Abstract
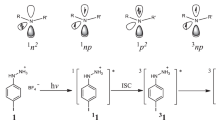
Nitrene radical compounds are short-lived intermediates in a variety of nitrogen-involved transformations. They feature either a singlet or a triplet ground state, depending on the electronic properties of the substituents. Triplet nitrenes are highly reactive and their isolation in the condensed phase under ambient conditions is challenging. Here we report the synthesis and isolation of a triplet arylnitrene supported by a bulky hydrindacene ligand. The arylnitrene is fully characterized by various spectroscopic and structural techniques including electron paramagnetic resonance spectroscopy and single-crystal X-ray diffraction. Its high stability is largely attributed to the steric hindrance and effective electron delocalization provided by the supporting ligand. Electron paramagnetic resonance spectroscopy in conjunction with highly correlated wavefunction-based ab initio calculations provides support for a triplet ground state nitrene with axial zero-field splitting D = 0.92 cm–1 and vanishing rhombicity E/D = 0.002.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
269,00 € per year
only 22,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Crystallographic data for the structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre (CCDC) under deposition numbers CCDC 2354664 (Supplementary Data 1), 2354665 (Supplementary Data 2), 2354666 (Supplementary Data 3), 2368517 (Supplementary Data 4), 2354667 (Supplementary Data 5), 2354668 (Supplementary Data 6) and 2368518 (Supplementary Data 7). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/. All data are available in the main text or Supplementary Information and are also available from the corresponding authors on reasonable request.
References
Platz, M. S. in Reactive Intermediate Chemistry (eds Moss, R. A., Paztz, M. S. & Jones, M. Jr.), 501–559 (Wiley-VCH, 2003).
Dequirez, G., Pons, V. & Dauban, P. Nitrene chemistry in organic synthesis: still in its infancy? Angew. Chem. Int. Ed. 51, 7384–7395 (2012).
Tiemann, F. Ueber die einwirkung von benzolsulfonsäurechlorid auf amidoxime. Ber. Dtsch. Chem. Ges. 24, 4162–4167 (1891).
Meyer, D. M. & Roth, K. C. Discovery of interstellar NH. Astrophys. J. Lett. 376, L49–L52 (1991).
Wasserman, E. in Progress in Physical Organic Chemistry (eds Streitwieser, A. Jr. & Taft, R. W.), 319–336 (Wiley-VCH, 1971).
Gritsan, N. P. & Platz, M. S. Kinetics, spectroscopy, and computational chemistry of arylnitrenes. Chem. Rev. 106, 3844–3867 (2006).
Wentrup, C. Nitrenes, carbenes, diradicals, and ylides. Interconversions of reactive intermediates. Acc. Chem. Res. 44, 393–404 (2011).
Kvaskoff, D., Lüerssen, H., Bednarek, P. & Wentrup, C. Phenylnitrene, phenylcarbene, and pyridylcarbenes. Rearrangements to cyanocyclopentadiene and fulvenallene. J. Am. Chem. Soc. 136, 15203–15214 (2014).
Gomberg, M. An instance of trivalent carbon: triphenylmethyl. J. Am. Chem. Soc. 22, 757–771 (1900).
Zard, S. Z. Recent progress in the generation and use of nitrogen-centred radicals. Chem. Soc. Rev. 37, 1603–1618 (2008).
Hirai, K., Itoh, T. & Tomioka, H. Persistent triplet carbenes. Chem. Rev. 109, 3275–3332 (2009).
Wentrup, C. Carbenes and nitrenes: recent developments in fundamental chemistry. Angew. Chem. Int. Ed. 57, 11508–11521 (2018).
Soleilhavoup, M. & Bertrand, G. Stable carbenes, nitrenes, phosphinidenes, and borylenes: past and future. Chem 6, 1275–1282 (2020).
He, M., Hu, C., Wei, R., Wang, X.-F. & Liu, L. L. Recent advances in the chemistry of isolable carbene analogues with group 13–15 elements. Chem. Soc. Rev. 53, 3896–3951 (2024).
Igau, A., Grutzmacher, H., Baceiredo, A. & Bertrand, G. Analogous α,α′-bis-carbenoid triply bonded species: synthesis of a stable λ3-phosphinocarbene-λ5-phosphaacetylene. J. Am. Chem. Soc. 110, 6463–6466 (1988).
Arduengo, A. J., Harlow, R. L. & Kline, M. A stable crystalline carbene. J. Am. Chem. Soc. 113, 361–363 (1991).
Bourissou, D., Guerret, O., Gabbaï, F. P. & Bertrand, G. Stable carbenes. Chem. Rev. 100, 39–92 (2000).
Sau, S. C., Hota, P. K., Mandal, S. K., Soleilhavoup, M. & Bertrand, G. Stable abnormal N-heterocyclic carbenes and their applications. Chem. Soc. Rev. 49, 1233–1252 (2020).
Bellotti, P., Koy, M., Hopkinson, M. N. & Glorius, F. Recent advances in the chemistry and applications of N-heterocyclic carbenes. Nat. Rev. Chem. 5, 711–725 (2021).
Badiei, Y. M., Krishnaswamy, A., Melzer, M. M. & Warren, T. H. Transient terminal Cu–nitrene intermediates from discrete dicopper nitrenes. J. Am. Chem. Soc. 128, 15056–15057 (2006).
Carsch, K. M. et al. Synthesis of a copper-supported triplet nitrene complex pertinent to copper-catalyzed amination. Science 365, 1138 (2019).
Das, A. et al. In crystallo snapshots of Rh2-catalyzed C–H amination. J. Am. Chem. Soc. 142, 19862–19867 (2020).
Grünwald, A. et al. Palladium terminal imido complexes with nitrene character. J. Am. Chem. Soc. 144, 8897–8901 (2022).
Jung, H. et al. Mechanistic snapshots of rhodium-catalyzed acylnitrene transfer reactions. Science 381, 525–532 (2023).
Keilwerth, M. et al. From divalent to pentavalent iron imido complexes and an Fe(V) nitride via N–C bond cleavage. J. Am. Chem. Soc. 145, 873–887 (2023).
Mao, W. et al. Synthesis and reactivity of a cobalt-supported singlet nitrene. J. Am. Chem. Soc. 145, 13650–13662 (2023).
Dielmann, F. et al. A crystalline singlet phosphinonitrene: a nitrogen atom–transfer agent. Science 337, 1526–1528 (2012).
Sun, J. et al. A platinum(II) metallonitrene with a triplet ground state. Nat. Chem. 12, 1054–1059 (2020).
Schmidt-Räntsch, T. et al. Nitrogen atom transfer catalysis by metallonitrene C−H insertion: photocatalytic amidation of aldehydes. Angew. Chem. Int. Ed. 61, e202115626 (2022).
Domenianni, L. I. et al. Photoinduced metallonitrene formation by N2 elimination from azide diradical ligands. Angew. Chem. Int. Ed. 62, e202309618 (2023).
Matsuo, T. et al. Synthesis and structures of a series of bulky ‘Rind-Br’ based on a rigid fused-ring s-hydrindacene skeleton. Bull. Chem. Soc. Jpn. 84, 1178–1191 (2011).
Olaru, M., Mebs, S. & Beckmann, J. Cationic carbene analogues: donor-free phosphenium and arsenium ions. Angew. Chem. Int. Ed. 60, 19133–19138 (2021).
He, Y., Dai, C., Wang, D., Zhu, J. & Tan, G. Phosphine-stabilized germylidenylpnictinidenes as synthetic equivalents of heavier nitrile and isocyanide in cycloaddition reactions with alkynes. J. Am. Chem. Soc. 144, 5126–5135 (2022).
Janssen, M., Mebs, S. & Beckmann, J. Kinetically stabilized diarylpnictogenium ions. ChemPlusChem 88, e202200429 (2023).
Pang, Y. et al. Synthesis and isolation of a triplet bismuthinidene with a quenched magnetic response. Science 380, 1043–1048 (2023).
Wang, D. et al. Monosubstituted doublet Sn(I) radical featuring substantial unquenched orbital angular momentum. J. Am. Chem. Soc. 145, 6914–6920 (2023).
Wang, D. et al. An isolable germylyne radical with a one-coordinate germanium atom. Nat. Chem. 15, 200–205 (2023).
Wu, M. et al. Triplet bismuthinidenes featuring unprecedented giant and positive zero field splittings. Natl Sci. Rev. 10, nwad169 (2023).
Wu, M. et al. A triplet stibinidene. Chem 9, 2573–2584 (2023).
Chen, H. et al. An isolable one-coordinate lead(I) radical with strong g-factor anisotropy. Angew. Chem. Int. Ed. 63, e202402093 (2024).
Wang, D. et al. An isolable phosphinogermylyne as a synthon of one-coordinate gei radical. Chin. J. Chem. 42, 736–742 (2024).
Janssen, M. et al. Synthesis of a stable crystalline nitrene. Science 385, 318–321 (2024).
Burdzinski, G. T., Middleton, C. T., Gustafson, T. L. & Platz, M. S. Solution phase isomerization of vibrationally excited singlet nitrenes to vibrationally excited 1,2-didehydroazepine. J. Am. Chem. Soc. 128, 14804–14805 (2006).
Biegger, P. et al. Bisalkynylated 3,6-diiminocyclohexa-1,4-diene-1,4-diamine. Chem. Commun. 51, 14844–14847 (2015).
Li, T. et al. Magnetic bistability in a discrete organic radical. J. Am. Chem. Soc. 138, 10092–10095 (2016).
Pilbrow, J. R., Sinclair, G. R., Hutton, D. R. & Troup, G. J. Asymmetric lines in field-swept EPR: Cr3+ looping transitions in ruby. J. Mag. Res. (1969) 52, 386–399 (1983).
Mabbs, F. E. & Collison, D. Electron Paramagnetic Resonance of d Transition Metal Compounds 466–579 (Elsevier, 1992).
Ye, S. Probing electronic structures of transition metal complexes using electron paramagnetic resonance spectroscopy. Mag. Res. Lett. 3, 43–60 (2023).
Roos, B. O. in Advances in Chemical Physics (ed. Lawley, K. P.), 399–445 (Wiley-VCH, 1987).
Angeli, C., Cimiraglia, R., Evangelisti, S., Leininger, T. & Malrieu, J. P. Introduction of n-electron valence states for multireference perturbation theory. J. Chem. Phys. 114, 10252–10264 (2001).
Evans, D. F. The determination of the paramagnetic susceptibility of substances in solution by nuclear magnetic resonance. J. Chem. Soc. 1959, 2003–2005 (1959).
Stoll, S. & Schweiger, A. Easyspin, a comprehensive software package for spectral simulation and analysis in EPR. J. Mag. Res. 178, 42–55 (2006).
Sheldrick, G. SHELXT–integrated space-group and crystal-structure determination. Acta Crystallogr. A 71, 3–8 (2015).
Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. C 71, 3–8 (2015).
Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. Olex2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 42, 339–341 (2009).
Neese, F., Wennmohs, F., Becker, U. & Riplinger, C. The ORCA quantum chemistry program package. J. Chem. Phys. 152, 224108 (2020).
Lee, C., Yang, W. & Parr, R. G. Development of the Colle–Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 37, 785–789 (1988).
Becke, A. D. Density‐functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98, 5648–5652 (1993).
Weigend, F. & Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: design and assessment of accuracy. Phys. Chem. Chem. Phys. 7, 3297–3305 (2005).
Grimme, S., Ehrlich, S. & Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 32, 1456–1465 (2011).
Malmqvist, P.-Å. & Roos, B. O. The CASSCF state interaction method. Chem. Phys. Lett. 155, 189–194 (1989).
Weigend, F. Hartree–Fock exchange fitting basis sets for H to Rn. J. Comput. Chem. 29, 167–175 (2008).
Kollmar, C., Sivalingam, K., Helmich-Paris, B., Angeli, C. & Neese, F. A perturbation-based super-CI approach for the orbital optimization of a CASSCF wave function. J. Comput. Chem. 40, 1463–1470 (2019).
Neese, F. Importance of direct spin–spin coupling and spin-flip excitations for the zero-field splittings of transition metal complexes: a case study. J. Am. Chem. Soc. 128, 10213–10222 (2006).
Angeli, C., Cimiraglia, R. & Malrieu, J.-P. n-Electron valence state perturbation theory: a spinless formulation and an efficient implementation of the strongly contracted and of the partially contracted variants. J. Chem. Phys. 117, 9138–9153 (2002).
Becke, A. D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 38, 3098–3100 (1988).
Perdew, J. P. Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys. Rev. B 33, 8822–8824 (1986).
Plasser, F., Wormit, M. & Dreuw, A. New tools for the systematic analysis and visualization of electronic excitations. I. Formalism. J. Chem. Phys. 141, 024106 (2014).
Acknowledgements
The synthetic work was supported by the National Natural Science Foundation of China (grant numbers 22322112, 22488101 and 22071164), and the Suzhou Science & Technology NOVA Program (grant number ZXL2022445). The study of electronic structures was supported by the National Natural Science Foundation of China (grant number 92161204) and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences (grant number DICP I202312). The computational work was supported by the National Supercomputer Center (Tianhe-2 Supercomputer), Guangzhou. We also thank Instrumental Analysis and Research Center, Sun Yat-sen University for the mass spectrometric and elemental analysis.
Author information
Authors and Affiliations
Contributions
G.T. and S.Y. conceived and supervised the project. D.W., H.C. and Y.C. carried out the experiments. W.C. performed the electronic structure studies.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Dominik Munz and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–47, Tables 1–3, starting material preparation, experimental procedures and product characterization, and computational details.
Supplementary Data 1
Crystallographic data for 1 (CCDC 2354664).
Supplementary Data 2
Crystallographic data for 2 (CCDC 2354665).
Supplementary Data 3
Crystallographic data for 3 (CCDC 2354666).
Supplementary Data 4
Crystallographic data for 4 (CCDC 2368517).
Supplementary Data 5
Crystallographic data for 5 (CCDC 2354667).
Supplementary Data 6
Crystallographic data for 6 (CCDC 2354668).
Supplementary Data 7
Crystallographic data for 7 (CCDC 2368518).
Supplementary Data 8
Coordinates for the optimized structures.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, D., Chen, W., Chen, H. et al. Isolation and characterization of a triplet nitrene. Nat. Chem. 17, 38–43 (2025). https://doi.org/10.1038/s41557-024-01669-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-024-01669-9
This article is cited by
-
Bottleable triplet nitrenes
Nature Chemistry (2025)