Abstract
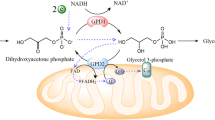
Glucose metabolism has been studied extensively, but the role of glucose-derived excretory glycerol remains unclear. Here we show that hypoxia induces NADH accumulation to promote glycerol excretion and this pathway consumes NADH continuously, thus attenuating its accumulation and reductive stress. Aldolase B accounts for glycerol biosynthesis by forming a complex with glycerol 3-phosphate dehydrogenases GPD1 and GPD1L. Blocking GPD1, GPD1L or glycerol 3-phosphate phosphatase exacerbates reductive stress and suppresses cell proliferation under hypoxia and tumour growth in vivo. Overexpression of these enzymes increases glycerol excretion but still reduces cell viability under hypoxia and tumour proliferation due to energy stress. AMPK inactivates aldolase B to mitigate glycerol synthesis that dissipates ATP, alleviating NADH accumulation-induced energy crisis. Therefore, glycerol biosynthesis/excretion regulates the trade-off between reductive stress and energy stress. Moreover, this mode of regulation seems to be prevalent in reductive stress-driven transformations, enhancing our understanding of the metabolic complexity and guiding tumour treatment.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
209,00 € per year
only 17,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
MS data have been deposited in ProteomeXchange with the primary accession code PXD056351 (http://proteomecentral.proteomexchange.org)50,51. The human cancer data were derived from the TCGA Research Network at http://cancergenome.nih.gov/. The dataset derived from this resource that supports the findings of this study is available in Supplementary Table 1. All other data supporting the findings of this study are available from the corresponding author on reasonable request. Source data are provided with this paper.
References
Hanahan, D. Hallmarks of cancer: new dimensions. Cancer Discov. 12, 31–46 (2022).
Terry, A. R. & Hay, N. Fuelling cancer cells. Nat. Rev. Endocrinol. 15, 71–72 (2019).
Yu, B. et al. Measuring tumor cycling hypoxia and angiogenesis using a side-firing fiber optic probe. J. Biophotonics 7, 552–564 (2014).
Carmeliet, P. & Jain, R. K. Angiogenesis in cancer and other diseases. Nature 407, 249–257 (2000).
Chappell, J. C., Payne, L. B. & Rathmell, W. K. Hypoxia, angiogenesis, and metabolism in the hereditary kidney cancers. J. Clin. Invest. 129, 442–451 (2019).
Vander Heiden, M. G. & DeBerardinis, R. J. Understanding the intersections between metabolism and cancer biology. Cell 168, 657–669 (2017).
Liu, M. et al. Inhibiting both proline biosynthesis and lipogenesis synergistically suppresses tumor growth. J. Exp. Med. 217, e20191226 (2020).
Yang, R. et al. Identification of purine biosynthesis as an NADH-sensing pathway to mediate energy stress. Nat. Commun. 13, 7031 (2022).
Titov, D. V. et al. Complementation of mitochondrial electron transport chain by manipulation of the NAD+/NADH ratio. Science 352, 231–235 (2016).
Missiaen, R., Lesner, N. P. & Simon, M. C. HIF: a master regulator of nutrient availability and metabolic cross-talk in the tumor microenvironment. EMBO J. 42, e112067 (2023).
Yang, R., Ying, G. & Li, B. Potential of electron transfer and its application in dictating routes of biochemical processes associated with metabolic reprogramming. Front. Med. 15, 679–692 (2021).
Sun, L., Suo, C., Li, S. T., Zhang, H. & Gao, P. Metabolic reprogramming for cancer cells and their microenvironment: beyond the Warburg Effect. Biochim. Biophys. Acta Rev. Cancer 1870, 51–66 (2018).
Mugabo, Y. et al. Identification of a mammalian glycerol-3-phosphate phosphatase: role in metabolism and signaling in pancreatic beta-cells and hepatocytes. Proc. Natl Acad. Sci. USA 113, E430–E439 (2016).
Nielsen, S. et al. Aquaporins in the kidney: from molecules to medicine. Physiol. Rev. 82, 205–244 (2002).
Zhang, L. & Tew, K. D. Reductive stress in cancer. Adv. Cancer Res 152, 383–413 (2021).
Sullivan, L. B. et al. Supporting aspartate biosynthesis is an essential function of respiration in proliferating cells. Cell 162, 552–563 (2015).
Billiard, J. et al. Quinoline 3-sulfonamides inhibit lactate dehydrogenase A and reverse aerobic glycolysis in cancer cells. Cancer Metab. 1, 19 (2013).
Kim, W. et al. Polyunsaturated fatty acid desaturation is a mechanism for glycolytic NAD+ recycling. Cell Metab. 29, 856–870.e7 (2019).
Obukowicz, M. G. et al. Novel, selective Δ6 or Δ5 fatty acid desaturase inhibitors as antiinflammatory agents in mice. J. Pharmacol. Exp. Ther. 287, 157–166 (1998).
Graziano, F. et al. Glycolysis gene expression analysis and selective metabolic advantage in the clinical progression of colorectal cancer. Pharmacogenomics J. 17, 258–264 (2017).
Finley, L. W. et al. SIRT3 opposes reprogramming of cancer cell metabolism through HIF1α destabilization. Cancer Cell 19, 416–428 (2011).
O’Donnell, J. L. et al. Oncological implications of hypoxia inducible factor-1α (HIF-1α) expression. Cancer Treat. Rev. 32, 407–416 (2006).
Tennant, D. A., Duran, R. V. & Gottlieb, E. Targeting metabolic transformation for cancer therapy. Nat. Rev. Cancer 10, 267–277 (2010).
Li, B. et al. Fructose-1,6-bisphosphatase opposes renal carcinoma progression. Nature 513, 251–255 (2014).
Grandjean, G. et al. Definition of a novel feed-forward mechanism for glycolysis-HIF1α signaling in hypoxic tumors highlights aldolase A as a therapeutic target. Cancer Res. 76, 4259–4269 (2016).
Cascone, T. et al. Increased Tumor Glycolysis Characterizes Immune Resistance to Adoptive T Cell Therapy. Cell Metab. 27, 977–987.e4 (2018).
Ou, X. et al. Crystal structures of human glycerol 3-phosphate dehydrogenase 1 (GPD1). J. Mol. Biol. 357, 858–869 (2006).
Cool, B. et al. Identification and characterization of a small molecule AMPK activator that treats key components of type 2 diabetes and the metabolic syndrome. Cell Metab. 3, 403–416 (2006).
Schaffer, B. E. et al. Identification of AMPK phosphorylation sites reveals a network of proteins involved in cell invasion and facilitates large-scale substrate prediction. Cell Metab. 22, 907–921 (2015).
Hardie, D. G., Schaffer, B. E. & Brunet, A. AMPK: an energy-sensing pathway with multiple inputs and outputs. Trends Cell Biol. 26, 190–201 (2016).
Steinberg, G. R. & Hardie, D. G. New insights into activation and function of the AMPK. Nat. Rev. Mol. Cell Biol. 24, 255–272 (2023).
Park, S. H. et al. Phosphorylation-activity relationships of AMPK and acetyl-CoA carboxylase in muscle. J. Appl Physiol. (1985) 92, 2475–2482 (2002).
Kim, J. & Guan, K. L. mTOR as a central hub of nutrient signalling and cell growth. Nat. Cell Biol. 21, 63–71 (2019).
Liu, Q. et al. Discovery of 1-(4-(4-propionylpiperazin-1-yl)-3-(trifluoromethyl)phenyl)-9-(quinolin-3-yl)benzo[h][1,6]naphthyridin-2(1H)-one as a highly potent, selective mammalian target of rapamycin (mTOR) inhibitor for the treatment of cancer. J. Med. Chem. 53, 7146–7155 (2010).
Zhou, G. et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Invest. 108, 1167–1174 (2001).
Li, M. et al. Aldolase B suppresses hepatocellular carcinogenesis by inhibiting G6PD and pentose phosphate pathways. Nat. Cancer 1, 735–747 (2020).
Liu, G. et al. Fructose-1,6-bisphosphate aldolase b depletion promotes hepatocellular carcinogenesis through activating insulin receptor signaling and lipogenesis. Hepatology 74, 3037–3055 (2021).
He, X. et al. Loss of hepatic aldolase B activates Akt and promotes hepatocellular carcinogenesis by destabilizing the Aldob/Akt/PP2A protein complex. PLoS Biol. 18, e3000803 (2020).
He, J. et al. Downregulation of ALDOB is associated with poor prognosis of patients with gastric cancer. Onco Targets Ther. 9, 6099–6109 (2016).
Sanders, E. & Diehl, S. Analysis and interpretation of transcriptomic data obtained from extended Warburg effect genes in patients with clear cell renal cell carcinoma. Oncoscience 2, 151–186 (2015).
Bu, P. et al. Aldolase B-Mediated Fructose Metabolism Drives Metabolic Reprogramming of Colon Cancer Liver Metastasis. Cell Metab. 27, 1249–1262.e4 (2018).
Chen, W. L. et al. Enhanced fructose utilization mediated by SLC2A5 is a unique metabolic feature of acute myeloid leukemia with therapeutic potential. Cancer Cell 30, 779–791 (2016).
Zhou, P. et al. High dietary fructose promotes hepatocellular carcinoma progression by enhancing O-GlcNAcylation via microbiota-derived acetate. Cell Metab. 35, 1961–1975.e6 (2023).
Cui, Y. et al. Fructose-induced mTORC1 activation promotes pancreatic cancer progression through inhibition of autophagy. Cancer Res. 83, 4063–4079 (2023).
Wang, Y. et al. Coordinative metabolism of glutamine carbon and nitrogen in proliferating cancer cells under hypoxia. Nat. Commun. 10, 201 (2019).
Qian, X. et al. Conversion of PRPS hexamer to monomer by AMPK-mediated phosphorylation inhibits nucleotide synthesis in response to energy stress. Cancer Discov. 8, 94–107 (2018).
Theret, M. et al. AMPKα1–LDH pathway regulates muscle stem cell self-renewal by controlling metabolic homeostasis. EMBO J. 36, 1946–1962 (2017).
Burg, J. S. & Espenshade, P. J. Regulation of HMG-CoA reductase in mammals and yeast. Prog. Lipid Res. 50, 403–410 (2011).
Li, L. et al. ALKBH5–PYCR2 positive feedback loop promotes proneural–mesenchymal transition via proline synthesis In GBM. J. Cancer 14, 1579–1591 (2023).
Chen, T. et al. iProX in 2021: connecting proteomics data sharing with big data. Nucleic Acids Res. 50, D1522–D1527 (2022).
Ma, J. et al. iProX: an integrated proteome resource. Nucleic Acids Res. 47, D1211–D1217 (2019).
Acknowledgements
We thank X. Liu (Metabolomics Facility at Tsinghua University Branch of China National Center for Protein Sciences, China) for technical help. This work was supported by the National Natural Science Foundation of China (82325038 and 82030093 to B.L.) and China Postdoctoral Science Foundation (GZB20230454 to R.Y.)
Author information
Authors and Affiliations
Contributions
B.L. conceived the study and designed experiments; X.Z. and R.Y. performed experiments; Q.C. prepared some constructs and cell lines; Z.G., P.H., X.L., C.B. and Z.L. collected and analysed data; L.Q., Y.F. and J.N. provided conceptual advice and gave technical support; B.L. wrote the manuscript; X.Z. and R.Y. edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Cell Biology thanks Lluis Fajas, Constantinos Koumenis and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Hypoxia promotes glycerol 3-phosphate biosynthesis.
a, The relative abundance of metabolites of glycolysis in HeLa cells under normoxia, hypoxia, or antimycin A (AntA, 2 μM) treatment for 8 h. b, The relative abundance of glycerol 3-phosphate and its precursor DHAP in HeLa cells under normoxia, hypoxia, or AntA (2 μM) treatment for 8 h. c, Isotopomer tracing analysis of glycerol 3-phosphate biosynthesis in HeLa cells cultured with 13C6-glucose (25 mM) or 13C5-glutamine (2 mM) for 8 h. d,e, Using N,O-bis(trimethylsilyl)trifluoroacetamide (BSTFA) to modify the hydroxyl groups of glycerol and glycerol 3-phosphate and detect them by gas chromatography-mass spectrometry (GC-MS). f, Isotopomer tracing analysis of excreted glycerol in HeLa cells cultured with 13C6-glucose (25 mM) under normoxia or hypoxia for 8 h. g,h, The levels of excreted glycerol determined by GC-MS or a liquid sample glycerol assay kit in HeLa cells cultured under normoxia and hypoxia for 24 h. Values are shown as mean ± SD, n = 3 biologically independent samples, two-tailed Student’s t-tests.
Extended Data Fig. 2 Hypoxia promotes glucose uptake and lactate excretion.
a, Glucose uptake of several cell types including tumor cells and endothelial cells cultured under normoxia and hypoxia for 24 h. b, Lactate excretion of several cell types including tumor cells and endothelial cells cultured under normoxia and hypoxia for 24 h. c-h, The glycerol excretion, glucose uptake, and lactate excretion in HeLa and A549 cells cultured under hypoxia as indicated. i, Western blot analysis of PGP, GPD1, GPD1L, GPD2, ALDOA, ALDOB, TPI1, and β-Actin expression in different cells as indicated. j, Immunoblot verification of GPD2 knockout in HeLa and A549 cells. k, Effects of GPD2 knockout on glycerol excretion of HeLa cells cultured under normoxia and hypoxia for 24 h. Values are shown as mean ± SD, n = 3 biologically independent samples (a-h, k), two-tailed Student’s t-tests. In i, data are verified in two replicates with similar results.
Extended Data Fig. 3 Effects of glycerol excretion on cell viability under hypoxia.
a, Effects of GPD1 and/or GPD1L knockout on colony formation in HeLa cells cultured for 10 days. b, Effects of PGP knockout and PGP re-expression on colony formation in HeLa cells cultured for 10 days. c, Effects of PGP knockout and PGP re-expression on glycerol excretion of A549 cells cultured under normoxia and hypoxia for 24 h. d, Effects of PGP knockout and PGP re-expression on cell viability of A549 cells cultured under normoxia and hypoxia for 48 h. e, Effects of PGP knockout and PGP re-expression on colony formation of A549 cells cultured for 10 days. f, Effects of PGP knockout and PGP re-expression on glycerol excretion of HepG2 cells cultured under normoxia and hypoxia for 24 h. g, Effects of PGP knockout and PGP re-expression on cell viability of HepG2 cells cultured under normoxia and hypoxia for 48 h. h, Effects of PGP knockout and PGP re-expression on colony formation of HepG2 cells cultured for 10 days. i, Analysis of the mRNA levels of aquaporins (AQPs) based on the data from Cancer Cell Line Encyclopedia (CCLE). j, Effects of AQP3 knockout on glycerol excretion and cell viability of HeLa and A549 cells cultured under normoxia and hypoxia for 24 h (glycerol excretion) or 72 h (cell proliferation). Values are shown as mean ± SD, n = 3 biologically independent samples, two-tailed Student’s t-tests.
Extended Data Fig. 4 Effects of NADH accumulation on glucose uptake, lactate excretion, and glycerol excretion.
a, Immunoblot verification of CHOP and ATF4 activation in GPD1/GPD1L DKO, PGP KO and PGP re-expression HeLa cells cultured under normoxia or hypoxia for 24 h. b, Effects of different concentrations of αKB on glucose uptake and lactate excretion of HeLa, A549, and HepG2 cells under hypoxia for 24 h. c, Effects of different concentrations of LDHi on glucose uptake and lactate excretion of HeLa, A549, and HepG2 cells under normoxia for 24 h. d, Glucose uptake and lactate excretion of HeLa, A549 and HepG2 cells cultured as indicated for 24 h. e, Effects of different concentrations of SC-26196 on the ratios of NADH/NAD+, glycerol excretion, glucose uptake, and lactate excretion of HeLa, A549, and HepG2 cells cultured under normoxia for 8 h (NADH/NAD+ ratios) or 24 h (glycerol excretion, glucose uptake, and lactate excretion). f, Effects of αKB (2 mM) on cell viability of HeLa PGP KO or GPD1/GPD1L DKO cells cultured under hypoxia for 48 h. Values are shown as mean ± SD, n = 3 biologically independent samples (b-f), two-tailed Student’s t-tests. In a, data are verified in three replicates with similar results.
Extended Data Fig. 5 Effects of glucose uptake on glycerol excretion.
a, Western blots for the expression of PKM2, LDHA, GAPDH, and β-Actin in HeLa and A549 cells treated with normoxia, hypoxia, or AntA (2 μM) for 24 h. b, Effects of HIF2α knockdown on glycerol excretion of HeLa and A549 cells cultured under hypoxia for 24 h. c, Immunoblot verification of GLUT1 knockdown in HeLa and A549 cells. d-f, Effects of GLUT1 knockdown on glucose uptake, lactate excretion, and glycerol excretion of HeLa and A549 cells cultured under normoxia and hypoxia for 24 h. g, Immunoblot verification of GLUT1 over-expression in HeLa and A549 cells. h-j, Effects of GLUT1 over-expression on glucose uptake, lactate excretion, and glycerol excretion of HeLa and A549 cells cultured under normoxia, hypoxia, or AntA (2 μM) treatment for 24 h. Values are shown as mean ± SD, n = 3 biologically independent samples (b, d-f, h-j), two-tailed Student’s t-tests. In a, data are verified in two replicates with similar results.
Extended Data Fig. 6 Effects of PGP, GPD1, or GPD1L over-expression on glucose uptake, lactate excretion, glycerol excretion, and cell growth.
a, Effects of PGP, GPD1, or GPD1L over-expression on glucose uptake and lactate excretion of HeLa and A549 cells cultured under normoxia and hypoxia for 24 h. b, Immunoblot verification of LKB1 expression in HeLa, A549, SK-Hep-1, and HCC-LM3 cells. c, Immunoblot verification of over-expression of PGP, GPD1L, or GPD1 in SK-Hep-1 and HCC-LM3 cells. d, Effects of PGP, GPD1, or GPD1L over-expression on glycerol excretion of SK-Hep-1 and HCC-LM3 cells cultured under normoxia and hypoxia for 24 h. e, Effects of PGP, GPD1, or GPD1L over-expression on cell viability of SK-Hep-1 and HCC-LM3 cells cultured with low nutrient medium (10% fetal bovine serum medium containing 2 mM of glucose, without glutamine and pyruvate) under normoxia and hypoxia for 8 h. f, Effects of PGP, GPD1, or GPD1L over-expression on cell viability of SK-Hep-1 and HCC-LM3 cells cultured in normal or nutrient-deprived media. Nutrient-deprived media contained 10% fetal bovine serum but without glucose, glutamine, and pyruvate. g, Effects of PGP, GPD1L, or GPD1 over-expression on AMPK activation in SK-Hep-1 and HCC-LM3 cells cultured in nutrient-deprived medium. h, Tumor formation ability in nude mice of SK-Hep-1 cells with PGP, GPD1, or GPD1L over-expression. Values are shown as mean ± SD, n = 3 biologically independent samples (a,d,e,f) or n = 5 biologically independent mice (h), two-tailed Student’s t-tests. In g, data are verified in two replicates with similar results.
Extended Data Fig. 7 Effects of aldolases and AMPK on glucose uptake, lactate excretion, and glycerol excretion.
a, Effects of ALDOA over-expression on glycerol excretion, glucose uptake, and lactate excretion of HeLa and A549 cells cultured under normoxia and hypoxia for 24 h. b, Effects of ALDOC over-expression on glycerol excretion, glucose uptake, and lactate excretion of HeLa and A549 cells cultured under normoxia and hypoxia for 24 h. c, Effects of A769662 treatment on glycerol excretion, glucose uptake, and lactate excretion of A549, SK-Hep-1, and HCC-LM3 cells cultured under normoxia and hypoxia for 12 h. d, Effects of Compound C on AMPK pathway in HeLa cells. e, Effects of Compound C on glycerol excretion, glucose uptake, and lactate excretion of HeLa and A549 cells cultured under normoxia and hypoxia for 12 h. f, Effects of AMPKα knockdown and AMPKα1 re-expression on glycerol excretion, glucose uptake, and lactate excretion of A549 cells cultured under normoxia and hypoxia for 24 h. g. Immunoprecipitation (IP) analysis of the interaction between endogenous ALDOB, GPD1 and GPD1L in HepG2 cells with anti-ADLOB antibody. Rabbit IgG was used as a negative control. WCL, whole-cell lysate. pALDOB T245, AMPK, pAMPK T172, ACC1 and pACC1 S79 were also blotted. Values are shown as mean ± SD, n = 3 biologically independent samples (a-c, e-f), two-tailed Student’s t-tests. In d,g, data are verified in two replicates with similar results.
Extended Data Fig. 8 Inactivation of ADLOB by AMPK-mediated phosphorylation.
a, HEK293T cells were transfected with vector, Flag-GPD1, or Flag-GPD1L plasmids, and then cultured under normoxia or hypoxia for 8 h. IP assays were performed using anti-FLAG affinity M2 beads followed by immunoblotting for the phosphorylation of GPD1 or GPD1L. b, Mass spectrometry was used to detect the phosphorylation site of ALDOB in HeLa cells after treatment with A769662 for 8 h. c, Conservation of the phosphorylation site in ALDOB among different species. Amino acid residues around Thr245 are shown. d, The activity of GST-ALDOB-WT, T245A, and T245D mutants purified from an E. coli expression system incubated with or without active AMPK as indicated. Values are shown as mean ± SD, data are verified in two replicates with similar results (a) and n = 3 biologically independent samples (d).
Extended Data Fig. 9 The trade-off regulation between reductive stress and energy stress.
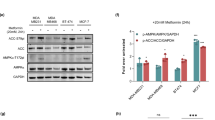
a, Effects of αKB and LbNOX on AMPK activation of A549 cells cultured under respiratory chain inhibition or hypoxia for 24 h. αKB, 2 mM; Dox, 0.1 μg/mL. b,c, Effects of αKB and LbNOX on cellular NADH/NAD+ ratio (b) and ATP/AMP ratio (c) of A549 cells cultured under respiratory chain inhibition or hypoxia for 24 h. αKB, 2 mM; Dox, 0.1 μg/mL. d, Effects of Torin-1 (0.1 μM) and Rapamycin (1 μM) on AMPK activation of A549 cells cultured under respiratory chain inhibition or hypoxia for 24 h. e,f, Effects of Torin-1 (0.1 μM) and Rapamycin (1 μM) on cellular NADH/NAD+ ratio (e) and ATP/AMP ratio (f) of A549 cells cultured under respiratory chain inhibition or hypoxia for 24 h. g, Effects of Compound C (2 μM) on cell death of A549 cells cultured in nutrient-deprived medium under respiratory chain inhibition or hypoxia for 12 h. h, Immunoblot verification of AMPKα knockout and AMPKα re-expression in HeLa cells. i, The effect of metformin (100 mg/kg/day, i.g.) or Compound C (20 mg/kg/day, i.p.) treatment alone or in combination on tumor formation ability in nude mice of HeLa cells. Metformin (Metf, 4 mM) and Ant A (2 μM) were used. Values are shown as mean ± SD, n = 3 biologically independent samples (b,c,e,f,g) or n = 6 biologically independent mice (i), two way ANOVA (i), and two-tailed Student’s t-tests for others. In a,d, data are verified in two replicates with similar results.
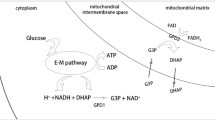
Extended Data Fig. 10 The working model for trade-off between reductive stress and energy stress.
a, Schematic diagram illustrating hypoxic regulation of glycerol biosynthesis. Hypoxia promotes glycerol biosynthesis and excretion by inducing NADH accumulation and glucose uptake. Glycerol biosynthesis consumes NADH to reduce reductive stress, but this process is accompanied by ATP consumption and thus may potentially provoke an energy crisis. Cells have evolved a negative feedback loop to suppress glycerol synthesis through AMPK-mediated phosphorylation of ALDOB. b, The trade-off regulation between reductive stress and energy stress. Reductive stress induced by hypoxia or ETC inhibition primarily contributes to energy stress and AMPK activation. To alleviate reductive stress, some metabolic pathways are promoted to consume NADH, along with ATP. In turn, the activated AMPK negatively regulates these metabolic reactions to prevent catastrophic energy depletion.
Supplementary information
Supplementary Table 1
Survival P-value analysis based on TCGA datasets.
Source data
Source Data Fig. 1
Unprocessed western blots.
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Unprocessed western blots.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Unprocessed western blots.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Unprocessed western blots.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Unprocessed western blots.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Unprocessed western blots.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 7
Unprocessed western blots.
Source Data Fig. 7
Statistical source data.
Source Data Fig. 8
Unprocessed western blots.
Source Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 2
Unprocessed western blots.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 3
Unprocessed western blots.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 4
Unprocessed western blots.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 5
Unprocessed western blots.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 6
Unprocessed western blots.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 7
Unprocessed western blots.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 8
Unprocessed western blots.
Source Data Extended Data Fig. 9
Statistical source data.
Source Data Extended Data Fig. 9
Unprocessed western blots.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhai, X., Yang, R., Chu, Q. et al. AMPK-regulated glycerol excretion maintains metabolic crosstalk between reductive and energetic stress. Nat Cell Biol 27, 141–153 (2025). https://doi.org/10.1038/s41556-024-01549-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41556-024-01549-x