Abstract
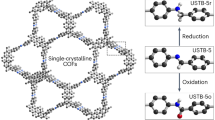
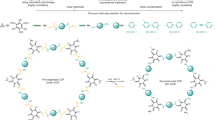
sp2-carbon-linked covalent organic frameworks (sp2c-COFs) are crystalline porous polymers with repeat organic units linked by sp2 carbons, and have attracted increasing interest due to their robust skeleton and tunable semiconducting properties. Single-crystalline sp2c-COFs with well-defined structures can represent an ideal platform for investigating fundamental physics properties and device performance. However, the robust olefin bonds inhibit the reversible-reaction-based crystal self-correction, thus yielding polycrystalline or amorphous polymers. Here we report an imine-to-olefin transformation strategy to form single-crystal sp2c-COFs. The isolated single crystals display rectangular nanotube-like domains with sizes up to approximately 24 μm × 0.8 μm × 0.8 μm, and permanent pore distribution around 1.1 nm. The highly conjugated olefin linkage endows the crystals with enhanced electronic connectivity which determines a remarkable room-temperature metal-free ferromagnetism (8.6 × 10−3 emu g−1). Our protocol is robust and generally applicable for the synthesis of single-crystalline sp2c-COFs for future spin-electron devices.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
269,00 € per year
only 22,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data supporting the findings of this study are available in the article and its Supplementary Information. Crystallographic data for the sc-COFs in this article have been deposited at the Cambridge Crystallographic Data Centre (CCDC) (CCDC 2340230 for sc-sp2c-COF-1, CCDC 2370333 for sc-sp2c-COF-2 and CCDC 2340227 for COF-303). Source data are provided with this paper.
References
Cote, A. P. et al. Porous, crystalline, covalent organic frameworks. Science 310, 1166–1170 (2005).
Diercks, C. S. et al. The atom, the molecule and the covalent organic framework. Science 355, eaal1585 (2017).
Tan, K. T. et al. Covalent organic frameworks. Nat. Rev. Methods Primer 3, 1 (2023).
Zhang, W. et al. Reconstructed covalent organic frameworks. Nature 604, 72–79 (2022).
Yu, B. et al. Linkage conversions in single-crystalline covalent organic frameworks. Nat. Chem. 16, 114–121 (2024).
Zhang, L. et al. Activation of pyroptosis using AIEgen-based sp2 carbon-linked covalent organic frameworks. J. Am. Chem. Soc. 145, 17689–17699 (2023).
Wang, X. et al. Topology-selective manipulation of two-dimensional covalent organic frameworks. J. Am. Chem. Soc. 145, 26900–26907 (2023).
Grunenberg, L. et al. Postsynthetic transformation of imine- into nitrone-linked covalent organic frameworks for atmospheric water harvesting at decreased humidity. J. Am. Chem. Soc. 145, 13241–13248 (2023).
Zhao, Y. et al. Construction of layer-blocked covalent organic framework heterogenous films via surface-initiated polycondensations with strongly enhanced photocatalytic properties. ACS Cent. Sci. 10, 775–781 (2024).
Yuan, C. et al. Crystalline C–C and C=C bond-linked chiral covalent organic frameworks. J. Am. Chem. Soc. 143, 369–381 (2021).
Li, S. et al. Direct construction of isomeric benzobisoxazole–vinylene-linked covalent organic frameworks with distinct photocatalytic properties. J. Am. Chem. Soc. 144, 13953–13960 (2022).
Zhang, P. et al. Fabricating industry‐compatible olefin‐linked COF resins for oxoanion pollutant scavenging. Angew. Chem. Int. Ed. 61, e202213247 (2022).
Jin, E. et al. Two-dimensional sp2 carbon-conjugated covalent organic frameworks. Science 357, 673–676 (2017).
Jiang, W. et al. A Lieb-like lattice in a covalent-organic framework and its Stoner ferromagnetism. Nat. Commun. 10, 2207 (2019).
Liu, R. et al. Linkage-engineered donor–acceptor covalent organic frameworks for optimal photosynthesis of hydrogen peroxide from water and air. Nat. Catal. 7, 195–206 (2024).
Chen, Q., Wang, Y. & Luo, G. Photoenzymatic CO2 reduction dominated by collaborative matching of linkage and linker in covalent organic frameworks. J. Am. Chem. Soc. 146, 586–598 (2024).
Kang, J. et al. 2D porous polymers with sp2‐carbon connections and sole sp2‐carbon skeletons. Adv. Funct. Mater. 30, 2000857 (2020).
Lyu, H. et al. Porous crystalline olefin-linked covalent organic frameworks. J. Am. Chem. Soc. 141, 6848–6852 (2019).
Liu, Y. et al. Vinylene‐linked 2D conjugated covalent organic frameworks by wittig reactions. Angew. Chem. Int. Ed. 61, e202209762 (2022).
Li, S. et al. Two-dimensional benzobisthiazole–vinylene-linked covalent organic frameworks outperform one-dimensional counterparts in photocatalysis. ACS Catal. 13, 1089–1096 (2023).
Wang, M. et al. Single-crystal polymers (SCPs): from 1D to 3D architectures. Chem. Soc. Rev. 52, 8165–8193 (2023).
Liu, R. et al. Covalent organic frameworks: an ideal platform for designing ordered materials and advanced applications. Chem. Soc. Rev. 50, 120–242 (2021).
Evans, A. M. et al. Emissive single-crystalline boroxine-linked colloidal covalent organic frameworks. J. Am. Chem. Soc. 141, 19728–19735 (2019).
Wang, S. et al. Single-crystal 2D covalent organic frameworks for plant biotechnology. J. Am. Chem. Soc. 145, 12155–12163 (2023).
Zhan, G. et al. Observing polymerization in 2D dynamic covalent polymers. Nature 603, 835–840 (2022).
Ma, T. et al. Single-crystal X-ray diffraction structures of covalent organic frameworks. Science 361, 48–52 (2018).
Han, J. et al. Fast growth of single-crystal covalent organic frameworks for laboratory X-ray diffraction. Science 383, 1014–1019 (2024).
Evans, A. M. et al. Seeded growth of single-crystal two-dimensional covalent organic frameworks. Science 361, 52–57 (2018).
Li, H. et al. Nucleation–elongation dynamics of two-dimensional covalent organic frameworks. J. Am. Chem. Soc. 142, 1367–1374 (2020).
Zhou, Z. et al. Growth of single-crystal imine-linked covalent organic frameworks using amphiphilic amino-acid derivatives in water. Nat. Chem. 15, 841–847 (2023).
Guan, X. et al. Chemically stable polyarylether-based covalent organic frameworks. Nat. Chem. 11, 587–594 (2019).
Segura, J. L. et al. Post-synthetic modification of covalent organic frameworks. Chem. Soc. Rev. 48, 3903–3945 (2019).
Cusin, L. et al. Chemical conversion and locking of the imine linkage: enhancing the functionality of covalent organic frameworks. Angew. Chem. Int. Ed. 60, 14236–14250 (2021).
Liang, R.-R. et al. Fabricating organic nanotubes through selective disassembly of two-dimensional covalent organic frameworks. J. Am. Chem. Soc. 142, 70–74 (2020).
Koner, K. et al. Porous covalent organic nanotubes and their assembly in loops and toroids. Nat. Chem. 14, 507–514 (2022).
Zhao, W. et al. The development of catalysts and auxiliaries for the synthesis of covalent organic frameworks. Chem. Soc. Rev. 53, 7531–7565 (2024).
Acharjya et al. Vinylene‐linked covalent organic frameworks by base‐catalyzed aldol condensation. Angew. Chem. Int. Ed. 58, 14865–14870 (2019).
Zhang, C.-R. et al. An ionic vinylene-linked three-dimensional covalent organic framework for selective and efficient trapping of ReO4− or 99TcO4−. Nat. Commun. 13, 7621 (2022).
Gui, B. et al. Crystallization of dimensional isomers in covalent organic frameworks. J. Am. Chem. Soc. 145, 11276–11281 (2023).
Han, X. et al. Directing molecular weaving of covalent organic frameworks and their dimensionality by angular control. J. Am. Chem. Soc. 145, 22885–22889 (2023).
Cui, B. et al. Realization of Lieb lattice in covalent-organic frameworks with tunable topology and magnetism. Nat. Commun. 11, 66 (2020).
Kang, K. et al. 2D coherent charge transport in highly ordered conducting polymers doped by solid state diffusion. Nat. Mater. 15, 896–902 (2016).
Mahmood, J. et al. Organic ferromagnetism: trapping spins in the glassy state of an organic network structure. Chem 4, 2357–2369 (2018).
Mi, Z. et al. Stable radical cation-containing covalent organic frameworks exhibiting remarkable structure-enhanced photothermal conversion. J. Am. Chem. Soc. 141, 14433–14442 (2019).
Zhao, Y. et al. Heterocyclic aromatic N-oxidation in the biosynthesis of phenazine antibiotics from Lysobacter antibioticus. Org. Lett. 18, 2495–2498 (2016).
Boukhvalov, D. W. et al. Effect of oxygen adsorption on magnetic properties of graphite. Phys. Rev. B 83, 233408 (2011).
Augustin, M. et al. Molar spin-susceptibility measurement of manganese ferrite nanoparticles using electron spin resonance study. Mater. Lett. 176, 71–73 (2016).
Dediu, V. A. et al. Spin routes in organic semiconductors. Nat. Mater. 8, 707–716 (2009).
Ando, K. Seeking room-temperature ferromagnetic semiconductors. Science 312, 1883–1885 (2006).
Acknowledgements
T.Z. acknowledges the National Natural Science Foundation of China (grant number 52322316), the Leading Innovative and Entrepreneur Team Introduction Program of Zhejiang (grant number 2021R01005) and the Key Research and Development Program of Ningbo (2022ZDYF020023). Z.Z acknowledges the National Natural Science Foundation of China (grant number 22371146). S.X. and P.S. acknowledge financial support from the Interdisciplinary Thematic Institute SysChem via the IdEx Unistra (ANR-10-IDEX-0002) within the program Investissement d’Avenir program, the Foundation Jean-Marie Lehn and the Institut Universitaire de France (IUF). We thank W. Wang for valuable discussion.
Author information
Authors and Affiliations
Contributions
T.Z. initiated the project. T.Z. and S.L. designed the experiments. S.L. performed the COF syntheses, chemical structure characterizations, crystal structure characterizations and magnetization experiments. E.L., T.W. and Z.Z performed the single-crystal analysis. H.Y. and J.H. performed the SEM characterization and schematic drawing. Y.Z. performed the 13C ssNMR experiments. T.Z., S.X., P.S., Q.X and S.L. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
T.Z. and S.L. have applied for a patent for the imine-to-olefin transformation strategy (CN 202410295514.8). The other authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–39 and Tables 1–6.
Supplementary Data 1
Crystallographic data for COF-303 (CCDC 2340227).
Supplementary Data 2
Crystallographic data for sc-sp2c-COF-1 (CCDC 2340230).
Supplementary Data 3
Crystallographic data for sc-sp2c-COF-2 (CCDC 2370333).
Source data
Source Data Fig. 1
Statistical source data for Fig. 1.
Source Data Fig. 2
Statistical source data for Fig. 2.
Source Data Fig. 3
Statistical source data for Fig. 3.
Source Data Fig. 4
Statistical source data for Fig. 4.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, S., Xu, S., Lin, E. et al. Synthesis of single-crystalline sp2-carbon-linked covalent organic frameworks through imine-to-olefin transformation. Nat. Chem. 17, 226–232 (2025). https://doi.org/10.1038/s41557-024-01690-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-024-01690-y