + /- s.d. o) Overlap of RELA targets defined by RELA ChIP occupancy and decreased expression after shRELA (p < 0.05 and log2FC < -0.5) with downregulated genes after PBRM1 restoration in PBRM1-KO 786-O cells (p < 0.05 and |log2FC | > 0.3). P value of the overlap was computed by hypergeometric test.
Extended Data Fig. 6 PBRM1-deficient tumors depend on SMARCA4 and ARID2 for NF-κB activity and tumor formation.
a) NCC432 cells expressing either empty vector (EV) or PBRM1 were treated with SMARCA2/4 ATPase inhibitor BRM014 for 3 days and the expression of NF-κB targets was evaluated by RT-qPCR (n = 3 technical replicates representative of 2 biological replicates). Data are presented as mean values + /- s.d. b) Colony formation of 786-O cells expressing shRNA against non-targeting control (NTC) or ARID2. c) Colony formation of 786-O cells expressing shRNA against non-targeting control (NTC) or SMARCA4. d) Immunoblotting and e) colony formation of NCC432 cells expressing shRNA against non-targeting control (NTC) or ARID2 (n = 3 biological replicates). Data are presented as mean values + /- s.d. P-value (two-sided test). f) Immunoblotting and g) colony formation of NCC432 cells expressing shRNA against non-targeting control (NTC) or SMARCA4 (n = 4 biological replicates). Data are presented as mean values + /- s.d. *p < 0.05, **p < 0.01, ***p < 0.001, **** p < 0.0001 (two-sided t-test). In vivo tumor formation in h) 786-O parental cells and i) 786-O PBRM1 KO cells expressing shRNA against Non-targeting control (NTC), ARID2 (clone 1) or SMARCA4 (clone 2) (n = 10 tumors per group). Data are presented as mean values + /- s.e.m.) for tumor growth and mean values + /- s.d. for tumor weight. **** p < 0.0001 (two-sided t-test).
Extended Data Fig. 7 NF-κB activation is mediated by changes in the chromatin.
a) Geneset enrichment of genes mapped to gained ARID2 and gained BRD7 peaks by ChIP-Enrich (Hypergeometric test). b) Protein expression of NF-κB subunits in cytoplasmic (cyto), soluble nuclear (sol nuc) or chromatin (chrom) fractions in 786-O PBRM1 WT, PBRM1 KO, PBRM1 KO expressing empty vector or PBRM1 (representative blot of 2 biological replicates). c) RELA peaks overlap significantly with total and gained ARID2 peaks (p-value calculated by hypergeometric test). d) UCSC browser view of selected NF-κB target genes.
Extended Data Fig. 8 Bortezomib abrogates RELA occupancy and selectively targets PBRM1 deficiency.
a) Colony formation of 786-O cells with and without PBRM1 treated with DMSO or NF-κB inhibitor, Bay 11-7085. b) Single sample gene-set enrichment (ssGSEA) of TNFa signaling via NF-κB in 786-O PBRM1 WT or KO cells treated with DMSO or 0.005 μM bortezomib (n = 3 biological replicates). Data are presented as median + /- 25%. Lower whisker is smallest observation greater than or equal to 25% quantile - 1.5 * interquantile range (IQR). Upper whisker represents the largest observation less than or equal to 75% + 1.5 * IQR. Amount of normalized RELA protein in the c) input or d) co-immunoprecipitated with SMARCA4 from 3 independent runs. Data are presented as mean values + /- s.d. e) Tornado plots of RELA and SMARCA4 ChIP-seq signals in parental 786-O or PBRM1-KO 786-O cells treated with DMSO or 0.005 μM bortezomib for 9 hours. f) Kaplan-Meier curves of NSG mice bearing 786-O isogenic cells treated with PBS vehicle control or 0.5 mg/kg bortezomib (WT: n = 10 PBS arm, median survival = 39 days, n = 10 bortezomib arm, median survival = 52 days, HR = 4.1, p = 3E-6, log rank test; KO: n = 10 PBS arm, median survival = 35 days, n = 10 bortezomib arm, median survival = 66 days, HR = 4.1, p = 3E-6, log rank test). g) Kaplan-Meier curves of NSG mice bearing NCC432 isogenic cells treated with PBS vehicle control or 0.5 mg/kg bortezomib (EV: n = 16 PBS arm, median survival = 42 days, n = 14 bortezomib arm, median survival = 49 days, HR = 2.5, p = 0.0009, log rank test; PBRM1: n = 12 PBS arm, median survival = 55 days, n = 14 bortezomib arm, median survival = 58 days, HR = 0.9, p = 0.7, log rank test). h) 786-O PBRM1 WT or KO cells were screened against the high-specific SGC epigenetic probe collection and cell viability was measured by colony formation after 1 week of treatment relative to DMSO control.
Supplementary information
Supplementary Table 1
Supplementary Table 1. Pathways upregulated in PBRM1-deficient KIRC TCGA tumours. Supplementary Table 2. A list of TNF targets upregulated by PBRM1 loss.
Source data
Fig. 1
Statistical source data.
Fig. 1
Unprocessed western blots.
Fig. 2
Statistical source data.
Fig. 2
Unprocessed western blots.
Fig. 5
Statistical source data.
Fig. 6
Statistical source data.
Fig. 6
Unprocessed western blots.
Fig. 8
Statistical source data.
Extended Data Fig. 1
Statistical source data.
Extended Data Fig. 2
Unprocessed western blots.
Extended Data Fig. 5
Statistical source data.
Extended Data Fig. 6
Statistical source data.
Extended Data Fig. 6
Unprocessed western blots.
Extended Data Fig. 7
Unprocessed western blots.
Extended Data Fig. 8
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yao, X., Hong, J.H., Nargund, A.M. et al. PBRM1-deficient PBAF complexes target aberrant genomic loci to activate the NF-κB pathway in clear cell renal cell carcinoma. Nat Cell Biol 25, 765–777 (2023). https://doi.org/10.1038/s41556-023-01122-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41556-023-01122-y
This article is cited by
-
Chromatin remodeling and cancer: the critical influence of the SWI/SNF complex
Epigenetics & Chromatin (2025)
-
PBRM1 directs PBAF to pericentromeres and protects centromere integrity
Nature Communications (2025)
-
Genomic landscape of diffuse glioma revealed by whole genome sequencing
Nature Communications (2025)
-
Alternative splicing of PBRM1 mediates resistance to PD-1 blockade therapy in renal cancer
The EMBO Journal (2024)
-
Chromatin remodellers as therapeutic targets
Nature Reviews Drug Discovery (2024)