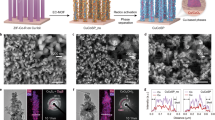
Abstract
Developing highly effective catalysts for ammonia (NH3) synthesis is a challenging task. Even the current, prevalent iron-derived catalysts used for industrial NH3 synthesis require harsh reaction conditions and involve massive energy consumption. Here we show that anchoring buckminsterfullerene (C60) onto non-iron transition metals yields cluster-matrix co-catalysts that are highly efficient for NH3 synthesis. Such co-catalysts feature separate catalytic active sites for hydrogen and nitrogen. The ‘electron buffer’ behaviour of C60 balances the electron density at catalytic transition metal sites and enables the synergistic activation of nitrogen on transition metals in addition to the activation and migration of hydrogen on C60 sites. As demonstrated in long-term, continuous runs, the C60-promoting transition metal co-catalysts exhibit higher NH3 synthesis rates than catalysts without C60. With the involvement of C60, the rate-determining step in the cluster-matrix co-catalysis is found to be the hydrogenation of *NH2. C60 incorporation exemplifies a practical approach for solving hydrogen poisoning on a wide variety of oxide-supported Ru catalysts.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
269,00 € per year
only 22,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data supporting the findings of this study are available within this Article and its Supplementary Information. Source data are provided with this paper.
References
Ertl, G. Reactions at surfaces: from atoms to complexity (Nobel lecture). Angew. Chem. Int. Ed. 47, 3524–3535 (2008).
Kandemir, T. et al. The Haber–Bosch process revisited: on the real structure and stability of “ammonia iron” under working conditions. Angew. Chem. Int. Ed. 52, 12723–12726 (2013).
Martirez, J. M. P. & Carter, E. A. Prediction of a low-temperature N2 dissociation catalyst exploiting near–IR–to–visible light nanoplasmonics. Sci. Adv. 3, eaao4710 (2017).
Chang, F. et al. Potassium hydride-intercalated graphite as an efficient heterogeneous catalyst for ammonia synthesis. Nat. Catal. 5, 222–230 (2022).
Tang, Y. et al. Metal-dependent support effects of oxyhydride-supported Ru, Fe, Co catalysts for ammonia synthesis. Adv. Energy Mater. 8, 1801772 (2018).
Ye, T.-N. et al. Vacancy-enabled N2 activation for ammonia synthesis on an Ni-loaded catalyst. Nature 583, 391–395 (2020).
Wang, Q. et al. Ternary ruthenium complex hydrides for ammonia synthesis via the associative mechanism. Nat. Catal. 4, 959–967 (2021).
Wang, T. et al. Weakening hydrogen adsorption on nickel via interstitial nitrogen doping promotes bifunctional hydrogen electrocatalysis in alkaline solution. Energy Environ. Sci. 12, 3522–3529 (2019).
Sato, K. et al. Barium oxide encapsulating cobalt nanoparticles supported on magnesium oxide: active non-noble metal catalysts for ammonia synthesis under mild reaction conditions. ACS Catal. 11, 13050–13061 (2021).
Jacobsen, C. J. H. et al. Catalyst design by interpolation in the periodic table: bimetallic ammonia synthesis catalysts. J. Am. Chem. Soc. 123, 8404–8405 (2001).
Peng, W. et al. Spontaneous atomic ruthenium doping in Mo2CTx MXene defects enhances electrocatalytic activity for the nitrogen reduction reaction. Adv. Energy Mater. 10, 2001364 (2020).
Kammert, J. et al. Nature of reactive hydrogen for ammonia synthesis over a Ru/C12A7 electride catalyst. J. Am. Chem. Soc. 142, 7655–7667 (2020).
Baik, Y. et al. Splitting of hydrogen atoms into proton–electron pairs at BaO–Ru interfaces for promoting ammonia synthesis under mild conditions. J. Am. Chem. Soc. 145, 11364–11374 (2023).
Zheng, J. et al. Efficient non-dissociative activation of dinitrogen to ammonia over lithium-promoted ruthenium nanoparticles at low pressure. Angew. Chem. Int. Ed. 58, 17335–17341 (2019).
Han, G.-F. et al. Mechanochemistry for ammonia synthesis under mild conditions. Nat. Nanotechnol. 16, 325–330 (2021).
Mehta, P. et al. Overcoming ammonia synthesis scaling relations with plasma-enabled catalysis. Nat. Catal. 1, 269–275 (2018).
Mao, C. et al. Hydrogen spillover to oxygen vacancy of TiO2–xHy/Fe: breaking the scaling relationship of ammonia synthesis. J. Am. Chem. Soc. 142, 17403–17412 (2020).
Ye, T.-N. et al. Contribution of nitrogen vacancies to ammonia synthesis over metal nitride catalysts. J. Am. Chem. Soc. 142, 14374–14383 (2020).
Zhang, K. et al. Spin-mediated promotion of Co catalysts for ammonia synthesis. Science 383, 1357–1363 (2024).
Zheng, J. et al. Ambient-pressure synthesis of ethylene glycol catalyzed by C60-buffered Cu/SiO2. Science 376, 288–292 (2022).
Fischer, J. E. et al. Compressibility of solid C60. Science 252, 1288–1290 (1991).
Wu, S. et al. Removal of hydrogen poisoning by electrostatically polar MgO support for low-pressure NH3 synthesis at a high rate over the Ru catalyst. ACS Catal. 10, 5614–5622 (2020).
Hattori, M., Okuyama, N., Kurosawa, H. & Hara, M. Low-temperature ammonia synthesis on iron catalyst with an electron donor. J. Am. Chem. Soc. 145, 7888–7897 (2023).
Kitano, M. et al. Ammonia synthesis using a stable electride as an electron donor and reversible hydrogen store. Nat. Chem. 4, 934–940 (2012).
Zhou, Y. et al. Essential role of Ru–anion interaction in Ru-based ammonia synthesis catalysts. ACS Catal. 12, 7633–7642 (2022).
Ojeda, M. et al. Manganese-promoted Rh/Al2O3 for C2-oxygenates synthesis from syngas: effect of manganese loading. Appl. Catal. A Gen. 261, 47–55 (2004).
Aika, K.-i Role of alkali promoter in ammonia synthesis over ruthenium catalysts—effect on reaction mechanism. Catal. Today 286, 14–20 (2017).
Sham, T. K. et al. Ru L‐edge X‐ray absorption studies of the formation of Ru–Cu bimetallic aggregates on Cu(100). J. Chem. Phys. 95, 8725–8731 (1991).
Deng, S. et al. Synergistic doping and intercalation: realizing deep phase modulation on MoS2 arrays for high-efficiency hydrogen evolution reaction. Angew. Chem. Int. Ed. 58, 16289–16296 (2019).
Lu, Y. et al. Water durable electride Y5Si3: electronic structure and catalytic activity for ammonia synthesis. J. Am. Chem. Soc. 138, 3970–3973 (2016).
Li, L. et al. Size sensitivity of supported Ru catalysts for ammonia synthesis: from nanoparticles to subnanometric clusters and atomic clusters. Chem 8, 749–768 (2022).
Zhou, S. et al. Boron nitride nanotubes for ammonia synthesis: activation by filling transition metals. J. Am. Chem. Soc. 142, 308–317 (2020).
Honkala, K. et al. Ammonia synthesis from first-principles calculations. Science 307, 555–558 (2005).
Yao, Y. et al. A Spectroscopic study of electrochemical nitrogen and nitrate reduction on rhodium surfaces. Angew. Chem. Int. Ed. 59, 10479–10483 (2020).
Bian, X. et al. Quantifying the contribution of hot electrons in photothermal catalysis: a case study of ammonia synthesis over carbon-supported Ru catalyst. Angew. Chem. Int. Ed. 62, e202304452 (2023).
Ravel, B. & Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Rad. 12, 537–541 (2005).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Wellendorff, J. et al. Density functionals for surface science: exchange-correlation model development with Bayesian error estimation. Phys. Rev. B 85, 235149 (2012).
Ulissi, Z. W., Medford, A. J., Bligaard, T. & Nørskov, J. K. To address surface reaction network complexity using scaling relations machine learning and DFT calculations. Nat. Commun. 8, 14621 (2017).
Alavi, A. et al. CO Oxidation on Pt(111): an ab initio density functional theory study. Phys. Rev. Lett. 80, 3650–3653 (1998).
Liu, Z.-P. & Hu, P. General rules for predicting where a catalytic reaction should occur on metal surfaces: a density functional theory study of C−H and C−O bond breaking/making on flat, stepped, and kinked metal surfaces. J. Am. Chem. Soc. 125, 1958–1967 (2003).
Anderson, G. M. Thermodynamics of Natural Systems (Cambridge Univ. Press, 2005).
Asthagiri, A. & Janik, M. J. Computational Catalysis (Royal Society of Chemistry, 2013).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Acknowledgements
The work was supported by the National Natural Science Foundation of China (22221005, L.J.; 22222801, X.W.; 92361303, S.X.; 22038002, L.J.; 92061204, S.X.; 22322302, B.Y.; 21721001, Y.Z.), the National Key Research and Development Program (2021YFB4000400, L.J.; and 2022YFA1604101, X.W.) and the Key R&D plan of the Shanghai Science and Technology Commission (21DZ1209002, L.J.). We thank Y. Gong from Northwestern Polytechnical University for providing electride supports. Moreover, we acknowledge the facility resources from the Electron Microscopy Center of Fuzhou University.
Author information
Authors and Affiliations
Contributions
L.J. conceived the project and organized experiments as well as participated in paper revision. X.W. proposed the detail research idea and supervised the entire project including designing the research scheme and the interpretation of results as well as wrote the manuscript. Y.Zhang and X.P. performed the synthesis of samples. Y.Zhang, X.P., T.Z. and M.Z. performed characterization and catalytic measurements. H.-R.T. performed mass spectra measurements. L.Z. provided the instrument platform for XAS. J.L., B.Y. and Z.-C.C. carried out the model construction and density functional theory calculations. X.W. and Y.Zhou performed in situ X-ray absorption fine-structure measurements. Y.T. performed the fitting of XAFS data, X.L. and Z.Y. carried out the AC–TEM and AC–STEM as well as iDPC measurements. J.-W.Z. and C.-t.A. participated in discussion and revision. S.-Y.X. provided the raw C60 and proposed the concept of cluster matrix, and participated in interpretation of results and made comments on the manuscript. All authors participated in the interpretation of results and made comments on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Peer review
Peer review information
Nature Chemistry thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–53, Tables 1–8, methods and discussion.
Supplementary Data 1
Source data of supplementary figures.
Source data
Source Data Fig. 1
Source data of Fig. 1.
Source Data Fig. 2
Source data of Fig. 2.
Source Data Fig. 3
Source data of Fig. 3.
Source Data Fig. 4
Source data of Fig. 4.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, Y., Peng, X., Tian, HR. et al. Fullerene on non-iron cluster-matrix co-catalysts promotes collaborative H2 and N2 activation for ammonia synthesis. Nat. Chem. 16, 1781–1787 (2024). https://doi.org/10.1038/s41557-024-01626-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-024-01626-6
This article is cited by
-
Fullerenes promote transition-metal-catalysed ammonia synthesis
Nature Chemistry (2024)