Abstract
Errors during cell division lead to aneuploidy, which is associated with genomic instability and cell transformation. In response to aneuploidy, cells activate the tumour suppressor p53 to elicit a surveillance mechanism that halts proliferation and promotes senescence. The molecular sensors that trigger this checkpoint are unclear. Here, using a tunable system of chromosome mis-segregation, we show that mitotic errors trigger nuclear deformation, nuclear softening, and lamin and heterochromatin alterations, leading to rapid p53/p21 activation upon mitotic exit in response to changes in nuclear mechanics. We identify mTORC2 and ATR as nuclear deformation sensors upstream of p53/p21 activation. While triggering mitotic arrest, the chromosome mis-segregation-induced alterations of nuclear envelope mechanics provide a fitness advantage for aneuploid cells by promoting nuclear deformation resilience and enhancing pro-invasive capabilities. Collectively, this work identifies a nuclear mechanical checkpoint triggered by altered chromatin organization that probably plays a critical role in cellular transformation and cancer progression.
Similar content being viewed by others
Main
Aneuploidy is a pathological condition that originates from errors in chromosome segregation during mitosis1. Aneuploidy is one of the central hallmarks of cancer, since over 90% of all solid tumours display aneuploid phenotypes2.
Faithful chromosome segregation depends on the proper attachment of microtubules to the kinetochore—a protein complex assembled on centromeres3. Centromere assembly and function rely on centromere protein A (CENP-A; a histone H3 variant) and its interactions with other centromeric factors, such as CENP-C, which is essential for building the proper kinetochore platform3. Degradation of one of these two proteins is sufficient to trigger chromosome mis-segregation, leading to aneuploidy4.
In response to aneuploidy, a cellular surveillance mechanism activates p53, a sequence-specific DNA-binding protein that regulates transcription5,6. TP53 is the most commonly mutated gene in tumours and its dysfunction causes a high level of chromosomal instability that is associated with poor prognosis7. Under basal conditions, the p53 protein is maintained at low levels by a series of proteolytic regulators, including MDM2—a ubiquitin ligase that promotes its degradation6. In response to cellular stress, p53 is stabilized and activates the cyclin-dependent kinase inhibitor p21, leading to cell cycle arrest and cellular senescence, and an accumulation of p16, alone or together with the stress kinase p38-dependent induction of apoptosis8.
Two main mechanisms of p53 activation following chromosome mis-segregation have been proposed. The first postulates that aneuploid cells experience high levels of replication stress during the S phase after faulty mitosis, causing DNA damage9,10,11,12. Here, p53-dependent cell cycle arrest would be induced after structural, rather than numerical, aneuploidy and only once a critical threshold of DNA damage is reached. The second hypothesis is based on the notion that chromosome mis-segregation associates with prolonged mitosis, which activates the mitotic stopwatch complex, stabilizing p53 and causing cell cycle arrest independent of DNA damage13,14,15. However, cells with a weakened spindle assembly checkpoint exhibit faulty mitosis with similar or even faster speed relative to normal cells. Nevertheless, a large proportion of aneuploid cells exiting mitosis fail to enter the next cell cycle due to p53 activation16,17. These inconsistencies highlight our incomplete mechanistic understanding of how aneuploidy activates the p53 pathway.
The nuclear envelope (NE) is a dynamic protective shell for the genome. Its functional integrity is essential for the maintenance of genome stability and controlled exchange of nucleocytoplasmic content18,19. During cell division, the NE dissociates to allow chromosome segregation and then it faithfully reforms around the two daughter nuclei at the end of mitosis. Its rupture is associated with the occurrence of DNA damage, potentially leading to senescence or apoptosis20. This is particularly evident in malignant cells21 and micronuclei22 (encapsulated chromosomes or chromatin fragments excluded from the main nucleus). Additionally, this phenomenon might occur in laminopathies, such as Hutchinson–Gilford progeria syndrome23, which are characterized by an altered assembly of the nuclear lamina underlying the inner nuclear membrane of the NE.
Laminopathies, aneuploidy and cancerous states have one unifying feature—the emergence of aberrant nuclear shape—which has been utilized for decades in the clinic to diagnose various pathologies24. Nuclear shape reflects the mechanical state of the nuclear periphery that is determined by the levels of heterochromatin and the composition of the nuclear lamina25. Recent findings suggest that chromosome mis-segregation can directly affect nuclear shape26,27,28. In addition, cells within tissues experience a wide range of mechanical forces that deform them. Mechanical deformation of the nucleus has been shown to impact DNA damage repair, cell cycle progression, migration, differentiation and cell fate specification24. Although the precise molecular mechanisms are not yet delineated, recent literature suggests that mechanical stress of the NE may be central to all nuclear mechanosensation29,30,31,32,33. In support of this notion, mechanical stretching of the NE has been shown to promote the activity of ataxia telangiectasia and Rad3-related kinase (ATR)34. Furthermore, nuclear deformations that stretch and tense the NE trigger calcium-sensitive signalling, leading to the remodelling of heterochromatin, alterations of transcription and modification of nuclear/chromatin mechanics33,34,35. Similarly, perinuclear signals also trigger actomyosin contractility to alter cell migration36,37. Thus, the nucleus—and particularly its periphery—is emerging as a hub for cellular mechanosensing and mechanotransduction that is able to influence cell fate decisions and impact cellular functions beyond the maintenance of genomic integrity.
To uncover the molecular responses to aneuploidy, we used an inducible system to generate rapid chromosome mis-segregation that we show to be associated with aberrant nuclear shape, p53/p21 accumulation and perturbed assembly of the nuclear lamina. We identified ATR, mammalian target of rapamycin complex 2 (mTORC2) and AKT activation in response to these changes. Restoration of nuclear shape or inhibition of ATR, mTORC2 or AKT prevented p53 activation, demonstrating that NE mechanics act as a G1 checkpoint to maintain genome integrity.
Results
Chromosome mis-segregation induces rapid p21/p53 activation
To investigate the immediate cellular consequences of aneuploidy, we used a rapid and tunable cellular model to induce chromosome mis-segregation in human cells. Based on the critical role of centromeres in faithful chromosome segregation, we used an auxin-inducible degron (AID) approach38,39 to rapidly deplete endogenous centromeric proteins (CENP-A or CENP-C) in stable diploid40, non-transformed hTERT-immortalized retinal pigment epithelial cells (hTERT RPE-1 cells)—referred to as CENP-AAID or CENP-CAID—with the addition of indole-3-acetic acid (IAA) (Fig. 1a). Depletion of either of these two key centromeric factors leads to defective kinetochore assembly and misaligned chromosomes, with CENP-C depletion causing the most deleterious effect on segregation fidelity4,40. Accordingly, immunofluorescence analysis of cells at mitotic exit (arrested in G1 and released in the presence of IAA for 15–18 h; Extended Data Fig. 1a) revealed the induction of chromosome mis-segregation, as quantified by the presence of micronuclei (Fig. 1b,c). Similar results were obtained in asynchronous cells (Extended Data Fig. 1b,c). Consistent with chromosome mis-segregation, IAA-treated CENP-A/C cells showed stabilization of p53 and its downstream target p21, but not of p16 or p38 (Fig. 1b,d,e and Extended Data Fig. 1d,e). p21 and p53 activation was evident even in G1-arrested cells that underwent only one round of faulty mitosis (Fig. 1d and Extended Data Fig. 1d), and p21 nuclear accumulation was only observed in cycling cells, as administration of IAA in G1-arrested cells did not cause p21 activation (Extended Data Fig. 1f). Fluorescence in situ hybridization (FISH) quantification of chromosomes particularly prone to mis-segregation40 confirmed that p21-positive cells had high levels of aneuploidy in CENP-A-depleted cells (Fig. 1f and Extended Data Fig. 1g,h). A comparable p21 response was achieved by perturbing mitosis by dual inhibition of the mitotic kinase MPS1, which is involved in the spindle assembly checkpoint41 and the kinase motor CENP-E42 (Fig. 1d). Combination of these inhibitors generates misaligned chromosomes and aneuploidy, indicating that it is not centromeric protein degradation per se that causes p21/p53 activation, but rather chromosome mis-segregation itself. To establish the temporal kinetics of p21 activation downstream of chromosome mis-segregation, we quantified p21 accumulation after G1 release using a p21 live sensor tagged endogenously with green fluorescent protein (GFP)43 in cells co-treated with CENP-E and MPS1 inhibitors (hereafter referred to as CENP-Ei and MPS1i, respectively) or by immunofluorescence in fixed IAA-treated cells. These analyses revealed that p21 accumulates rapidly in the nucleus after mitotic errors, starting around 1–2 h from telophase exit (Fig. 1g and Extended Data Fig. 1i,j) and continuing at later time points (Extended Data Fig. 1e). This slight increase in p21 expression that increased over time was sufficient to block the cell cycle, as the majority of cells arrested permanently in G1 after just one defective mitosis cycle. This occurred in a p53-dependent manner (Fig. 1h and Extended Data Fig. 1k–m), indicating that this accumulation is unlikely to be caused by the remaining cycling cells.
a, Schematic of the scope of the manuscript. A, CENP-A; C, CENP-C; ?, phenomenon sensed by p53/p21. b, Immunofluorescence images of p21 (red) activation and micronucleus formation upon IAA treatment (18 h) of CENP-CAID RPE-1 cells. Nuclei were counterstained with DAPI (grey). Scale bars, 5 μm. NT, not treated. c, Quantification of cells with micronuclei in untreated and IAA-treated CENP-AAID and CENP-CAID RPE-1 cells at the indicated times after release from G1 (palbociclib (Palbo) washout). n = 8 (untreated), 4 (IAA at 15 h) and 8 (IAA at 18 h) independent replicates, with 197, 59 and 159 CENP-AAID cells for these respective groups and 166, 61 and 160 CENP-CAID cells for these respective groups. Each dot represents the mean of one experiment. Statistical significance was determined by ordinary one-way analysis of variance (*P = 0.0229; ****P < 0.0001). d, Quantification of the normalized p21 immunofluorescence intensity in CENP-A/CAID or CENP-Ei+MPS1i RPE-1 cells after cell synchronization in G1, release and treatments performed according to the schematic to the right. Each dot represents the mean of one experiment. n = 6, 10 and 16 independent replicates with 60 cells per experiment for NT, IAA and CENP-Ei + MPS1i, respectively. Statistical significance was determined by one-sample two-sided t-test (**P = 0.0015; ***P = 0.0002). e, Immunoblot analysis with the indicated antibodies of CENP-AAID RPE-1 cells treated with IAA for 72 h where indicated. Two representative experiments are shown (#1 and #2). Vinculin served as the loading control. f, Quantification of IAA-induced aneuploidy relative to chromosomes 3, 6, 9 and X in CENP-AAID RPE-1 cells sorted by p21 negativity versus positivity, assessed by FISH in interphase. n = 150 nuclei counted per condition from one experiment. Statistical significance was determined by two-sided Fisher’s exact test (****P < 0.0001). g, Quantification of p21 signal during live-cell imaging of synchronized eGFP–p21 RPE-1 cells treated as indicated. T0 denotes telophase exit. The signals are normalized to T0 of the untreated group. n = 3 (background), 8 (NT) and 20 (CENP-Ei + MPS1i) cells from three independent replicates. Statistical significance was determined by Kruskal–Wallis test (****P < 0.0001), calculated at the last time point. h, Percentages of BrdU-negative and -positive CENP-AAID RPE-1 cells after cell cycle synchronization, release from G1 and treatments performed according to the schematic to the right. CENP-Ei and MPS1i were used at 0.5 and 1.0 μM, respectively. n = 2 independent replicates with 10,000 cells. In c, d, g and h, the error bars represent s.e.m.
Mis-segregated nuclei harbour aberrant shapes and mechanics
Next, we set out to uncover the mechanisms of p53/p21 activation in CENP-A/C-depleted mis-segregated cells. Interestingly, we did not detect an increase in DNA damage in primary nuclei following chromosome mis-segregation, as measured by γH2A.X and 53BP1 immunofluorescence analyses (Extended Data Fig. 2a,b). Consistent with the absence of DNA damage, no differences in p21 or p53 levels were observed in CENP-A-depleted cells treated with an inhibitor of ATM kinase (ATMi) required for the DNA damage response (Extended Data Fig. 2c–e). Moreover, the p21 increase did not depend on the formation of micronuclei (Extended Data Fig. 2f)—a well-known source of DNA damage44—despite their abundance following centromere inactivation-mediated mis-segregation (Fig. 1c and Extended Data Fig. 1b,c). Furthermore, p21 activation did not originate from defects during previous DNA replication, as CENP-C depletion in either the G1 or G2 phase before mitosis had a similar effect on p21 induction (Extended Data Fig. 2g). Similarly, re-blocking cells in G1 after the first mitotic exit to prevent possible entry in the subsequent S phase did not halt p53 and p21 accumulation (Extended Data Fig. 2g,h), consistent with our results showing that p21 activation occurs in early G1 (Fig. 1g and Extended Data Fig. 1i,j). Finally, we investigated the involvement of the recently identified mitotic stopwatch complex15 in this process. We previously showed that CENP-A depletion has only a minor effect on mitotic duration4, and further shortening of mitotic timing with MPS1 inhibition45 did not suppress p53/p21 induction in either CENP-A- or CENP-C-depleted cells (Extended Data Fig. 2i). Importantly, DNA damage-independent p53/p21 accumulation in CENP-AAID auxin-depleted cells was also observed in U-2 OS cells (Extended Data Fig. 2j–l), which are known to lack a functional mitotic stopwatch complex15. Collectively, these results indicate that the mis-segregation checkpoint following CENP-A or CENP-C removal occurs immediately after exit from mitosis and, in most cases, does not require the known canonical pathways of p21/p53 activation, such as prolonged mitosis, defective DNA replication and/or DNA damage within the primary nuclei or micronuclei.
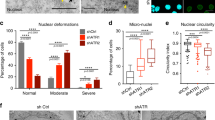
The most prominent phenotypic feature of CENP-A/-C-driven chromosome mis-segregation is the consistent presence of nuclear shape abnormalities (Fig. 2a–c). Detailed quantification of nuclear morphology (solidity, circularity and elliptic Fourier coefficient ratio46) revealed strong nuclear deformation that was more frequent, but not more pronounced, in CENP-C-depleted cells, suggesting correlation of checkpoint activation with the frequency rather than severity of chromosome mis-segregation (Fig. 2a–c and Extended Data Fig. 3a). Importantly, nuclear deformation was observed immediately after mitotic exit, preceding the accumulation of p21 by a few hours (Fig. 2d,e, Extended Data Fig. 3b,c and Supplementary Video 1).
a, Electron microscopy of untreated and IAA-treated (24 h) RPE-1 cells Scale bar, 5 μm. b, Lamin immunofluorescence of untreated and IAA-treated (24 h) CENP-AAID RPE-1 cells. Scale bar, 5 μm. c, Quantification of nuclei deformation (solidity). n = 23, 11, 14 and 7 independent replicates with 60 cells per experiment per condition for NT, CENP-AAID, CENP-CAID and CENP-Ei + MPS1i, respectively. Statistical significance was determined by Kruskal–Wallis test (****P < 0.0001). d, Imaging of IAA-treated RPE-1 cells from metaphase until G1. The arrows denote misaligned chromosomes causing nuclear deformations. The values in the bottom right corners represent the time (in min) since the beginning of the recording (metaphase). Scale bar, 5 μm. e, Nuclear solidity (arbitrary units) during CENP-AAID RPE-1 live-cell imaging in untreated cells or upon IAA treatment. n = 25 cells per condition. Statistical significance was determined by unpaired two-sided t-test (****P < 0.0001), calculated at the last time point. f, Left, lamin immunofluorescence of G1 RPE-1 cells. Right, lamin intensity variation in untreated versus IAA-treated (24 h post-palbociclib release) RPE-1 cells. n = 6 and 9 independent replicates with 570 and 627 cells for NT and IAA, respectively. Statistical significance was determined by unpaired two-sided t-test (**P = 0.0014; ***P = 0.0004). Scale bar, 5 μm. g, SIM of lamins in untreated versus IAA-treated (24 h) RPE-1 cells. Arrows denote irregular lamin patches and invaginations. Scale bar, 5 μm. h, Left, H3K9me3 and p21 immunofluorescence. The arrows denote cells with reduced H3K9me3 and increased p21. Right, relative H3K9me3, HP1α and H3K27me3 signals in untreated versus IAA-treated (24 h post-palbociclib release) RPE-1 cells. n = 3 (three NT groups), 7 (HP1α; IAA), 15 (H3K9me3; IAA) and 8 (H3K27me3; IAA) independent replicates with 50 cells per experiment per condition. Statistical significance was determined by unpaired two-sided t-test (*P = 0.02; **P = 0.002). Scale bar, 5 μm. i, Lamin B1 ChIP-qPCR of lamin-associated domains in IAA-treated (24 h post-G1 release) RPE-1 cells. Immunoglobulin G (IgG) was used as the isotype control. Enrichment was computed as the percentage of input normalized to the NT group. Each dot represents enrichment relative to a discrete LAD locus (7). n = 3 independent replicates. Statistical significance was determined by one-sample two-sided t-test (***P = 0.0002). j, Nuclear stiffness normalized to the NT or dimethyl sulfoxide (DMSO) treatment groups. n = 3 independent replicates with 235, 157, 173, 80 and 96 cells for NT, CENP-A + IAA, CENP-C + IAA, DMSO and CENP-Ei, respectively. Statistical significance was determined by Kruskal–Wallis test (****P < 0.0001). k, Nuclear stiffness post-G1 release (18 h). n = 107 and 100 cells for NT and IAA, respectively, from three independent replicates. Statistical significance was determined by unpaired two-sided t-test (****P < 0.0001). In c, e, f and h–k, the error bars represent s.e.m. In c, f, h, j and k, each dot represents the mean of one experiment.
To investigate the causes and consequences of nuclear shape abnormalities, we assessed the levels of nuclear lamins and nuclear lamina-anchored peripheral heterochromatin, both of which are important regulators of nuclear shape and stability33,47,48. We did not detect a substantial change in total lamin A/C protein levels in IAA-treated cells, but there was an expected decrease in lamin B1 (which is known to decrease following p53 activation49) after 3 d of IAA treatment and cell cycle block (Extended Data Fig. 3d). However, quantitative immunofluorescence analysis and three-dimensional (3D) structured illumination microscopy (SIM) revealed notable variation in lamin A/C and lamin B1 signal intensity at the nuclear periphery (measured by the coefficient of variation), with formation of both lamin-enriched invaginations and patches with high or low lamin levels. Such alterations were not observed in control cells (Fig. 2f,g, Extended Data Fig. 3e and Supplementary Videos 2–4).
We then measured key heterochromatin marks that have been implicated in proper nuclear shape and mechanics maintenance33,50. These analyses were performed within a few hours of mitotic exit, when lamina-anchored heterochromatin is re-established, and revealed a decrease in the levels of heterochromatin protein 1 alpha (HP1α), as well as H3K9me3- and H3K27me3-marked heterochromatin in CENP-A/C-depleted cells among both RPE-1 and U-2 OS cells (Fig. 2h and Extended Data Fig. 3f,g). Consistently, using chromatin immunoprecipitation coupled with quantitative real-time PCR (ChIP-qPCR), we detected reduced lamin B1 levels at previously defined lamina-associated domains (LADs)51 in IAA-treated RPE-1 cells (Fig. 2i). A similar decrease in peripheral heterochromatin was also observed by ChromEM transmission electron microscopy analyses52 in CENP-C-depleted cells versus control cells (Extended Data Fig. 3h). This indicates defective reassociation of LADs with the nuclear lamina upon mitotic exit of mis-segregated cells.
These findings further prompted us to analyse the mechanical properties of CENP-A/C-depleted nuclei. Atomic force microscopy (AFM)-based force indentation measurements revealed significant softening of CENP-A/C-depleted nuclei (Fig. 2j and Extended Data Fig. 3i). Importantly, nuclear softening was detected in synchronized CENP-A-depleted cells directly after mitotic exit (Fig. 2k), indicating that changes in the mechanical properties of deformed nuclei are an early event following chromosome mis-segregation. Similar changes were observed following chromosome mis-segregation induced via CENP-E inhibition (Fig. 2j). We confirmed that this difference in stiffness arises from the nucleus and not from the cytoskeletal components by performing AFM measurements in cells pre-treated with cytochalasin D (200 nM for 15 min) to depolymerize the actin cytoskeleton (Extended Data Fig. 3j,k). Notably, we observed no changes in cytoskeletal stiffness or morphological alterations in the microtubule or actin cytoskeleton organization in CENP-A/-C-depleted cells, excluding the contribution of an abnormal cytoskeleton to the observed nuclear deformation (Extended Data Fig. 3l). Collectively, these data indicate that centromere dysfunction-induced chromosome mis-segregation leads to improper reestablishment of the nuclear lamina and anchoring of peripheral heterochromatin upon mitotic exit, leading to abnormal nuclear shapes and mechanics.
Restoration of chromatin architecture rescues p53 activation
As nuclear deformation precedes p21 activation and nuclear shape is largely dependent on the levels and state of heterochromatin25, we next asked whether the p53/p21 mis-segregation checkpoint is sensitive to the amount of heterochromatin (Fig. 3a). To test this, we treated CENP-A/C-depleted cells with two pharmacological inhibitors of histone lysine demethylases (methylstat and JIB-04) to elevate heterochromatin levels53,54. Increasing heterochromatin levels was sufficient to attenuate p53 and p21 activation in RPE-1 CENP-A/C-depleted cells (Fig. 3b–d and Extended Data Fig. 4a). In contrast, increasing chromatin relaxation by inhibition of histone deacetylases with trichostatin A did not dampen p53 activation in IAA-treated cells and triggered p53 activation in control cells (Extended Data Fig. 4b). Collectively these data implicate heterochromatin alteration in activation of the p21/p53 checkpoint.
a, Schematic of the rationale behind the experiments presented in this figure. b, Immunoblot of p53/p21 in CENP-AAID RPE-1 cells. The loading control was GAPDH. c, Quantification of p53 from immunoblots of CENP-AAID RPE-1 cells treated as indicated for 72 h at 3 μM and normalized to GAPDH and NT (dashed line). n = 9 independent replicates for IAA and n = 6 for methylstat, JIB-04, IAA + methylstat and IAA + JIB-04. Statistical significance was determined by Kruskal–Wallis test (**P = 0.0024; ****P < 0.0001). d, p21 immunofluorescence signals of CENP-AAID (circles) and CENP-CAID RPE-1 cells (squares) treated for 72 h with IAA and/or methylstat/JIB-04 (3 μM). n = 335, 274, 229 and 376 cells for the indicated conditions (left to right in the graph) from four independent replicates. Statistical significance was determined by Kruskal–Wallis test (****P < 0.0001). e, Elliptic Fourier coefficient (EFC) ratios (DAPI) of CENP-AAID (circles) and CENP-CAID RPE-1 cells (squares). Both IAA and remodelin were administered for 24 h at 50 μM. n = 284, 337 and 316 cells for NT, IAA and remodelin + IAA, respectively, from four independent replicates. Statistical significance was determined by Kruskal–Wallis test (**P = 0.0015; ****P < 0.0001). f, H3K9me3 immunofluorescence signal of CENP-AAID RPE-1 cells treated with IAA, remodelin or both for 24 h at 50 μM. n = 260, 233 and 242 cells for NT, IAA and remodelin + IAA, respectively, from three independent replicates depicted with different shapes. Statistical significance was determined by Kruskal–Wallis test (****P < 0.0001). g, Nuclear stiffness of CENP-AAID RPE-1 cells treated with IAA, remodelin or both for 24 h at 50 μM. n = 68, 69, 75 and 70 cells for NT, IAA, remodelin and remodelin + IAA, respectively, from three independent replicates. Statistical significance was determined by Kruskal–Wallis test (**P = 0.0018; ****P < 0.0001). h, Immunoblot showing p53 and p21 signals in CENP-AAID cells. The loading control was vinculin. i, Quantification of p53 signals from immunoblots of CENP-AAID cells, normalized to vinculin and NT (dashed line). n = 4 independent replicates. Statistical significance was determined by Kruskal–Wallis test (*P = 0.0220; **P = 0.0041). j, p21 immunofluorescence nuclear signals in CENP-AAID (circles) and CENP-CAID RPE-1 cells (squares). n = 3 (CENP-AAID) and 4 (CENP-CAID) independent replicates with 591, 553 and 545 cells for NT, IAA and remodelin + IAA, respectively. Statistical significance was determined by one-sample two-sided t-test (**P = 0.0019; ***P = 0.0001). In c–g, i and j, the error bars represent s.e.m. Each dot represents the mean of one experiment.
To further strengthen the mechanistic link between the chromosome mis-segregation checkpoint and the observed defects in nuclear lamina, mechanics and heterochromatin, we utilized remodelin, an inhibitor of the multifunctional protein NAT10 (ref. 55). Remodelin is a small molecule with demonstrated ability to rectify the strong aberrant nuclear organization defects of laminopathies, such as the premature ageing disease Hutchinson–Gilford progeria syndrome—possibly, but not exclusively, by reorganizing the microtubule network. Remodelin treatment mildly restored nuclear shape abnormalities in CENP-A/C-depleted cells (as measured by the elliptic Fourier coefficient ratio; Fig. 3e and Extended Data Fig. 4c), as well as heterochromatin levels and nuclear stiffness (Fig. 3f,g and Extended Data Fig. 4d,e). Importantly, the restoration of nuclear shape rapidly prevented p53 and p21 activation downstream of CENP-A/C depletion (Fig. 3h–j and Extended Data Fig. 4f). However, this effect was independent of the cytoskeleton networks, as chemical inhibition of microtubule, myosin and actin polymerization using nocodazole, blebbistatin or dihydrocytochalasin B, respectively, did not prevent p21 activation (Extended Data Fig. 4g). This is in agreement with the notion that the cytoskeleton is not severely altered following centromere inactivation (Extended Data Fig. 3l).
Nuclear membrane tension triggers p53/p21 activation
Next, we proceeded to investigate the molecular mechanisms by which abnormalities in nuclear shape and stiffness—conferred by reduced heterochromatin and improperly assembled lamina—trigger p53/p21 activation. Heterochromatin levels have been shown to positively correlate with nuclear membrane tension33, defined here as the in-plane tension of the lipid bilayer, which serves as a sensor for extreme nuclear deformation36,37. We hypothesized that nuclear membrane tension could sense altered chromatin organization and mechanics in aneuploid cells (Fig. 4a). To test this, we measured nuclear membrane tension in CENP-A/C-depleted cells immediately after mitosis using an endoplasmic reticulum–NE-specific membrane tension probe, ER Flipper-TR56. These analyses revealed elevated membrane tension at mitotic exit in both CENP-A- and CENP-C-depleted cells (Fig. 4b,c). An orthogonal analysis of rapid nuclear fluctuations of LAP2β-GFP in CENP-A/C-depleted cells (using live-cell imaging at intervals of 100–150 ms)36 following chromosome mis-segregation revealed a decrease in the amplitude of NE fluctuations (Fig. 4d and Supplementary Video 5) consistent with an increase in apparent NE tension.
a, Schematic of the rationale behind the experiments presented in this figure. b,c, Distribution of fluorescence lifetimes with a Gaussian fit, measured using the ER Flipper-TR membrane tension probe, in CENP-CAID (b) and CENP-AAID RPE-1 cells (c) treated for 24 h with IAA. Sucrose treatment (1 M) served as a control. n = 3 independent replicates with 157, 152, 109, 169, 158 and 56 cells for NT, IAA and sucrose in CENP-AAID cells and NT, IAA and sucrose in CENP-CAID cells, respectively. Statistical significance was determined by Kruskal–Wallis test (***P = 0.0038; ****P < 0.0001). d, NE fluctuations measured in RPE-1 cells expressing LAP2β-GFP. n = 93, 91, 149 and 169 cells for NT and IAA in CENP-AAID cells and NT and IAA in CENP-CAID cells, respectively, from three independent replicates depicted with different shapes. Statistical significance was determined by Kruskal–Wallis test (***P = 0.0002; ****P < 0.0001). e, Representative images (left) and quantification (right) of p21 activation (eGFP–p21) in compressed RPE-1 cells (3 h). Each dot represents the mean of one experiment. n = 1,221 and 2,290 cells for 10 and 3 μm, respectively, from three independent replicates depicted with different shapes. Statistical significance was determined by unpaired two-sided t-test (*P = 0.0354). Scale bars, 30 μm. f, p21 intensity (eGFP–p21) in RPE-1 cells in suspension following compression. n = 82, 90, 98, 160, 90, 147, 63, 133, 79 and 90 cells for the conditions depicted from left to right, from two independent replicates. Etoposide (50 μM) was used as a positive control. g, Live p21 intensity (eGFP–p21) in RPE-1 cells in hypotonic versus isotonic medium. Continuous lines denote the average values of 28 (NT) and 35 (hypotonic buffer-treated) cells from n = 3 independent replicates. The horizontal dashed line marks the basal signal intensity to which the data have been normalized (T0). The vertical dashed line marks the time of hypotonic buffer addition. Statistical significance was determined by unpaired two-sided t-test (****P < 0.0001), calculated at the last time point. h, Left, cPLA2 immunofluorescence in CENP-CAID RPE-1 cells treated or not with IAA. Scale bars, 5 μm. Right, cPLA2 nuclear signal in CENP-AAID (circles) and CENP-CAID cells (squares). Hypotonic medium was used as the positive control. n = 627, 628, 650, 1,065 and 653 cells for 0, 18, 22 and 48 h IAA and hypotonic buffer, respectively, from four (48 h release) or six independent replicates. Statistical significance was determined by Kruskal–Wallis test (****P < 0.0001). In d, f and h, the central lines represent median values, the lower and upper bounds represent the 25th and 75th percentiles, the whiskers represent the furthest observations within 1.5× the interquartile range and the dots inside and outside the whiskers represent the means of one experiment and outlier data points, respectively. In e and g, the error bars represent s.e.m. NS, not significant.
Next, we asked whether changes in NE tension are sufficient to activate the p21/p53 response. To this end, we employed a cell confinement system (modified from ref. 57) with 3 μm beads to apply an approximately 30% nuclear compression to tense the nuclear membrane without causing DNA damage and with nuclear rupture only occurring in less than 1% of the cells (Extended Data Fig. 5a–c). Imaging of RPE-1 cells expressing endogenously tagged p21-GFP demonstrated that increased NE tension following cell or nuclear compression for 3 h was sufficient to increase p21 expression (Fig. 4e). A similar p21 activation was obtained by compressing cells in suspension using a micropillar-based system without causing nuclear rupture at 6 μm height (Fig. 4f and Extended Data Fig. 5d) or DNA damage32, further excluding indirect cell adhesion-mediated effects of compression. Consistently, p21 induction was also observed upon hypotonic treatment (Fig. 4g and Extended Data Fig. 5e), which also triggers a transient increase in NE membrane tension58 but not nuclear rupture (Extended Data Fig. 5c). Here, we observed a biphasic response with an initial decrease in p21 intensity, followed by a sustained increase as cells and nuclei adapted to the altered tonicity. Altogether, these analyses indicate that a tensed nuclear membrane is responsible for activating the mechanosensitive checkpoint and that the heterochromatin-mediated softening facilitates nuclear deformation rather than induction of nuclear blebbing or rupture upon mechanical challenge, thus leading to a lack of nuclear fragility59. Finally, we monitored cytosolic phospholipase A2 (cPLA2) activity, which is known to respond to increased membrane tension36,37. Quantifications of cPLA2 revealed a modest but consistent increase in its nuclear levels following mitotic exit of CENP-A/C-depleted cells (Fig. 4h). Taken together, these data indicate that centromere dysfunction-driven chromosome mis-segregation leads to an increase in nuclear membrane tension, which correlates with p21 activation.
mTORC2 senses NE membrane stress and regulates ATR activity
To identify the bona fide NE stress sensors involved in p53/p21 activation in response to chromosome mis-segregation, we performed an imaging-based chemical screen using a library consisting of 392 kinase inhibitors (see Methods for details) in CENP-AAID RPE-1 cells treated for 24 h with IAA, using p21 activation as the readout (Fig. 5a). By correlating the efficiency (the slope of the dose–response curve) and the effect of inhibition (the percentage of p21-positive cells), we identified 58 molecules that were able to reduce p21 levels (Supplementary Table 1). Analysis of these hits showed a broad range of signalling cascades halting p21 activation, in addition to expected inhibitors known to block proliferation and the cell cycle. Overall, this strengthened our finding that induction of the p21/p53 checkpoint requires exit from mitosis (Fig. 5b).
a, Schematic of the screening process used to identify kinase inhibitors involved in p53/p21 activation in response to chromosome mis-segregation. b, Efficiency (dose–response slope) plotted against effect (percentage of p21-positive cells at 5 nM) for hits at 5 and 50 nM. c, Left, immunoblot of phospho-AKT (Ser473), phospho-p70 S6 (Thr389) and p70 S6. Vinculin and AKT were used as loading controls. Right, quantification of the phospho-AKT signal normalized to that of the loading control and NT. n = 14 independent replicates. Statistical significance was determined by one-sample two-sided t-test (*P = 0.0348). d, Left, immunoblot of p53 and p21 in CENP-AAID RPE-1 cells treated with the mTORC1/2 inhibitor INK-128, normalized to vinculin and NT. Vinculin was used as the loading control. Right, quantification of protein levels under the conditions shown for the immunoblot. n = 3 independent replicates. Statistical significance was determined by unpaired two-sided t-test (*P = 0.0068 and 0.0058). e, p21 immunofluorescence in G1 RPE-1 cells treated for 24 h with the mTORC1/2 inhibitor INK-128 (0.2 μM), the mTORC2 inhibitor JR-AB2-011 (5 μM) or the mTORC1 inhibitor rapamycin (0.1 μM). n = 11, 13, 7, 10 and 5 independent replicates with 400, 767, 423, 499 and 332 cells for the indicated conditions (left to right in the graph). Statistical significance was determined by one-sample two-sided t-test (**P = 0.046; ***P = 0.0010; ****P < 0.0001). mTORCi, mTORC inhibitor. f, p21 immunofluorescence in CENP-AAID RPE-1 cells treated with ipatasertib (an AKT inhibitor (AKTi)). n = 2 (0.1 μM) or four independent replicates with 225, 199, 104, 187, 194 and 185 cells for the conditions shown from left to right, respectively. The continuous line is the data interpolating curve. Statistical significance was determined by one-sample two-sided t-test (*P = 0.0112, 0.0198 and 0.0477, from left to right; ****P < 0.0001). g, Nuclear ATR immunofluorescence in CENP-AAID RPE-1 cells treated with IAA (with or without MPS1i). n = 250 and 449 cells for NT and IAA (−/+MPS1i), respectively, from three and five independent replicates. Statistical significance was determined by unpaired two-sided t-test (****P < 0.0001). h, p21 immunofluorescence in G1 CENP-A/CAID RPE-1 cells treated with VE-821 (5–10 μM). n = 103, 164 and 345 cells for NT, IAA and ATRi, respectively, from two (NT and IAA) or six (ATR) independent replicates. Statistical significance was determined by one-sample two-sided t-test (****P < 0.0001). i, Nuclear ATR immunofluorescence in G1 CENP-AAID (circles) and CENP-CAID RPE-1 cells (squares) treated with INK-128 (0.2 μM), JR-AB2-011 (5–10 μM) or ipatasertib (5–20 μM). n = 191, 166, 185, 192 and 182 cells for the indicated conditions (left to right in the graph), from four independent replicates. Statistical significance was determined by one-sample two-sided t-test (****P < 0.0001). In c–i, the error bars represent s.e.m. Each dot represents the mean of one experiment.
Intriguingly, inhibitors of the PI3K–AKT–mTOR pathway emerged as candidates (seven out of 58 compounds) (Fig. 5b and Supplementary Table 1). Both mTORC1 and mTORC2 are known to increase the stability of p53 and to induce cellular senescence in a p21-dependent manner60. In addition, mTORC2 has been identified to function as a plasma membrane tension sensor in yeast61 and to respond to stretching of the plasma membrane induced by hypo-osmotic shock in both mammalian and yeast cells62,63,64. To assess the involvement of the mTOR pathway, we first assessed induction of the most prominent downstream effectors of mTORC1 and mTORC2, as revealed by the phosphorylation of S6 kinase (T389) and AKT (S473), respectively. We observed consistent AKT activation following CENP-A/C-induced chromosome mis-segregation, but we did not detect a significant change in the phosphorylation of S6 kinase (Fig. 5c and Extended Data Fig. 6a,b). Consistently, both mTORC2 (INK-128 and JR-AB2-011) and AKT (ipatasertib) inhibitors, but not rapamycin-mediated mTORC1 inhibition, prevented p21 and p53 activation downstream of chromosome mis-segregation (Fig. 5d–f and Extended Data Fig. 6c). This was not simply due to a block of the cell cycle, as we administered mTORC2 or AKT inhibitors directly at mitotic exit following IAA treatment (Fig. 5e).
Among the positive hits, we also found an inhibitor of ATR (NU6027). By further filtering compounds that are efficient at reducing p21 intensity at a low concentration (5 nM) to avoid cell toxicity at higher doses, we found two additional ATR inhibitors: VE-821 and AZ20 (Supplementary Table 1). Besides its known role in the DNA damage response, ATR has been identified as a mechanosensor able to respond to topological stress at the NE–chromatin interface34. Consistently, we observed an increase in nuclear ATR following IAA treatment (Fig. 5g and Extended Data Fig. 6d). Inhibition of ATR with two different small molecules (VE-821 and AZD6738) attenuated both p53 and p21 activation in CENP-A/C-depleted cells (Fig. 5h and Extended Data Fig. 6e). ATR is known to directly promote p53 activation via serine 15 phosphorylation in the DNA damage response65. In the context of aberrant nuclear tension, we also observed mild phosphorylation of p53 at serine 15 that was not prevented by ATR inhibition (Extended Data Fig. 6f), which is in agreement with the absence of DNA damage (Extended Data Fig. 2a–d). However, inhibition of either mTORC2 or AKT reduced the levels of p53 phosphorylation at serine 15 (Extended Data Fig. 6g). mTORC2 inhibition reduced the nuclear ATR signal (Fig. 5i and Extended Data Fig. 6h), suggesting that mTORC2 activity is required to activate ATR in response to nuclear tension alterations. These data are consistent with a a primary role for mTORC2 activity in the mechanosensitive NE checkpoint. Importantly, ATR inhibition led to lower levels of AKT phosphorylation (Extended Data Fig. 6i), suggesting a cooperative function of mTORC2 and ATR in the downstream signalling cascade involving AKT activation.
Overall, these data indicate that mTORC2 and ATR have mechanosensory activity in inducing the p53/21 checkpoint. This is initiated by changes in membrane tension that are driven by alterations in the chromatin state at the nuclear periphery in response to chromosome mis-segregation. Similar results in preventing p21 and/or p53 activation were also observed in U-2 OS and hTERT-immortalized BJ fibroblasts following chromosome mis-segregation, either via CENP-A depletion or CENP-E/MPS1 co-inhibition (Extended Data Fig. 6j,k).
The NE checkpoint universally senses aberrant nuclear states
Given that our results suggested that altered nuclear morphology and mechanics trigger p53 activation downstream of aneuploidy, we next investigated whether this checkpoint could be activated in other scenarios of abnormal nuclear mechano-architecture, independent of chromosome mis-segregation. To this end, we induced the expression of progerin—a protein resulting from mutation of the gene that encodes lamin A. This mutation is known to cause progeria syndrome, which is characterized by nuclear deformation, improper lamina assembly and reduced heterochromatin66. Using a doxycycline-inducible system to express either progerin or wild-type lamin A, we achieved transient protein expression in RPE-1 cells (Fig. 6a,b) without significantly increasing chromosome mis-segregation or aneuploidy, and with only minor levels of DNA damage (Extended Data Fig. 7a–d). Notably, short-term induction of progerin, but not of wild-type lamin A, triggered activation of p53 in a DNA damage-independent manner and reduced the proliferation capability, as measured by lowered colony formation potential (Fig. 6b–d and Extended Data Fig. 7e). Similar to what we observed following chromosome mis-segregation, short-term expression of progerin, but not of wild-type lamin A, induced reduction of peripheral heterochromatin, led to nuclear softening and increased nuclear membrane tension (Fig. 6e,f and Extended Data Fig. 7f,g). Accordingly, mTORC2 inhibition via INK-128 reduced p53 activation (Extended Data Fig. 7h). Altogether, these data indicate that defects in nuclear architecture and mechanics are sufficient to trigger the p53-mediated checkpoint.
a, Inducible (i)-lamin A-GFP and progerin-GFP microscopy of doxycycline-treated RPE-1 cells. Scale bars, 5 μm. b, Lamin A-GFP and progerin-GFP immunoblot analysis. Vinculin was used as the loading control. Dox, doxycycline. c, Immunoblot quantification normalized to vinculin and the no-Dox condition. n = 3 independent replicates. Statistical significance was determined by unpaired two-sided t-test (*P = 0.0348). d, Colony formation assays after 7 d of doxycycline treatment (50 ng ml−1). e, AFM-measured nuclear stiffness. Doxycycline was administered for 72 h (10 ng ml−1 for lamin A and 50 ng ml−1 for progerin). Cytochalasin D was administered at 200 nM for 15 min. n = 97, 237, 90, 82, 162 and 107 cells for the indicated conditions (left to right in the graph), from three or four (Dox + cytochalasin D) independent replicates. Statistical significance was determined by Kruskal–Wallis test (*P = 0.0468 and 0.0396, from left to right; ****P < 0.0001). f, NE fluctuations following doxycycline treatment for 72 h. n = 311 and 268 cells for lamin A and progerin, respectively, from three independent replicates. Statistical significance was determined by two-sided Mann–Whitney test (****P < 0.0001). g, Workflow of the cell confinement experiment used to obtain the results shown in h and i. h, Percentages of cells with eGFP–FLAG–cGAS-E225A/D227A foci at the nuclear periphery in CENP-AAID RPE-1 cells left untreated or treated with IAA (24-48 h). n = 157 and 139 cells for NT and IAA, respectively, from three independent replicates represented by different symbols. Statistical significance was determined by two-sided Fisher’s exact test (*P = 0.0338). i, Numbers of eGFP–FLAG–cGAS-E225A/D227A foci at the nuclear periphery in CENP-AAID RPE-1 cells left untreated or treated with IAA (48 h). n = 157 and 139 cells for NT and IAA, respectively, from three independent replicates. Each dot represents a cell. Statistical significance was determined by two-sided Mann–Whitney test (****P < 0.0001). j, Crossing time through 4 and 2 μm microchannels (24 h imaging) of untreated or IAA-treated CENP-AAID RPE-1 cells. n = 120, 126, 72, 94, 83, 54, 28 and 47 cells for the indicated conditions (left to right in the graph), from two independent replicates. Statistical significance was determined by Mann–Whitney test (*P = 0.0416 and 0.0115, from left to right). k, Invasion path length through a collagen matrix of CENP-AAID RPE-1 cells. n = 234 and 568 cells for NT and IAA, respectively, from three independent replicates. Statistical significance was determined by two-sided Mann–Whitney test (****P < 0.0001). l, Model for the mechanosensitive NE checkpoint in response to chromosome mis-segregation. In f, j and k, the central lines represent median values, the lower and upper bounds represent 25th and 75th percentiles, the whiskers represent the furthest observations within 1.5× the interquartile range and the dots inside and outside the whiskers represent the means of one experiment and outlier data points, respectively. In c, e and h, the error bars represent s.e.m. Each dot represents the mean of one experiment.
Nuclear softening improves fitness in confined environments
Aneuploidy and aberrant nuclear shape are common features of many cancers1,2 and nuclear softening has been shown to improve the migratory potential of transformed cells67. We thus hypothesized that nuclear softening arising from chromosome mis-segregation could improve cellular fitness within confined environments. To test this hypothesis, we first utilized our micropillar-based cell confiner system57, along with a catalytically inactive cyclic GMP–AMP synthase (cGAS) reporter32, to detect NE rupture in RPE-1 cells in suspension. In agreement with our finding that mis-segregated cells have softer nuclei, CENP-A/C-depleted cells were found to be more resistant to confinement-induced nuclear rupture (Fig. 6g–i and Supplementary Video 6). To investigate the migratory and invasive capabilities of aneuploid cells, we quantified cell migration through microfabricated channels in 2D68 or in dense collagen matrices in 3D. CENP-A-depleted cells exhibited higher migratory capacity in both assays (Fig. 6j,k and Supplementary Videos 7 and 8). To confirm these findings and exclude indirect effects from the specific microenvironments, we performed a transwell migration assay and found that CENP-A-depleted cells were more efficient at invading through narrow pores (Extended Data Fig. 7i). Collectively, these findings demonstrate that centromere dysfunction-driven aneuploidy contributes to an enhanced nuclear resilience against deformation and heightened invasive capacities.
Discussion
Organismal growth, development and tissue repair require error-free DNA replication and chromosome segregation during cell division. Cell cycle control mechanisms safeguard cells against genomic instability by preventing DNA damage accumulation and mitotic errors. Here we identify NE stress sensing resulting from an abnormal chromatin–NE interface as a cell cycle checkpoint by which chromosome mis-segregation triggers p53 activation and cell cycle arrest.
Despite divergent underlying processes, several human pathologies, including cancers, laminopathies and trisomies, exhibit common phenotypes such as abnormal nuclear shape and aneuploidy26,69,70. Here we propose that nuclear deformation arises from incorrect chromosome alignment(s) resulting in reduced chromosome proximity and abnormal chromatin mass during NE reassembly (Fig. 6l). The widespread prevalence of these features in diseased tissues and their link to poor outcomes highlight their importance69,71,72. However, to which extent nuclear shape abnormalities and aneuploidy are a cause or a consequence of the various dysfunctional phenotypes has remained an open question. Recent works posit that the nucleus is a mechanosensitive organelle able to sense its own deformation. Nuclear deformation triggers signalling that leads to increased contractility, allowing single cells to escape from a deformation-inducing microenvironment36,37. Alternatively, the nucleus can tolerate deformation by increasing chromatin mobility and softening to avoid DNA damage33. The ability to trigger this mechanoadaptation response depends on the mechanical properties of the nuclear periphery resulting from the interactions of the peripheral heterochromatin and nuclear lamina. Our work suggests that similar molecular mechanisms are employed to sense deformation resulting from both nuclear-extrinsic and nuclear-intrinsic forces triggered by the abnormal mass and/or topology of aneuploid chromatin. Importantly, our work indicates that the mechanosensation and checkpoint activation downstream of chromosome mis-segregation operate at the level of the nucleus and do not engage the cytoskeleton, at least at the rapid time scales explored here. It is likely that the cytoskeleton becomes critical during the long-term cellular mechanoadaptation response, as is the case for genome mechanoprotection33.
We observe that chromosome mis-segregation leads to immediate perturbations in the spatial organization of chromatin, specifically in its tethering to the nuclear lamina, which itself becomes highly irregular. Chromatin–lamina interactions within the LADs are essential for proper genome function25 and might contribute to nuclear shape47. Furthermore, alterations in the chromatin–lamina interface, as well as in chromatin interactions with NE-resident proteins, can lead to NE stress and altered stability24. Our study demonstrates that, at mitotic exit, cells that experience chromosome mis-segregation have reduced heterochromatin occupancy within LADs and physical tethering to the NE, which are normally required to support proper nuclear mechanics and to buffer mechanical forces48. These results are consistent with the observations that cells derived from patients with Down syndrome have reduced interaction of LADs with the nuclear lamina and increased expression of genes within LADs73,74. Consequently, this altered chromatin organization leads to a perturbed nuclear mechanophenotype characterized by a softer nucleus with a tensed NE. These changes are then sensed by mTORC2 and ATR to cooperatively induce the p53/p21 checkpoint that ultimately prevents aneuploidy transmission by blocking cell cycle progression (Fig. 6l). Intriguingly, ATR and mTORC2 share common HEAT repeat domains that could in principle detect NE deformation and mechanical alterations arising from their structural properties as elastic connectors75,76. The precise molecular mechanisms underpinning the ATR/mTORC2 crosstalk to activate p53 in the context of the mechanosensitive checkpoint remains to be investigated. Our data suggest that p53 Ser15 phosphorylation could be directly deposited by mTOR and stimulated by AKT activity, as was previously reported60. Thus, mTORC2 might be upstream of ATR activation and ATR, in turn, may initiate the signalling cascade by contributing directly or indirectly to the phosphorylation of AKT. Nevertheless, it is uncertain whether the mild p53 Ser15 phosphorylation observed in IAA-treated cells is sufficient to trigger the checkpoint or stabilize p53 or if it is an indirect byproduct from secondary sources9,10,11,12,44,77. Importantly, in light of the fact that loss of the tumour suppressor gene TP53 is the most frequent genomic alteration in all cancers7, our study implicates an additional advantage for the loss of p53: cells that can tolerate an aneuploid genome by escaping the p53-dependent mechanical checkpoint gain high migratory potential due to nuclear softening78. This increased invasiveness may contribute to a poorer prognosis in solid tumours with a high prevalence of aneuploidy69.
Our results suggest that the activation of p53/p21 following chromosome mis-segregation is rapid (a few hours) in response to altered nuclear mechanics and continues to accumulate gradually over time. Here we posit that the mode of chromosome mis-segregation (polar, misaligned and lagging chromosomes) leads to variable types of nuclear alteration that might be sensed differently by the mechanosensor checkpoint. This notion is consistent with the fact that chromosome mis-segregation systems can bias for specific chromosome aneuploidies40,79,80. Indeed, acquisition of one extra chromosome gain is sufficient to induce abnormal nuclear shape in human cells, except for highly heterochromatic chromosomes, such as the silenced additional X chromosome of patients with X syndrome26. Thus, the identity of the chromosomes gained or lost from the main nucleus may differentially affect nuclear mechanics81, shape82 and the p21/p53 checkpoint. This difference in the mode of aneuploidy is probably the underlying cause in the varying degree of p21 activation and frequency in G1-arrested cells across different studies10,17. Our findings show that CENP-C-depleted cells harbour a higher level and percentage of p21-positive cells compared with CENP-A-depleted cells, in addition to a stronger mis-segregation rate4,40, despite similar degrees of nuclear deformation. This suggests that the efficiency of the p53/p21 response may be more directly related to the frequency of aneuploidy and the status of NE tension rather than the severity of nuclear deformation.
Mechanistically, alterations in NE lipid composition may contribute to its fluidification to favour the accommodation of extra chromosomes, as has been observed in trisomic cells26. Chromosome gain is also a source of proteotoxic stress, which triggers a hypo-osmotic stress response and cell swelling83 that may contribute to the observed increase in NE tension immediately after chromosome mis-segregation. The contribution of chromatin- and lipid-based mechanisms, along with the osmotic component, in the establishment and regulation of the nuclear mechanophenotype at the NE is an open frontier that is worth further exploration.
Finally, we demonstrate broad applicability of the proposed nuclear mechanosensor checkpoint by demonstrating its activation in progerin-expressing cells. Thus, we propose that the biophysical state of the nuclear periphery interface serves as a gatekeeper of proper genome organization and chromatin content. Consistent with this notion, the mTORC2 pathway84,85 and high levels of aneuploidy have been implicated in physiological ageing86,87, which is usually associated with chromatin and nuclear shape alterations. Unravelling the relevance of NE stress and the sensation of chromatin topology in the broader scope of cellular physiology and the natural process of chronological ageing represents an open and exciting avenue for investigation.
Methods
Cell culture and treatments
All cell lines were maintained at 37 °C under 5% CO2 and 21% O2. Immortalized hTERT RPE-1 cells (CRL-40000; American Type Culture Collection (ATCC); RRID:CVCL_43888) were grown in Dulbecco’s Modified Minimal Essential Medium DMEM/F-12 (Life Technologies) supplemented with 10% foetal bovine serum (FBS) or 10% tetracycline-free FBS (Atlanta Biologicals), 100 U ml−1 penicillin–streptomycin (Life Technologies), 0.1% sodium bicarbonate and 2 mM l-glutamine. U-2 OS cells (HTB-96; ATCC; RRID:CVCL_0042) were maintained in DMEM (Life Technologies) supplemented with 10% FBS, 100 U ml−1 penicillin–streptomycin (Life Technologies) and 2 mM l-glutamine. Immortalized hTERT BJ fibroblasts (CRL-4001; ATCC; RRID:CVCL_6573) were grown in DMEM (Life Technologies) mixed with Medium 199 (M4530; Sigma–Aldrich) at a 3.5:1 ratio, supplemented with 10% FBS, 100 U ml−1 penicillin–streptomycin (Life Technologies) and 2 mM l-glutamine. No commonly misidentified lines per International Cell Line Authentication Committee register were used.
Cell cycle synchronization was obtained by treatment with 0.5 μg ml−1 of the cyclin-dependent kinase 4/6 inhibitor palbociclib (PZ0383; Sigma–Aldrich) for 24 h. Palbociclib removal (washout) was performed with four washes in complete medium.
Hypotonic treatments were performed using cell incubation with growth medium diluted 1:1 with sterile cell culture-grade water (W3500; Sigma–Aldrich) for the indicated times. Hypertonic treatments were performed using cell incubation with growth medium containing 1 M sucrose (S0389; Sigma–Aldrich) for the indicated times.
The following chemicals were used as indicated in the main text and figure captions: IAA (0.5 μM; I5148; Sigma–Aldrich), doxycycline (D2507; Sigma–Aldrich), ATR inhibitors (that is, VE-821 (SML1415; Sigma–Aldrich) and AZD6738 (S7693; Selleckchem)), methylstat (SML0343; Sigma–Aldrich), JIB-04 (15338; Cayman Chemical), CENP-Ei (that is, GSK923295 (SML3220; Sigma–Aldrich)), MPS1i (that is, AZ3146 (HY-14710; MedChemExpress) MPI-0479605 (HY-12660; MedChemExpress) and reversine (HY-14711; MedChemExpress)), ATMi (that is, KU55933 (HY-12016; MedChemExpress)), etoposide (E1383; Sigma–Aldrich), cytochalasin D (C8273; Sigma–Aldrich), remodelin (SML1112; Sigma–Aldrich), trichostatin A (S1045; Selleckchem), nocodazole (M1404; Sigma–Aldrich), rapamycin (553210; Sigma–Aldrich), INK-128 (HY-13328; MedChemExpress), ipatasertib (HY-15186; MedChemExpress), blebbistatin (203390; Calbiochem), JR-AB2-011 (HY-122022; MedChemExpress), dihydrocytochalasin B (D1641; Sigma–Aldrich), AKTi (that is, MK-2206 (S1078; Selleckchem)) and insulin (BE02-033E; Lonza).
Plasmid generation
Wild-type lamin A and LAP2β coding sequences were PCR amplified from pTRIP-CMV-Puro-2AFlag-LaminA and pTRIP-SFFV-GFP-LAP2β plasmids, respectively, and cloned in frame downstream from the enhanced GFP (eGFP) sequence in a pDEST shuttle vector. Progerin plasmid was derived from the lamin A wild-type plasmid via inverse PCR deleting of 150 base pairs corresponding to Val607–Gln656 of lamin A. Coding sequences were finally cloned into a pInducer20 backbone (44012; Addgene) through the Gateway system using LR II recombinase (11791020; Invitrogen).
Cell line generation
Doxycycline-inducible eYFP–AID-CENP-A/eGFP–lamin A wild-type, eYFP–AID-CENP-A/eGFP–progerin and eYFP–AID-CENP-A/eGFP–LAP2β cell lines were generated by transduction with lentiviral particles delivering respective pInducer20 vectors (see the section ‘Plasmid generation’) for 16 h in the presence of 8 μg ml−1 polybrene. Cells were then selected using G418 (0.5 mg ml−1). Doxycycline was used at 100 ng ml−1 for eGFP–LAP2β, 10 ng ml−1 for lamin A and 50 ng ml−1 for progerin.
eYFP–AID-CENP-A p53 knockdown RPE-1 cells were generated by lentiviral transduction of the eYFP–AID-CENP-A cell line with lentiviral particles delivering short hairpin RNA against p53 (sequence 5′-GACTCCAGTGGTAATCTACT-3′). Stably expressing clones were derived and verified by immunoblot analysis.
eYFP–AID-CENP-A/eGFP–FLAG–cGAS-E225A/D227A RPE-1 cells were generated by lentiviral transduction of the eYFP–AID-CENP-A cell line with lentiviral particles delivering pTRIP-SFFV-eGFP-FLAG-cGAS-E225A/D227A (ref. 88). eYFP–AID-CENP-A/eGFP–NLS RPE-1 cells were generated by lentiviral transduction of the eYFP–AID-CENP-A cell line with lentiviral particles delivering pTRIP-SFFV-eGFP-NLS.
The RPE-1 OsTIR19–Myc/CENP-A−/−/eYFP–AID-CENP-A+ integrated fluorescent ubiquitination-based cell cycle indicator system was generated as described in ref. 4.
p21-GFP RPE-1 cells were generated as described in ref. 43 (a kind gift from the laboratory of A. Barr and C. Bakal).
Immunoblot analyses
Whole-cell extracts were obtained by denaturation and sonication in sodium dodecyl sulfate (SDS) lysis buffer (200 mM Tris (pH 8.8), 4% SDS and 10% glycerol); alternatively, a non-denaturing lysis buffer was used (20 mM Tris-HCl (pH 7.6), 300 mM NaCl, 7.5% glycerol and 0.2% IGEPAL). Images were acquired on a ChemiDoc imager (Bio-Rad) and analysed on Image Lab software (version 6.1; Bio-Rad). Lysates were quantified using the bicinchoninic acid assay (BCA) method (Thermo Fisher Scientific), diluted in Laemmli Sample Buffer and incubated at 95 °C for 5 min before being resolved by SDS polyacrylamide gel electrophoresis, transferred to nitrocellulose membranes and blotted with the following antibodies: CENP-A (1:1,000; 2186; Cell Signaling Technology; RRID:AB_10828491), p53 (1:200; OP43; Millipore; RRID:AB_213402), p21 (1:200; OP43; Millipore; RRID:AB_2335868), lamin A/C (1:1,000; sc-7292; Santa Cruz Biotechnology; RRID:AB_627875), lamin B1 (1:2,000; 12987 ProteinTech; RRID:AB_2136290), vinculin (1:5,000; V9264 Sigma–Aldrich; RRID:AB_10603627), p16 INK4A (D3W8G) (1:1,000; 2186; Cell Signaling Technology; RRID:AB_2750891), p38 MAPK (1:1,000; 9212; Cell Signaling Technology; RRID:AB_330713), GAPDH (1:5,000; 2118; Cell Signaling Technology; RRID:AB_561053), ph-AKT (S473) (1:1,000; 4060; Cell Signaling Technology; RRID:AB_2315049), AKT (1:1,000; 9272; Cell Signaling Technology; RRID:AB_329827), ph-p70 S6 kinase (Thr389) (1:1,000; 9234; Cell Signaling Technology; RRID:AB_2269803), ph-CHK2 (T68) (1:1,000; 2661; Cell Signaling Technology; RRID:AB_331479), CHK2 (1:1,000; 611570; BD Biosciences; RRID:AB_399016), p70 S6 kinase (1:1,000; 2708; Cell Signaling Technology; RRID:AB_390722), ph-p53 (S15) (1:1,000; 9284; Cell Signaling Technology; RRID:AB_331464) and γ-H2AX (1:1,000; 2718; Cell Signaling Technology; RRID:AB_659840). Band quantifications were performed by densitometry using Fiji and intensities were normalized to the loading control vinculin or GAPDH. Unprocessed, uncropped blots are provided as source data.
Fluorescence-activated cell sorting and analysis
For cell cycle analysis, bromodeoxyuridine (BrdU) pulse labelling was performed for 24 h or 30 min (10 μM final) before collection and fixation in 70% ethanol. Denaturation was performed with 2 N HCl followed by neutralization with 0.1 M sodium tetraborate (pH 8.5). Cells were stained with anti-BrdU (1:5; 347580; BD Biosciences) and proper secondary antibody. Propidium iodide was added at a final concentration of 2.5 μg ml−1 in the presence of 250 μg ml−1 RNase A (Thermo Fisher Scientific) and incubated for at least 1 h before acquisition on an LSR II Flow Cytometer (BD Biosciences) with FACSDiva software (BD Biosciences). The data were analysed using FlowJo software version 10 (BD Biosciences).
Cell staining for the sorting of p21-positive and -negative cells was performed as follows: cells were collected, counted and fixed in 1% formaldehyde for 20 min on ice. The reaction was blocked with a final concentration of 125 mM glycine for 3 min. After washes with 1× phosphate-buffered saline (PBS), cells were permeabilized with 1× permeabilization buffer (Foxp3/Transcription Factor Staining Buffer Set; 00-5523-00; Thermo Fisher Scientific) for 30 min at room temperature. Cells were resuspended in 1× permeabilization buffer containing 0.5 µg anti-p21 antibody (MABE325; Merck) incubated for 1 h at room temperature. After three washes in 1× permeabilization buffer, cells were resuspended in permeabilization buffer with fluorochrome-conjugated secondary antibody (1:500; Alexa Fluor 647; The Jackson Laboratory) for 30 min at room temperature. Following two washes in 1× permeabilization buffer, cells were resuspended in 30 µl 1× FACS buffer (1% bovine serum albumin (BSA) and 2 mM EDTA in PBS) containing 4′,6-diamidino-2-phenylindole (DAPI; 1 µg ml−1). For the immunoglobulin G isotype control sample, primary antibody was omitted. Cell sorting was performed on an SH800 cell sorter (Sony Biotechnology). Collected cells were further processed for FISH analysis (see the section ‘FISH’). A representative graph of the gating strategy was generated using FlowJo software version 10 (BD Biosciences).
Immunofluorescence and live-cell imaging microscopy
Cells were seeded on glass coverslips and fixed in PBS containing 4% formaldehyde at room temperature for 10 min. Blocking was performed with 2.5% FBS (vol/vol), 0.2 M glycine and 0.1% Triton X-100 (vol/vol) in PBS for 30 min at room temperature. Primary antibodies were incubated in blocking buffer for 1 h at room temperature or overnight at 4 °C. After washing with PBS containing 0.1% Triton X-100, coverslips were incubated with secondary antibodies conjugated to fluorochromes (Jackson ImmunoResearch) for 45 min at room temperature, washed and counterstained with DAPI (1 µg ml−1) for 10 min. Coverslips were mounted using the antifade reagent ProLong Gold (Life Technologies). For ATR, cPLA2 and p-AKT staining, cytoplasm was extracted before fixation by incubating coverslips in ice-cold hypotonic buffer (340 mM sucrose, 10 mM HEPES (pH 7.4), 10 mM KCl, 1 mM dithiothreitol, 7% glycerol, 0.1% IGEPAL and Protease Inhibitor Cocktail (Roche) for 5 min on ice. Permeabilization, saturation and staining were performed with PBS containing 0.2% BSA and 0.05% saponin. For 5-ethynyl-2′-deoxyuridine (EdU) staining, EdU incorporation (to a final concentration of 10 μM) was performed during the 30 min before fixation. After fixation and permeabilization, EdU click labelling was performed using a Click-iT EdU Cell Proliferation Kit following the manufacturer’s instructions (C10337; Thermo Fisher Scientific).
The following antibodies were used: ACA (1:500; 15-235-0001; Antibodies Incorporated; RRID: AB_2797146), p21 (1:200; OP43; Millipore; RRID:AB_2335868), CENP-A (1:1,000; 2186; Cell Signaling Technology; RRID:AB_10828491), 53BP1 (1:500; NB100-304; Novus Biologicals; RRID:AB_10003037), γH2A.X (1:1,000; 05-636; Millipore; RRID:AB_309864), lamin A/C (1:1,000; sc-7292; Santa Cruz Biotechnology; RRID:AB_627875), lamin B1 (1:1,000; 12987-1-AP; Proteintech; RRID:AB_2136290), H3K9me3 (1:1,000; ab8898; Abcam; RRID:AB_306848), H3K27me3 (1:1,000; 9733S; Cell Signaling Technology; RRID:AB_2616029), ATR (1:1,000; 2790; Cell Signaling Technology; RRID:AB_2227860), cPLA2 (1:1,000; ab58375; Abcam; RRID:AB_881998), ph-AKT (1:500; 4060; Cell Signaling Technology; RRID:AB_2315049), p53 (1:200; OP43; Millipore; RRID:AB_213402), α-tubulin (1:1,000; NB100-690; Novus Biologicals; RRID:AB_521686), HP1α (1:1,000; a gift from the laboratory of A. Straight at Stanford University) and Alexa Fluor 647 Phalloidin (1:250; A22287; Thermo Fisher Scientific; RRID:AB_2620155). Images were acquired on a DeltaVision Core system (Applied Precision) with a 60× or 100× Olympus UPlanSApo oil immersion objective (NA: 1.4), a 250 W xenon light source and a Photometrics CoolSNAP HQ2 camera. 4 μm z stacks were acquired (z step size: 0.2 μm).
Live-cell imaging was performed either on a DeltaVision Core system (Applied Precision) with softWoRx software (version 7.2.1) or on an inverted ECLIPSE Ti2 microscope (Nikon) equipped with a fully motorized spinning disk CSU-W1 (Yokogawa Electric) using NIS-Elements (version 5.41.0). Imaged cells were grown on high-optical-quality plastic slides (Ibidi) and treated as indicated in the figures and their corresponding captions.
Super-resolution SIM imaging and 3D analysis
Super-resolution 3D SIM imaging was performed on a ZEISS Elyra 7 microscope (Carl Zeiss) using an alpha Plan-Apochromat ×63/1.46 oil immersion objective and PCO edge sCMOS cameras. For SIM reconstruction, 13 images were acquired. SIM image reconstruction was performed with the software ZEN Black (Carl Zeiss). Axial chromatic shift correction was not applied on colocalization analysis. Imaris software (version 10) was utilized for visualization.
Intensity quantifications in microscopy experiments
The nuclei of interphase cells were detected using a thresholding method on DAPI images with the software Fiji/ImageJ or using Cellpose (version 2.0)89 with a manually trained model based on the Cellpose cyto model to improve the exclusion of micronuclei touching the main nuclei from the generated masks. Nuclear intensities were then measured using Fiji/ImageJ and the integrated density values were taken. To measure the signal specifically at the nuclear periphery, a ring was drawn by eroding the nuclear masks and the coefficient of variation of the mean intensity within the ring was calculated on the lamin channels to measure the variation of the lamina staining. To measure heterochromatin loss at the nuclear periphery, the mean H3K9me3 intensity of this ring was divided by the mean intensity in the rest of the nucleus (the nuclear interior). 53BP1 foci were counted within nuclear masks using the Find Maxima function in Fiji/ImageJ. The prominence parameter of the function was chosen empirically for each experiment to reach optimal foci detection. To measure NE breaks, cGAS foci appearing at the nuclear periphery were counted manually. Microtubule and actin architectures were quantified by thresholding the cytoskeletal signal area, which was then normalized by the total cell area.
FISH
P21-positive sorted cells were resuspended in PBS and cytospun on a glass slide, which was then fixed in Carnoy’s fixative (methanol:acetic acid at 3:1) for 15 min at room temperature, rinsed in 80% ethanol and air dried for 5 min. Centromere-specific probes for chromosomes 3 and X (XCE 3 Orange (D-0803-050-OR; MetaSystems Probes) and XCE X Green (D-0823-050-FI; MetaSystems Probes)) were mixed, applied and sealed with a coverslip. Slides were denatured at 75 °C for 2 min and incubated at 37 °C overnight in a humidified chamber. Slides were washed with 0.4× saline sodium citrate at 72 °C for 2 min, then 2× saline sodium citrate and 0.1% Triton X-100 at room temperature for 30 s and finally rinsed with PBS. Slides were incubated with DAPI solution for 10 min before mounting in antifade reagent. Slides were scanned and acquired on a Metafer system (version 3.13.5). The same slides were stripped with 70% formamide for 2 min at 75 °C and re-hybridized with centromere-specific probes for chromosomes 6 and 9 (XCE 6 Green (D-0806-050-FI; MetaSystems Probes) and XCE 9 Orange (D-0809-050-OR; MetaSystems Probes)). Selected coordinates were used to find the same metaphases acquired in the first acquisition using a Metafer system. The final analysis allowed the detection of aneuploidy for chromosomes X, 3, 6 and 9 on the same metaphase.
Metaphase spreads for aneuploidy evaluation
Cells were treated with colcemid (100 ng ml−1; Merck Millipore) for 3 h to enrich for mitotic cells, which were collected by mitotic shake-off after a short trypsin treatment. Collected cells were centrifuged at 200g for 5 min. Cell pellets were resuspended in 75 mM KCl and incubated for 15 min at 37 °C. Carnoy’s fixative solution was freshly prepared and added to the cells at a 1:10 volume ratio before centrifugation at 200g for 5 min. The cells were then fixed for 30 min at room temperature in the Carnoy’s solution, centrifuged and resuspended in a low volume of fixative for spreading onto glass slides. DAPI staining was performed for 5 min in DAPI solution (1 μg ml−1 in 1× PBS with 0.1% Triton X-100) and the slides were mounted in antifade mounting medium (P36941; Invitrogen). Images were acquired using an Upright Leica DM6000 B widefield microscope and the numbers of chromosomes per spread were manually counted.
ChIP-qPCR
ChIP assays were performed as previously described90. Briefly, 1% formaldehyde cross-linked chromatin was sonicated to an average size of 300–600 base pairs and quantified. 1 mg sonicated chromatin was incubated overnight at 4 °C with 10 μg lamin B1 antibody (12987-1-AP; Proteintech; RRID:AB_2136290) and recovered using Sepharose Protein A beads. The beads were washed three times in low-salt buffer and once in high-salt buffer. The DNA was decrosslinked and column purified. Lamin B1 enrichment at selected LADs (from ref. 91; Supplementary Table 2) was determined using LightCycler 480 SYBR Green I Master mix (04887352001; Roche) on a QuantStudio thermal cycler (Applied Biosystems). Enrichment was calculated as the percentage on bound input and normalized to the untreated condition.
Colony formation assay
Cells were plated in serial dilutions on six-well plates and the indicated treatments were added 24 h after seeding. After 7 d, colonies were fixed for 10 min in methanol, washed twice with 1× PBS, stained for 10 min in 1% crystal violet containing 20% EtOH and washed twice with 1× PBS.
Migration assays
For the transwell migration assays, cells were pre-treated with IAA for 24 h where indicated, counted and resuspended in DMEM/F-12 serum-free medium supplemented with 0.1% BSA. Transwell inserts with an 8 μm pore membrane (10061051; Falcon) were coated with 50 μg ml−1 Matrigel (E1270; Sigma–Aldrich) for 1 h at 37 °C and inserted in a well (in a 12-well plate) containing DMEM/F-12 medium supplemented with 20% serum. 75,000 cells were seeded and left to migrate through the membrane for 24 or 48 h. Inserts were gently washed in PBS, fixed with methanol, stained with crystal violet and imaged on a Nikon AZ100 microscope. Migrated cells were quantified with the cell counter plugin in the Fiji software.
For 3D migration, cells were left untreated or pre-treated with IAA for 24 h, then seeded into low-adherence multi-well plates (AggreWell400 (STEMCELL Technologies) or 4520 (Corning)) to generate spheroids with 1,000 cells per microwell in the presence of IAA where indicated. After 20 h, spheroids were embedded in 1.5 mg ml−1 pH-adjusted rat tail collagen I matrix (50201; Ibidi) and the cells were allowed to invade for 24 h while being live imaged every 30 min on an inverted Nikon ECLIPSE Ti microscope. The longest invasion paths were quantified per cell aggregate using the Fiji software.
Migration assay through microfabricated channels was performed as follows. Microchannels were prepared as previously described92. Briefly, polydimethylsiloxane (PDMS; 10/1 wt/wt PDMS A/crosslinker B; GE Silicones) was used to prepare 12-µm-wide and 5-µm-high microchannels with constrictions of 15 µm length and varying widths (2 and 4 µm) from a self-made mould. Channels with constrictions were incubated with 10 µg ml−1 fibronectin for 30 min then washed with PBS three times and finally incubated with medium for at least 5 h before adding the cells. 20,000 cells (pre-treated with IAA for 24 h where indicated) resuspended in 5 µl medium were added to each reservoir. After 30 min of incubation with 1 µg ml−1 Hoechst dye for 30 min, the medium was added above the PDMS device. The acquisition was made 12 h after seeding the cells in the device, with a LEICA DMi8 video microscope equipped with a 10×/0.45 Plan-Apochromat dry objective. The time-lapse series was continuously acquired every 1 min over 24 h. The crossing time was quantified by measurement of the time that a stained nucleus spent between the engagement in the constriction and the exit.
2D confinement assay
p21–eGFP RPE-1 cells grown on glass-bottomed plates were washed and replenished with fresh medium containing 1 μm latex beads (89904; Sigma–Aldrich) or 10 μm latex beads (72986; Sigma–Aldrich) (0.25 μl beads per 1 ml medium) at least 1 h before the experiment. On the microscope stage, suction cup-bound BSA-coated coverslips were driven on top of the cells using a controlled pressure pump (Elveflow). Compression was held on the cells for 3 h and the cells were fixed and imaged post-confinement on an inverted Nikon ECLIPSE Ti microscope. Cells were fixed directly at the end of the compression period by removing the medium and adding 8% paraformaldehyde in PBS before stopping the compression. Then, the vacuum was gradually switched off, the coverslip was removed and the solution was replaced with 4% paraformaldehyde in PBS for 10 min at room temperature before being further processed as described in the section ‘Immunofluorescence and live-cell imaging microscopy’.
Microfabrication-based confinement using a six-well confiner
To image the effect of confinement at several different confinement heights at the same time, cell confinement was performed using a version of the cell confiner adapted to multi-well plates60. The height of the microspacers (micropillars) determines the height of the spatial confinement of cells between the coverslip (top) and substrate (bottom). The surface of the confining side was always treated with non-adhesive PLL-g-PEG (PLL(20)-g[3.5]-PEG(2); SuSoS). To make PDMS (GE RTV615) micropillars of the desired height, 13 mm glass coverslips (1 μm height and 13 mm length; VWR) were plasma treated for 2 min and then placed on top of a PDMS mixture (10/1 wt/wt PDMS A/crosslinker B) on wafer moulds (containing holes and micropillars fabricated following standard photolithography procedures). After baking at 95 °C for 20 min, coverslips with PDMS micropillars were carefully removed from the wafers under isopropanol. They were then cleaned with isopropanol, well dried, treated with plasma for 2 min and then treated with 0.5 mg ml−1 PLL-g-PEG in 10 mM (pH 7.4) HEPES buffer for 1 h at room temperature. Coverslips with PDMS pillars were rinsed with water (Milli-Q; Merck Millipore) and incubated in complete medium overnight before confining the cells. The modified cover lid of a glass-bottomed multi-well plate (1.5 μm height, uncoated and irradiated; MatTeK) was used to apply confining glass coverslips to cells where large PDMS pillars were stuck on the cover lid of the multi-well plate to hold the confining glass coverslips containing a layer of microfabricated micropillars. These large PDMS pillars pushed the confining glass coverslips from the top of the plastic six-well cover lid to confine the cells in six-well glass- or plastic-bottomed plates. Once the cover lid with confining pillars and glass coverslips had been lowered down on the wells to achieve confinement, the structure was held together by adhesive tape.
RPE-1 cells expressing the p21-GFP reporter (1.2 × 106 cells per ml) were confined at the time of mitotic exit (15 h after release from G1 arrest) using the six-well confiner at heights of 4, 6 and 20 μm until the end of the experiment. In addition to the mentioned six-well plates, two wells without confinement (one with a negative control and one with a positive control) were prepared. Unconfined cells served as a negative control and unconfined cells treated with 50 μM etoposide, added upon cell seeding (to induce p21 expression), were used as a positive control. Six-well plates with confined and control cells were imaged after 2 h of confinement and at the 24 h time point (imaging of control cells was performed at the same time). Cells were imaged at ten random positions at every time point with the lowest possible exposure times that still allowed differentiation of the signal. Imaging of six-well confiner plates was performed at 37 °C in 5% CO2 with a DMi8 inverted fluorescence microscope (Leica) controlled with MetaMorph software (Molecular Devices version 7.8). The microscope was equipped with a 40× dry objective (HC PL FLUOTAR; NA = 0.8; Leica), a motorized stage and filter wheel, a cage incubation chamber with temperature and CO2 controls (Pecon, Meyer Instruments), an ORCA-Flash4.0 LT Digital camera (C11440-42U; Hamamatsu Photonics), a z axis guiding piezo motor (Märzhäuser Wetzlar) and a SOLA Light Engine solid-state illumination system (Lumencor).
Analysis of the p21-GFP signal was performed using Fiji software by quantifying the mean nuclear fluorescence levels (>70 cells per experimental condition) at each time point. To normalize the variation between conditions, the mean background fluorescence under each condition was subtracted from every measurement. Normalized mean nuclear fluorescence levels of confined cells were corrected according to the confinement height and the height of a resting RPE-1 cell nucleus (8 μm). Normalized mean nuclear fluorescence levels were multiplied by 2 for the 4 μm condition, by 1.33 for the 6 μm condition and by 1 for the 20 μm condition. This correction was introduced to take into account that the images were taken with a widefield epifluorescence microscope. The graph was generated and statistical analyses were performed using Prism GraphPad software.
A very similar experiment was performed with RPE-1 cells expressing a catalytically inactive cGAS (E225A/D227A)–mCherry reporter, where cells (1.2 × 106 cells per ml) in suspension were confined using the six-well confiner at heights of 2, 4, 6 and 20 μm for 2 h and the numbers of cGAS foci were quantified. Cells were imaged with the same microscope set up as for the p21 confinement experiment. The graph was generated and statistical analyses were performed using Prism GraphPad software.
Transmission electron microscopy
Samples were fixed with 2.5% electron microscopy-grade glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.4) (Electron Microscopy Sciences) at room temperature for 30 min, followed by 1 h of fixation on ice. Samples were then washed five times with 0.1 M sodium cacodylate buffer. After this primary fixation, samples were fixed in 1% osmium tetroxide for 1 h, then rinsed with 0.1 M sodium cacodylate buffer and dehydrated via graded alcohol series from 75 to 100%. This was followed by a 100% acetone wash, which was followed by an overnight incubation in a mixture of 50:50 acetone and embedding media. The following day samples were switched to 100% Epon (TAAB 812) to allow gradual infiltration. The samples were polymerized in Epon overnight at 60 °C, after which a pyramid was made with a razor blade. The razor blade was then used to cut 80 nm sections, which were mounted on copper slot grids to be imaged on a conventional 80 kV JEM-1400 (JEOL).
AFM
AFM measurements were performed on IAA pre-treated or untreated cells plated on 35 mm tissue culture dishes using a JPK NanoWizard 4 (Bruker) atomic force microscope mounted on a Nikon ECLIPSE Ti2 inverted fluorescence microscope (Nikon) and operated via JPK SPM Control Software version 6. Non-conductive triangular silicon nitride MLCT cantilevers (Bruker) were used for all of the AFM experiments of cells and nuclei. Forces of up to 2 nN were applied at a constant cantilever velocity (5 µm s−1) while ensuring an indentation depth of 500 nm. All analyses were performed with JPK Data Processing Software version 6 (Bruker). Briefly, the offset was removed from the baseline of raw force curves, then the contact point was identified and cantilever bending was subtracted. Finally, a Hertz model was fitted with correct tip geometry to obtain the Young’s modulus.
Nuclear envelope tension analyses
To obtain NE fluctuations to measure apparent NE tension, CENP-AAID and CENP-CAID RPE-1 cells expressing LAP2β-GFP or RPE-1 cells expressing Dox-inducible lamin A-GFP and progerin-GFP were imaged using a high-frame-rate acquisition mode (150 ms per frame) for 5 min. Then, the images were corrected for bleaching and linear rotational drift (Bleach Correction and StackReg plugins, respectively; ImageJ). NE fluctuations were calculated by measuring the standard deviation of the position of the NE from its mean position, as described previously38. ER Flipper-TR (SC021; Spirochrome; 1 mM) was used to quantify in-plane NE tension. Fluorescence lifetime imaging microscopy (FLIM) imaging was performed using a Leica SP8 STED 3X/Confocal Microscope with Falcon FLIM. Excitation was performed with a pulsed 488 nm laser operating at 20 MHz with emission collected through a bandpass (565/610 nm) filter. FLIMfit 5.1.1 software (Imperial College London) was used to fit the fluorescence decay data from regions of interest to a dual exponential model after deconvolution for the instrument response function (measured with backscattered emission light of saturated erythrosin B with potassium iodide). The longest lifetime with the higher fit amplitude was used to quantify membrane tension.
Kinase panel screening
A total of 7,000 cells were seeded into each well of glass-bottomed 96-well plates (P96-1.5H-N; Cellvis). The next day, the cells were treated with 500 μM IAA for 24 h and co-treated with either 5, 50 or 500 nM kinase inhibitors from the Selleckchem kinase inhibitors library (L1200; Selleckchem). Cells were treated with dimethyl sulfoxide as a negative control. Cells were treated with IAA only or 4 μM etoposide to provide p21-positive controls. Cells were imaged using a CV7000 high-throughput spinning disk confocal microscope (Yokogawa) with a 20× air objective (NA: 0.75) and two sCMOS 2,550 pixel × 2,160 pixel (5.5 megapixel) cameras. Six random fields of view were acquired for each well. Images were binned 2 × 2 (pixel size: 650 nm). Cells were stained with anti-p21 antibody and DAPI as described in the section ‘Immunofluorescence and live-cell imaging microscopy’. The images were analysed using Columbus 2.9.1 software (PerkinElmer). A Columbus image analysis pipeline segmented nuclei using the DAPI image and then measured p21 nuclear mean intensity levels. Wells treated with 500 nM compounds were discarded as most of them were toxic to the cells. Wells with a lower number of cells than in the IAA-only wells were also discarded. Wells treated with 5 and 50 nM compounds with an average percentage of p21-positive cells below that of the IAA-only wells were selected as hits.
Statistics and reproducibility
Statistical analyses of all of the graphs were performed using GraphPad Prism 10. The choice of statistical method was made following the guidelines from ref. 93. Briefly, an unpaired t-test was used to compare two groups; either ordinary one-way analysis of variance or a multiple Kruskal–Wallis test (depending on the data distribution) was used to compare more than two groups; a Fisher’s exact test was used to compare categorical samples and a one-sample t-test was used to compare samples with a fixed value. GraphPad Prism’s normality test was used to determine whether data followed a normal distribution. A summary of the data presented in all of the graphs is provided in the numerical source data file. No statistical methods were used to predetermine sample sizes, but our sample sizes are similar to those reported in previous publications4,33. All of the experiments were performed using several biological replicates. Numbers of replicates for each experiment are indicated in the corresponding figure captions. Several steps were taken to ensure the reproducibility of our experimental findings and key results were confirmed using complementary experimental approaches. With respect to data randomization, sample assignment to treatment and control groups, as well as data collection, was performed randomly without previous assessment of any cell culture characteristics. In general, data collection and analysis were not performed blind to the experimental conditions; however, quantitative image analyses, which are at the heart of all of the presented data, were agnostic to experimental conditions. No data were excluded from the analyses.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The data supporting the findings of this study are available from the corresponding authors upon request. Source data are provided with this paper.
References
Santaguida, S. & Amon, A. Short- and long-term effects of chromosome mis-segregation and aneuploidy. Nat. Rev. Mol. Cell Biol. 16, 473–485 (2015).
Gordon, D. J., Resio, B. & Pellman, D. Causes and consequences of aneuploidy in cancer. Nat. Rev. Genet. 13, 189–203 (2012).
McKinley, K. L. & Cheeseman, I. M. The molecular basis for centromere identity and function. Nat. Rev. Mol. Cell Biol. 17, 16–29 (2016).
Hoffmann, S. et al. CENP-A is dispensable for mitotic centromere function after initial centromere/kinetochore assembly. Cell Rep. 17, 2394–2404 (2016).
Hafner, A., Bulyk, M. L., Jambhekar, A. & Lahav, G. The multiple mechanisms that regulate p53 activity and cell fate. Nat. Rev. Mol. Cell Biol. 20, 199–210 (2019).
Lavin, M. F. & Gueven, N. The complexity of p53 stabilization and activation. Cell Death Differ. 13, 941–950 (2006).
Chen, X. et al. Mutant p53 in cancer: from molecular mechanism to therapeutic modulation. Cell Death Dis. 13, 974 (2022).
Levine, M. S. & Holland, A. J. The impact of mitotic errors on cell proliferation and tumorigenesis. Genes Dev. 32, 620–638 (2018).
Sheltzer, J. M. et al. Aneuploidy drives genomic instability in yeast. Science 333, 1026–1030 (2011).
Garribba, L. et al. Short-term molecular consequences of chromosome mis-segregation for genome stability. Nat. Commun. 14, 1353 (2023).
Passerini, V. et al. The presence of extra chromosomes leads to genomic instability. Nat. Commun. 7, 10754 (2016).
Burrell, R. A. et al. Replication stress links structural and numerical cancer chromosomal instability. Nature 494, 492–496 (2013).
Lambrus, B. G. et al. A USP28–53BP1–p53–p21 signaling axis arrests growth after centrosome loss or prolonged mitosis. J. Cell Biol. 214, 143–153 (2016).
Meitinger, F. et al. 53BP1 and USP28 mediate p53 activation and G1 arrest after centrosome loss or extended mitotic duration. J. Cell Biol. 214, 155–166 (2016).
Meitinger, F. et al. Control of cell proliferation by memories of mitosis. Science 383, 1441–1448 (2024).
Soto, M. et al. p53 prohibits propagation of chromosome segregation errors that produce structural aneuploidies. Cell Rep. 19, 2423–2431 (2017).
Santaguida, S. et al. Chromosome mis-segregation generates cell-cycle-arrested cells with complex karyotypes that are eliminated by the immune system. Dev. Cell 41, 638–651.e5 (2017).
Chow, K. H., Factor, R. E. & Ullman, K. S. The nuclear envelope environment and its cancer connections. Nat. Rev. Cancer 12, 196–209 (2012).
Hetzer, M. W. The nuclear envelope. Cold Spring Harb. Perspect. Biol. 2, a000539 (2010).
Shah, P., Wolf, K. & Lammerding, J. Bursting the bubble—nuclear envelope rupture as a path to genomic instability? Trends Cell Biol. 27, 546–555 (2017).
Vargas, J. D., Hatch, E. M., Anderson, D. J. & Hetzer, M. W. Transient nuclear envelope rupturing during interphase in human cancer cells. Nucleus 3, 88–100 (2012).
Hatch, E. M., Fischer, A. H., Deerinck, T. J. & Hetzer, M. W. Catastrophic nuclear envelope collapse in cancer cell micronuclei. Cell 154, 47–60 (2013).
Marcelot, A., Worman, H. J. & Zinn-Justin, S. Protein structural and mechanistic basis of progeroid laminopathies. FEBS J. 288, 2757–2772 (2021).
Kalukula, Y., Stephens, A. D., Lammerding, J. & Gabriele, S. Mechanics and functional consequences of nuclear deformations. Nat. Rev. Mol. Cell Biol. 23, 583–602 (2022).
Buchwalter, A., Kaneshiro, J. M. & Hetzer, M. W. Coaching from the sidelines: the nuclear periphery in genome regulation. Nat. Rev. Genet. 20, 39–50 (2019).
Hwang, S. et al. Suppressing aneuploidy-associated phenotypes improves the fitness of trisomy 21 cells. Cell Rep. 29, 2473–2488.e5 (2019).
Fonseca, C. L. et al. Mitotic chromosome alignment ensures mitotic fidelity by promoting interchromosomal compaction during anaphase. J. Cell Biol. 218, 1148–1163 (2019).
Hart, M., Adams, S. D. & Draviam, V. M. Multinucleation associated DNA damage blocks proliferation in p53-compromised cells. Commun. Biol. 4, 451 (2021).
Aureille, J. et al. Nuclear envelope deformation controls cell cycle progression in response to mechanical force. EMBO Rep. 20, e48084 (2019).
Bastianello, G. et al. Cell stretching activates an ATM mechano-transduction pathway that remodels cytoskeleton and chromatin. Cell Rep. 42, 113555 (2023).
Kidiyoor, G. R. et al. ATR is essential for preservation of cell mechanics and nuclear integrity during interstitial migration. Nat. Commun. 11, 4828 (2020).
Nader, G. P. F. et al. Compromised nuclear envelope integrity drives TREX1-dependent DNA damage and tumor cell invasion. Cell 184, 5230–5246.e22 (2021).
Nava, M. M. et al. Heterochromatin-driven nuclear softening protects the genome against mechanical stress-induced damage. Cell 181, 800–817.e22 (2020).
Kumar, A. et al. ATR mediates a checkpoint at the nuclear envelope in response to mechanical stress. Cell 158, 633–646 (2014).
Le, H. Q. et al. Mechanical regulation of transcription controls Polycomb-mediated gene silencing during lineage commitment. Nat. Cell Biol. 18, 864–875 (2016).
Lomakin, A. J. et al. The nucleus acts as a ruler tailoring cell responses to spatial constraints. Science 370, eaba2894 (2020).
Venturini, V. et al. The nucleus measures shape changes for cellular proprioception to control dynamic cell behavior. Science 370, eaba2644 (2020).
Holland, A. J., Fachinetti, D., Han, J. S. & Cleveland, D. W. Inducible, reversible system for the rapid and complete degradation of proteins in mammalian cells. Proc. Natl Acad. Sci. USA 109, E3350–E3357 (2012).
Nishimura, K., Fukagawa, T., Takisawa, H., Kakimoto, T. & Kanemaki, M. An auxin-based degron system for the rapid depletion of proteins in nonplant cells. Nat. Methods 6, 917–922 (2009).
Dumont, M. et al. Human chromosome-specific aneuploidy is influenced by DNA-dependent centromeric features. EMBO J. 39, e102924 (2020).
Santaguida, S. & Musacchio, A. The life and miracles of kinetochores. EMBO J. 28, 2511–2531 (2009).
Yao, X., Abrieu, A., Zheng, Y., Sullivan, K. F. & Cleveland, D. W. CENP-E forms a link between attachment of spindle microtubules to kinetochores and the mitotic checkpoint. Nat. Cell Biol. 2, 484–491 (2000).
Barr, A. R. et al. DNA damage during S-phase mediates the proliferation-quiescence decision in the subsequent G1 via p21 expression. Nat. Commun. 8, 14728 (2017).
Krupina, K., Goginashvili, A. & Cleveland, D. W. Causes and consequences of micronuclei. Curr. Opin. Cell Biol. 70, 91–99 (2021).
Gemble, S. et al. Centromere dysfunction compromises mitotic spindle pole integrity. Curr. Biol. 29, 3072–3080.e5 (2019).
Tamashunas, A. C. et al. High-throughput gene screen reveals modulators of nuclear shape. Mol. Biol. Cell 31, 1392–1402 (2020).
Schibler, A. C., Jevtic, P., Pegoraro, G., Levy, D. L. & Misteli, T. Identification of epigenetic modulators as determinants of nuclear size and shape. eLife 12, e80653 (2023).
Schreiner, S. M., Koo, P. K., Zhao, Y., Mochrie, S. G. & King, M. C. The tethering of chromatin to the nuclear envelope supports nuclear mechanics. Nat. Commun. 6, 7159 (2015).
Freund, A., Laberge, R. M., Demaria, M. & Campisi, J. Lamin B1 loss is a senescence-associated biomarker. Mol. Biol. Cell 23, 2066–2075 (2012).
Strom, A. R. et al. HP1α is a chromatin crosslinker that controls nuclear and mitotic chromosome mechanics. eLife 10, e63972 (2021).
Brueckner, L. et al. Local rewiring of genome-nuclear lamina interactions by transcription. EMBO J. 39, e103159 (2020).
Ou, H. D. et al. ChromEMT: visualizing 3D chromatin structure and compaction in interphase and mitotic cells. Science 357, eaag0025 (2017).
Luo, X. et al. A selective inhibitor and probe of the cellular functions of Jumonji C ___domain-containing histone demethylases. J. Am. Chem. Soc. 133, 9451–9456 (2011).
Wang, L. et al. A small molecule modulates Jumonji histone demethylase activity and selectively inhibits cancer growth. Nat. Commun. 4, 2035 (2013).
Larrieu, D., Britton, S., Demir, M., Rodriguez, R. & Jackson, S. P. Chemical inhibition of NAT10 corrects defects of laminopathic cells. Science 344, 527–532 (2014).
Colom, A. et al. A fluorescent membrane tension probe. Nat. Chem. 10, 1118–1125 (2018).
Le Berre, M., Zlotek-Zlotkiewicz, E., Bonazzi, D., Lautenschlaeger, F. & Piel, M. Methods for two-dimensional cell confinement. Methods Cell. Biol. 121, 213–229 (2014).
Roffay, C. et al. Passive coupling of membrane tension and cell volume during active response of cells to osmosis. Proc. Natl Acad. Sci. USA 118, e2103228118 (2021).
Srivastava, N. et al. Nuclear fragility, blaming the blebs. Curr. Opin. Cell Biol. 70, 100–108 (2021).
Jung, S. H. et al. mTOR kinase leads to PTEN-loss-induced cellular senescence by phosphorylating p53. Oncogene 38, 1639–1650 (2019).
Berchtold, D. et al. Plasma membrane stress induces relocalization of Slm proteins and activation of TORC2 to promote sphingolipid synthesis. Nat. Cell Biol. 14, 542–547 (2012).
Chen, C. H. et al. ER stress inhibits mTORC2 and Akt signaling through GSK-3β-mediated phosphorylation of rictor. Sci. Signal. 4, ra10 (2011).
Riggi, M. et al. Decrease in plasma membrane tension triggers PtdIns(4,5)P(2) phase separation to inactivate TORC2. Nat. Cell Biol. 20, 1043–1051 (2018).
Thorner, J. TOR complex 2 is a master regulator of plasma membrane homeostasis. Biochem. J. 479, 1917–1940 (2022).
Pabla, N., Huang, S., Mi, Q. S., Daniel, R. & Dong, Z. ATR–Chk2 signaling in p53 activation and DNA damage response during cisplatin-induced apoptosis. J. Biol. Chem. 283, 6572–6583 (2008).
Scaffidi, P. & Misteli, T. Reversal of the cellular phenotype in the premature aging disease Hutchinson–Gilford progeria syndrome. Nat. Med. 11, 440–445 (2005).
Friedl, P., Wolf, K. & Lammerding, J. Nuclear mechanics during cell migration. Curr. Opin. Cell Biol. 23, 55–64 (2011).
Vargas, P., Terriac, E., Lennon-Dumenil, A. M. & Piel, M. Study of cell migration in microfabricated channels. J. Vis. Exp. 84, e51099 (2014).
Ben-David, U. & Amon, A. Context is everything: aneuploidy in cancer. Nat. Rev. Genet. 21, 44–62 (2020).
Smith, E. R., Capo-Chichi, C. D. & Xu, X. X. Defective nuclear lamina in aneuploidy and carcinogenesis. Front. Oncol. 8, 529 (2018).
Mitmaker, B., Begin, L. R. & Gordon, P. H. Nuclear shape as a prognostic discriminant in colorectal carcinoma. Dis. Colon Rectum 34, 249–259 (1991).
Liu, B. et al. Genomic instability in laminopathy-based premature aging. Nat. Med. 11, 780–785 (2005).
Letourneau, A. et al. Domains of genome-wide gene expression dysregulation in Down’s syndrome. Nature 508, 345–350 (2014).
Meharena, H. S. et al. Down-syndrome-induced senescence disrupts the nuclear architecture of neural progenitors. Cell Stem Cell 29, 116–130.e7 (2022).
Kidiyoor, G. R., Kumar, A. & Foiani, M. ATR-mediated regulation of nuclear and cellular plasticity. DNA Repair (Amst.) 44, 143–150 (2016).
Perry, J. & Kleckner, N. The ATRs, ATMs, and TORs are giant HEAT repeat proteins. Cell 112, 151–155 (2003).
Giunta, S. et al. CENP-A chromatin prevents replication stress at centromeres to avoid structural aneuploidy. Proc. Natl Acad. Sci. USA 118, e2015634118 (2021).
Roberts, A. B. et al. Tumor cell nuclei soften during transendothelial migration. J. Biomech. 121, 110400 (2021).
Klaasen, S. J. et al. Nuclear chromosome locations dictate segregation error frequencies. Nature 607, 604–609 (2022).
Worrall, J. T. et al. Non-random mis-segregation of human chromosomes. Cell Rep. 23, 3366–3380 (2018).
Stephens, A. D. et al. Chromatin histone modifications and rigidity affect nuclear morphology independent of lamins. Mol. Biol. Cell 29, 220–233 (2018).
Berg, I. K. et al. Transcription inhibition suppresses nuclear blebbing and rupture independently of nuclear rigidity. J. Cell Sci. 136, jcs261547 (2023).
Tsai, H. J. et al. Hypo-osmotic-like stress underlies general cellular defects of aneuploidy. Nature 570, 117–121 (2019).
Arriola Apelo, S. I. et al. Ovariectomy uncouples lifespan from metabolic health and reveals a sex-hormone-dependent role of hepatic mTORC2 in aging. eLife 9, e56177 (2020).
Papadopoli, D. et al. mTOR as a central regulator of lifespan and aging. F1000Res 8, F1000 Faculty Rev-998. (2019).
Barroso-Vilares, M. et al. Small-molecule inhibition of aging-associated chromosomal instability delays cellular senescence. EMBO Rep. 21, e49248 (2020).
Macedo, J. C. et al. FoxM1 repression during human aging leads to mitotic decline and aneuploidy-driven full senescence. Nat. Commun. 9, 2834 (2018).
Raab, M. et al. ESCRT III repairs nuclear envelope ruptures during cell migration to limit DNA damage and cell death. Science 352, 359–362 (2016).
Pachitariu, M. & Stringer, C. Cellpose 2.0: how to train your own model. Nat. Methods 19, 1634–1641 (2022).
Scelfo, A. et al. Functional landscape of PCGF proteins reveals both RING1A/B-dependent-and RING1A/B-independent-specific activities. Mol. Cell 74, 1037–1052.e7 (2019).
Van Schaik, T., Vos, M., Peric-Hupkes, D., Hn Celie, P. & van Steensel, B. Cell cycle dynamics of lamina-associated DNA. EMBO Rep. 21, e50636 (2020).
Heuze, M. L., Collin, O., Terriac, E., Lennon-Dumenil, A. M. & Piel, M. Cell migration in confinement: a micro-channel-based assay. Methods Mol. Biol. 769, 415–434 (2011).
Pollard, D. A., Pollard, T. D. & Pollard, K. S. Empowering statistical methods for cellular and molecular biologists. Mol. Biol. Cell 30, 1359–1368 (2019).
Acknowledgements
We thank all of the members of D.F.’s laboratory, scientists at UMR144 and UMR3663, M. Maurin (Institut Curie) and C. Bartocci (Institut Curie) for discussions and suggestions. We thank S. A. Wickström for critical review of the manuscript. We also thank A. Barr (Imperial College London) for sharing cell lines and R. Ceccaldi (Institut Curie) and N. Mailand (Copenhagen University) for sharing critical reagents. We thank C. Combs (National Heart Lung and Blood Institute Light Microscopy Core) for assistance with FLIM imaging acquisition. We also acknowledge the Flow Cytometry Core Facility and Cell and Tissue Imaging Platform (PICT-IBiSA, a member of the French national research infrastructure France-BioImaging; ANR10-INBS-04), especially A. Dauphin of the Institut Curie. D.F. receives salary support from the Centre National de la Recherche Scientifique. D.F. has received support for this project from LabEx CellnScale, the Institut Curie, the programme Investissements d’Avenir (launched by the French Government and implemented by the Agence Nationale de la Recherche with the references ANR-10-LABX-0038 and ANR-10-IDEX-0001-02 PSL), an Emergence Grant 2018 from the City of Paris and ANR-21-CE13-0030. A.S. was supported by Italian Association for Cancer Research and the European Union’s Horizon 2020 research and innovation programme under Marie Skłodowska-Curie grant agreement 800924. Y.A.M. is supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases at the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
S.H., A.S., G.B.-M., M. Grison, M. Dumont, A.A., K.V., M. Deygas, G.P.F.N., Y.A.M. and D.F. obtained the experimental data. A.-S.M., A.W. and M. Gentili provided the key technical expertise relating to the imaging analysis, fluctuation analysis and cGAS experiment, respectively. M.P. and N.M. provided key suggestions. G.P. designed and analysed the kinase drug screen. A.K. performed the image analyses of the kinase drug screen. N.M., M.P., Y.A.M. and D.F. obtained funding. Y.A.M. and D.F. supervised the experimental work. D.F. conceived the project and supervised it along with Y.A.M. S.H., A.S., Y.A.M. and D.F. interpreted the results, designed the experiments and wrote the manuscript. All authors participated in reviewing and editing the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Cell Biology thanks Andrew Blackford, Elsa Logarinho and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Chromosome mis-segregation triggers a p53-p21 response.
a. Number of mitotic CENP-AAID RPE-1 cells from live imaging post-G1 release. b. RPE-1 cells with micronuclei. Each dot: mean of one experiment. n = 3 or 4 (CENP-AAID 48 h) independent replicates with 100 cells/experiment/condition. Ordinary one-way ANOVA: *p = 0.0801; **p = 0.0026; ****p < 0.0001. c. Distribution of the number of micronuclei/cell from (b). n = 2 independent replicates with 40 cells/experiment/condition. d. p53 immunofluorescence in RPE-1 cells. N = 64, 82, 65 and 91 cells for NT, 24hIAA, 2nd G1 arrest and IAA+2nd G1 arrest, respectively, from one experiment. Unpaired two-sided t-test: ∗∗∗p = 0.0003; ∗∗∗∗p < 0.0001. e. p21 immunofluorescence in RPE-1 cells. N = 211, 222, 218, 116, 162 and 120 cells for the treatment groups shown from left to right, from one experiment. Kruskal-Wallis test: ***p = 0.0008; ****p < 0.0001. f. p21 immunofluorescence in RPE-1 cells. N = 40, 46, 58 and 52 cells for the treatment groups shown from left to right, from one experiment. Unpaired two-sided t-test: ns, not significant. g. FACS plots of p21-positive and -negative sorted (Alexa Fluor-647) CENP-AAID RPE-1 cells (IAA: 36 h). h. Chromosome-specific FISH in p21-negative and p21-positive cells. N = 460, 942, 753, 559 cells for chromosomes X, 3, 9 and 6, respectively, from one experiment. i. p21 intensity (from Fig. 1g) in eGFPp21 RPE-1 live-cell imaging (normalized to T = 0 for untreated cells). Coloured lines: eGFPp21 intensity in single CENP-Ei+Mps1i-treated cells; black lines: in untreated cells; grey lines: background. n = 3 independent replicates with 3, 8 and 20 cells for Bkg, NT and CENP-Ei+Mps1i, respectively. j. p21 immunofluorescence in G1 RPE-1 cells. N = 57, 50, 62, 67, 57, and 72 cells for the treatment groups shown from left to right, from one experiment. Kruskal-Wallis test: ***p = 0.0002; ****p < 0.0001. k. Percentages of cells in G1, S and G2/M phases. N = 201, 216, 355 and 235 cells for 0 h, 24 h, 48 h and 72 h IAA respectively, from two independent replicas. Two-sided Fisher’s exact test: ****p < 0.0001. l. Percentages of S-phase cells (EdU positive) of p53 and scramble shRNA in RPE-1 cells. N = 50 cells/condition from one experiment. m. Percentages of BrdU-negative and -positive CENP-AAID RPE-1 cells. n = 3 independent replicates with 10,000 cells. In d–f and j, the center line is the median, the lower and upper bounds are the 25th and 75th percentiles, the whiskers are the furthest observations within 1.5× the interquartile range and the dots inside and outside the whiskers are the mean of one experiment and outlier data points, respectively. In b and m, the error bars represent s.e.m.
Extended Data Fig. 2 Chromosome mis-segregation triggers p53/p21 response independent of DNA damage, replication stress and extended mitotic duration.
a. γH2A.X immunofluorescence intensity in RPE-1 cells. N = 75, 65, 94 and 105 cells for the treatment groups shown from left to right, from one experiment. Unpaired two-sided t-test. b. Number of 53BP1 foci/nucleus in RPE-1 cells. N = 211, 222, 136 and 162 cells for the treatment groups shown from left to right, from one experiment. Unpaired two-sided t-test. c. p21 immunofluorescence intensity in RPE-1 cells. N = 56, 52 and 61 cells for NT, IAA and IAA+ATMi, respectively, from one experiment. Kruskal-Wallis test: *p = 0.0207. d. Immunoblot analysis of RPE-1 cells. IAA: 72 h. Loading control: Vinculin. e. Immunoblot analysis in RPE-1 cells for ATMi efficacy. Positive control: Etoposide. Loading control: Vinculin. f. p21 immunofluorescence intensity in micronuclei-negative and -positive RPE-1 cells treated with IAA (24 h). N = 73, 47, 73 and 47 cells forthe treatment groups shown from left to right, from one experiment. Unpaired two-sided t-test. g. p21 immunofluorescence intensity in RPE-1 cells, normalized to the untreated group. n = 3, 3, 3, 4, 7 and 4 independent replicates with 30 cells/experiment/condition for the treatment groups shown from left to right. Wilcoxon t-test: **p = 0.0060, 0.0021, 0.0011 and 0.0062 from left to right; ****p < 0.0001. h. Immunoblot analysis of RPE-1 cells. Loading control: Vinculin. i. p21 immunofluorescence intensity in CENP-AAID (circle) or CENP-CAID (square) RPE-1 cells (MPSi: 24 h MPI-0479605 or Reversine). n = 3, 7, 7, 7 and 12 independent replicates with 79, 91, 74, 68 and 63 cells/experiment for NT, IAA, IAA + 0.2 μM Mps1i, IAA + 0.5 μM Mps1i and 0.5 μM Mps1i, respectively. Kruskal-Wallis test: **p = 0.0051. j. p21 immunofluorescence intensity. N = 70 and 47 cells for −/+ IAA respectively, from one experiment. Unpaired two-sided t-test: ****p < 0.0001. k. Immunoblot analysis. Positive control: Etoposide. Loading control: Vinculin. l. 53BP1 immunofluorescence intensity. N = 70 and 46 cells for −/+ IAA respectively, from one experiment. Unpaired two-sided t-test. In a–c, f, j and l, the center lines are median values, the lower and upper bounds are 25th and 75th percentiles, the whiskers are the furthest observations within 1.5× the interquartile range and the dots outside the whiskers are the outlier data points. In g and i, the error bars represent s.e.m. Each dot is the mean of one experiment.
Extended Data Fig. 3 NE alterations are triggered by chromosome mis-segregation.
a. Solidity, circularity and EFC ratio of palbociclib-released RPE-1 cells. N = 85, 114, 98, 96, 67, 61, 34, 66 cells for the treatment groups shown from left to right. Unpaired two-sided t-test: ****p < 0.0001. b. Nuclear solidity of CENP-AAID RPE-1 cells. Each dot: one nucleus. N = 66, 53, 156, 139 cells for the treatment groups shown from left to right, from one experiment. Unpaired two-sided t-test ****p < 0.0001. c. Solidity and p21 immunofluorescence of CENP-AAID RPE-1 cells. n = 4 independent replicates with N = 60 cells/experiment/condition. d. RPE-1 cell immunoblot (IAA: 72 h). Loading control: Vinculin. e. Coefficient of variation of Lamins (IAA 24 h). N = 105, 113, 85 and 96 cells for the treatment groups shown from left to right, from one experiment. Unpaired two-sided t-test: ***p = 0.0008; ****p < 0.0001. f. H3K9me3 immunofluorescence of RPE-1 cells. N = 64, 100, 62, 59, 43, 52, 50, 83, 63, 42, 64 and 101 cells for the treatment groups shown from left to right. Unpaired two-sided t-test: **p = 0.0028, ***p = 0.0007; ****p < 0.0001. g. H3K9me3 immunofluorescence of U-2 OS cells. Each dot: mean of one experiment. n = 7 independent replicates with 469 and 486 cells for −/+IAA, respectively. One sample two-sided t-test: *p = 0.0273. h. Left, TEM of CENP-CAID RPE-1 cells. Right, heterochromatin thickness. Each dot: one nucleus (N = 29 and 33 cells for −/+IAA, respectively, from one experiment). Scale bar: 2 μm. Unpaired two-sided t-test: **p = 0.0012. i. Nuclear stiffness of CENP-A/CAID RPE-1 cells. N = 31, 41, 36 and 51 cells for the treatment groups shown from left to right, from one experiment. Unpaired two-sided t-test: ****p < 0.0001. j. Force-distance curve of an indentation experiment (Cytochalasin D: 200 nM, 15 min). k. RPE-1 cell nuclear stiffness (Cytochalasin D: 200 nM, 15 min). N = 54, 56, 53 and 64 cells for the treatment groups shown from left to right for 3 independent replicates. Unpaired two-sided t-test. l. Left, actin (phalloidin) and microtubule (α-tubulin) imaging in G1 CENP-AAID RPE-1 cells (24 h post-release). Right, average areas of actin stress fibers (SFs) and microtubules (MT) per cell (N = 10 cells/field of view, each dot is one field of view of one experiment). Scale bars: 25 μm. In a, e, f, i and k, the center lines are medians, the lower and upper bounds are 25th and 75th percentiles, the whiskers are the furthest observations within 1.5× the interquartile range and the dots outside the whiskers are outlier data points. In b, c, g, h and l, the error bars are s.e.m.
Extended Data Fig. 4 Restoration of heterochromatin and nuclear shape, but not perturbation of the cytoskeleton, prevent p53 activation.
a. p21 immunofluorescence signal of RPE-1 CENP-AAID cells (methylstat 3 μM). N = 87, 82, 51 and 67 cells for the indicated conditions (left to right in the graph), from one experiment. Kruskal-Wallis test: **p = 0.0016, ****p < 0.0001. b. p53 immunoblot analysis in RPE-1 CENP-AAID cells. Loading control: Vinculin. c. EFC ratio of CENP-A/CAID RPE-1 cells. N = 85, 114, 101, 34, 66 and 59 cells for the treatment groups shown from left to right, from one experiment. Kruskal-Wallis test: *p = 0.0398 and 0.0398. d. H3K9me3 immunofluorescence intensity in RPE-1 CENP-AAID cells (Remodelin: 50 μM). N = 103, 98 and 99 cells for the treatment groups shown from left to right, from one experiment. Kruskal-Wallis test: ****p < 0.0001. e. Nuclear stiffness of RPE-1 CENP-AAID cells (Remodelin: 50 μM, 24 h). N = 19, 25, 24, 24, 23, 22, 22, 27, 26, 23, 22 and 25 cells for the indicated conditions (left to right in the graph), from 3 independent replicates. Kruskal-Wallis test: *p = 0.0264; ***p = 0.0006. f. p21 immunofluorescence intensity in RPE-1 CENP-AAID cells (IAA 72 h). N = 87, 82, 69 and 59 cells for the indicated conditions (left to right in the graph), from one experiment. Kruskal-Wallis test: ****p < 0.0001. g. p21 immunofluorescence intensity of CENP-AAID RPE-1 cells (Blebbistatin: 100 μM; Dihydrocytochalasin B: 4 μM; Nocodazole: 100 ng/ml). N = 56, 52, 51, 60 and 71 cells for the indicated conditions (left to right in the graph), from one experiment. Kruskal-Wallis test. In a and c–g, the center line is the median, the lower and upper bounds are the 25th and 75th percentiles, the whiskers are the furthest observations within 1.5× the interquartile range and the dots outside the whiskers are the outlier data points.
Extended Data Fig. 5 p53/p21 activation is triggered by increased membrane tension.
a. γH2A.X immunofluorescence intensity (left) and quantification (right) after 3 h of compression at 3 μm or 10 μm (no compression) in RPE-1 cells. Each dot: mean of one experiment. Unpaired two-sided t-test *p = 0.0272. Scale bar 30 μm. n = 3 independent replicates with 1,221 and 2,290 cells for the treatment groups shown from left to right. b. Percentages of EGFP-FLAGcGASE225A/D227A RPE-1 cells with cGAS foci at the nuclear periphery before compression and foci induced by compression at 3 μm. n = 3 independent replicates with 1,888 cells. Two-sided Fisher’s exact test: **p = 0.0013. c. Percentages of cells positive for cytosolic NLS-GFP in CENP-AAID (circle) and CENP-CAID (square) RPE-1 cells (hypotonic buffer: 1 h, compression: immediately at 3 μm, IAA: 48 h). n = 3 independent replicates with 571, 2,412 and 1,658 cells for hypotonic buffer and compression −/+IAA, respectively. d. Percentages of mCherry-FLAGcGASE225A/D227A RPE-1 cells in suspension with cGAS foci at the nuclear periphery following compression for 2 h. n = 6 independent replicates with 231, 291, 349, 161 and 307 cells for 2/4/6/20/- μm compression, respectively. The center line is the median, the lower and upper bounds are the 25th and 75th percentiles, the whiskers are the furthest observations within 1.5× the interquartile range and the dots outside the whiskers are the outlier data points. Kruskal-Wallis test: **p = 0.0057. e. GFP signal intensity by live imaging of eGFPp21 RPE-1 cells in the presence of isotonic (left) or hypotonic (right) medium (added 30 min after video recording started; dotted line). The data are normalized to T0. Dashed line: 100 min. Continuous colored lines: single cells. In a–c, the error bars are s.e.m. Each dot is the mean of one experiment.
Extended Data Fig. 6 AKT and ATR are activated upon nuclear envelope stress.
a. Left, phospho-AKT (Ser473) immunofluorescence signal in CENP-AAID RPE-1 cells. Scale bars: 5 μm. Right, quantification of phospho-AKT in CENP-A/CAID RPE-1 cells. Positive control: hypotonic buffer. N = 1,913, 1,484 and 1,651 cells for the treatment groups shown from left to right, from 4 independent replicates. Kruskal-Wallis test: ****p < 0.0001. b. Immunoblot showing mTOR activation. Loading control: Vinculin. c. p53/p21 immunoblot with AKTi. Repeated 5 times with similar results. Loading control: Vinculin. d. ATR immunofluorescence signal in CENP-AAID RPE-1 cells. Scale bar: 5 μm. e. Left, p53 immunoblot of CENP-AAID RPE-1 cells (ATRi: 10 μM). Loading control: Vinculin. Right, quantification of p53 levels, normalized to Vinculin and untreated (dashed line). n = 6 independent replicates. Unpaired two-sided t-test *p = 0.0229. f. p53 phosphorylation immunoblot. Loading control: Vinculin. Positive control: Etoposide 20 μM. Repeated 2 times with similar results. g. p53 phosphorylation immunoblot (72 h; INK-128: 200 nM, MK-2206: 5 μM, Ipatasertib: 5 μM). Loading control: H3. Positive control: Etoposide 20 μM. Repeated 3 times with similar results. h. ATR nuclear intensity in RPE-1 cells. N = 43, 37, 44, 46 and 47 cells for the indicated conditions (left to right in the graph), from one experiment. Kruskal-Wallis test: **p = 0.0068; ****p < 0.0001. i. Left, AKT phosphorylation immunoblot. Loading control: Vinculin. Right, quantification of AKT phosphorylation normalized to Vinculin and untreated (dashed line). n = 3 independent replicates. Kruskal-Wallis test: *p = 0.0459. j. p21 immunofluorescence intensity (INK-128: 200 nM, JIB-04: 1.5 μM, Remodelin: 50 μM). n = 8, 7, 5, 6 and 4 independent replicates with 616, 684, 451, 287 and 285 cells for the indicated conditions (left to right in the graph). One-sample two-sided t-test: *p = 0.0170; ***p = 0.0001; ****p < 0.0001. k. Left, p53/p21 immunoblot in BJ-hTERT1 (MPS1i 0.5 μM + CENP-Ei 0.2 μM; ATRi VE-821). Loading control: Vinculin. Right, quantification of p53 levels normalized to Vinculin and DMSO. N = 6 (DMSO/Mps1i+CENP-Ei) and 3 (all other conditions) independent replicates. One sample two-sided t test: *p = 0.0112; **p = 0.0058; ****p < 0.0001. In a and h, the center lines are the medians, the lower and upper bounds are the 25th and 75th percentiles, the whiskers are the furthest observations within 1.5× the interquartile range and the dots outside the whiskers are the outlier data points. In e, i, j and k, the error bars are s.e.m. In e and i, each dot represents one experiment. In j and k, each dot is the mean of one experiment.
Extended Data Fig. 7 mTORC2 senses nuclear membrane alterations in Progerin-expressing cells.
a. Cells with micronuclei. n = 3 independent replicates with 276, 215, 281 and 190 cells for the treatment groups shown from left to right. Kruskal-Wallis test. Doxycycline: 50 ng/mL for 72 h. b. Cell ploidy distribution. n = 3, 3, 4 and 4 independent replicates with 62, 61, 58 and 61 cells for the treatment groups shown from left to right. Kruskal-Wallis test. Doxycycline: 50 ng/mL for 72 h. c. γH2A.X immunoblot. Loading control: Vinculin. d. γH2A.X immunofluorescence. N = 551, 623, 363, 385, 216 cells for the treatment groups shown from left to right, from 3 or 2 (Etoposide) independent replicates. Etoposide: 20 μM for 24 h. Kruskal-Wallis test: ****p < 0.0001. e. Left, immunoblot in Progerin-expressing RPE-1 cells. Loading control: Vinculin. Right, quantification of p53 levels normalized to no Dox and Vinculin. n = 2, 4, 2 and 4 replicates for the treatment groups shown from left to right. f. Nuclear stiffness quantification. Doxycycline: 72 h 10 ng/mL for Lamin A cells, 72 h 50 ng/mL for Progerin cells; cytochalasin-D:200 nM; 15 min for all cells. n = 3 or 4 (Cytochalasin D) independent replicates with 97, 237, 90, 82, 162 and 118 cells for the indicated conditions (left to right in the graph). Kruskal-Wallis test: *p = 0.0468, 0.0396; ****p < 0.0001. g. Left, H3K9me3 immunofluorescence in Progerin-GFP RPE-1 cells (−/+ 72 h 50 ng/mL doxycycline). Right, ratio of the H3K9me3 signal at the nuclear periphery over that at the nuclear interior. Each dot: mean of one experiment. n = 3 independent replicates with 225 and 184 cells for −/+dox, respectively. Unpaired two-sided t-test: *p = 0.0472. Scale bar: 10 μm. h. Left, immunoblot of Lamin A-GFP or Progerin-GFP RPE-1 cells. INK-128: 200 nM. Loading control: Vinculin. Right, quantification of p53 levels normalized to no Dox and Vinculin. n = 3 independent replicates. One sample two-sided t-test: *p = 0.0390. i. Left, crystal violet-stained transwell membrane with invaded cells. Right, numbers of CENP-AAID RPE-1 cells treated for 48 (circle) or 72 h (square) with IAA below the Matrigel-coated transwell membrane. N = 3 or 7 independent replicates with 7,927 and 3,829 cells for NT and IAA, respectively. Two-sided Fisher’s exact test: ****p < 0.0001. In d and f, the center lines are the medians, the lower and upper bounds are the 25th and 75th percentiles, the whiskers are the furthest observations within 1.5× the interquartile range and the dots inside and outside the whiskers are the mean of one experiment and outlier data points, respectively. In a, b, e and g–i, the error bars are s.e.m. In e, h and i, each dot represents one experiment.
Supplementary information
Supplementary Information
Supplementary Figs. 1 and 2.
Supplementary Table 1
Identified kinase inhibitors capable of reducing p21 levels following chromosome mis-segregation via CENP-A depletion for 24 h.
Supplementary Table 2
Genomic coordinates of LADs and relative qPCR primers for ChIP-qPCR analysis.
Supplementary Video 1
Representative time-lapse microscopy of IAA-treated CENP-AAID RPE-1 cells expressing mCherry-H2B from the metaphase until early G1. Cells were imaged every 3 min.
Supplementary Video 2
Representative super-resolution (SIM) images of nuclear lamin A/C (magenta) and lamin B1 (green) in untreated CENP-AAID RPE-1 cells. Scale bar, 5 μm.
Supplementary Video 3
Representative super-resolution (SIM) images of nuclear lamin A/C (magenta) and lamin B1 (green) in IAA-treated CENP-AAID RPE-1 cells for 24 h. Scale bar, 5 μm.
Supplementary Video 4
Representative super-resolution (SIM) images of nuclear lamin A/C (magenta) and lamin B1 (green) in IAA-treated CENP-CAID RPE-1 cells for 24 h. Scale bar, 5 μm.
Supplementary Video 5
LAP2β-GFP time-lapse microscopy used to measure NE fluctuations in CENP-AAID RPE-1 cells. Cells were imaged every 150 ms for 5 min.
Supplementary Video 6
eGFP–FLAG-cGAS-E225A/D227A time-lapse microscopy was used to measure nuclear rupture after cell compression in CENP-AAID RPE-1 cells left untreated or treated with IAA for 24 h. The DNA was stained with NucBlue Live probe (magenta). Cells were imaged every 5 min.
Supplementary Video 7
Live-cell imaging of the 3D migration of CENP-AAID RPE-1 cells (pre-treated for 24 h with dimethyl sulfoxide or IAA, as indicated) embedded in collagen I. Cells were imaged every 30 min.
Supplementary Video 8
Migration assay through microfabricated channels using Hoechst dye to stain nuclei in CENP-AAID RPE-1 cells. Cells were imaged every minute over 24 h.
Source data
Source Data Figs. 1–6 and Extended Data Figs. 1–7
Source numerical data.
Source Data Figs. 1, 3, 5 and 6 and Extended Data Figs. 2–4, 6 and 7
Unprocessed blots.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hervé, S., Scelfo, A., Bersano Marchisio, G. et al. Chromosome mis-segregation triggers cell cycle arrest through a mechanosensitive nuclear envelope checkpoint. Nat Cell Biol 27, 73–86 (2025). https://doi.org/10.1038/s41556-024-01565-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41556-024-01565-x
This article is cited by
-
USP25-driven KIFC1 regulates MYCBP expression and promotes the progression of cervical cancer
Cell Death & Disease (2025)
-
Misshapen chromosomes in check by mechanics
Nature Cell Biology (2025)