Abstract
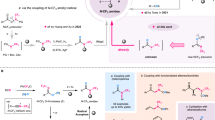
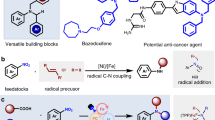
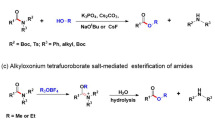
N-monofluoromethyl (N-CH2F) amides, combining amide and monofluoromethyl motifs, represent a practical modification of the amide bond that can mimic N-CH3 amides. Despite the potential value in transforming peptides and peptidomimetics with N-CH2F, the very existence of this structure has been controversial. Here we report the preparation of N-CH2F amides and carbamates via simple and robust chemical methods. The syntheses of N-CH2F amides were achieved via successive acylation and fluorination of imines and directly used in the modification of drugs, peptides and heteroaryl amides without racemization or epimerization. The use of triethylamine is the key to the separation of N-CH2F amides. The stability of nine structurally diverse N-CH2F amides was tested in eight different media, showing that most compounds remained 60–100% intact for 24 h.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
269,00 € per year
only 22,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The authors declare that all the data supporting the findings of this study are available within the article and its Supplementary Information. Crystallographic data for structure 20 reported in this article have been deposited at the Cambridge Crystallographic Data Centre, under deposition number CCDC 2330509. Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/.
References
Pattabiraman, V. R. & Bode, J. W. Rethinking amide bond synthesis. Nature 480, 471–479 (2011).
Roughley, S. D. & Jordan, A. M. The medicinal chemist’s toolbox: an analysis of reactions used in the pursuit of drug candidates. J. Med. Chem. 54, 3451–3479 (2011).
Bode, J. W. Emerging methods in amide- and peptide-bond formation. Curr. Opin. Drug Discov. Dev. 9, 765–775 (2006).
Müller, K., Faeh, C. & Diederich, F. Fluorine in pharmaceuticals: looking beyond intuition. Science 317, 1881–1886 (2007).
Purser, S., Moore, P. R., Swallow, S. & Gouverneur, V. Fluorine in medicinal chemistry. Chem. Soc. Rev. 37, 320–330 (2008).
Brand, S. et al. Lead optimization of a pyrazole sulfonamide series of trypanosoma brucei N-myristoyltransferase inhibitors: identification and evaluation of CNS penetrant compounds as potential treatments for stage 2 human African trypanosomiasis. J. Med. Chem. 57, 9855–9869 (2014).
Liu, Y. et al. Recent progress in monofluoromethylation. Chin. J. Org. Chem. 40, 2322–2337 (2020).
Shen, X., Zhou, M., Ni, C., Zhang, W. & Hu, J. Direct monofluoromethylation of O-, S-, N-, and P-nucleophiles with PhSO(NTs)CH2F: the accelerating effect of a-fluorine substitution. Chem. Sci. 5, 117–122 (2014).
Liu, Y., Lu, L. & Shen, Q. Monofluoromethyl-substituted sulfonium ylides: electrophilic monofluoromethylating reagents with broad substrate scopes. Angew. Chem. Int. Ed. 56, 9930–9934 (2017).
Senatore, R., Malik, M., Spreitzer, M., Holzer, W. & Pace, V. Direct and chemoselective electrophilic monofluoromethylation of heteroatoms (O-, S-, N-, P-, Se-) with fluoroiodomethane. Org. Lett. 22, 1345–1349 (2020).
Chatterjee, J., Gilon, C., Hoffman, A. & Kessler, H. N-methylation of peptides: a new perspective in medicinal chemistry. Acc. Chem. Res. 41, 1331–1342 (2008).
Chatterjee, J., Rechenmacher, F. & Kessler, H. N-methylation of peptides and proteins: an important element for modulating biological functions. Angew. Chem. Int. Ed. 52, 254–269 (2013).
Wang, J. et al. Fluorine in pharmaceutical industry: fluorine-containing drugs introduced to the market in the last decade (2001-2011). Chem. Rev. 114, 2432–2506 (2014).
Gillis, E. P., Eastman, K. J., Hill, M. D., Donnelly, D. J. & Meanwell, N. A. Applications of fluorine in medicinal chemistry. J. Med. Chem. 58, 8315–8359 (2015).
Zhang, W., Zhu, L. & Hu, J. Electrophilic monofluoromethylation of O-, S-, and N-nucleophiles with chlorofluoromethane. Tetrahedron 63, 10569–10575 (2007).
Reichel, M. & Karaghiosoff, K. Monofluorinated nitrogen containing heterocycles: synthesis, characterization and fluorine effect. Z. Anorg. Allg. Chem. 646, 1790–1794 (2020).
Zhang, M. R., Ogawa, M., Furutsuka, K., Yoshida, Y. & Suzuki, K. [18F]Fluoromethyl iodide ([18F]FCH2I): preparation and reactions with phenol, thiophenol, amide and amine functional groups. J. Fluor. Chem. 125, 1879–1886 (2004).
Moreira, R., Mendes, E., Calheiros, T., Bacelo, M. J. & Iley, J. A new direct synthesis of tertiary N-acyloxymethylamide prodrugs of carboxylic acid drugs. Tetrahedron Lett. 35, 7107–7110 (1994).
Zhang, Y., DeSchepper, D. J., Gilbert, T. M., Sai, K. K. & Klumpp, D. A. Superacid promoted reactions of N-acyliminium salts and evidence for the involvement of superelectrophiles. Chem. Commun. https://doi.org/10.1039/b708760h (2007).
Klumpp, D. A., Zhang, Y., O’Connor, M. J., Esteves, P. M. & de Almeida, L. S. Aza-Nazarov reaction and the role of superelectrophiles. Org. Lett. 9, 3085–3088 (2007).
Valeur, E. & Bradley, M. Amide bond formation: beyond the myth of coupling reagents. Chem. Soc. Rev. 38, 606–631 (2009).
Hudlicky, M. Chemistry of Organic Fluorine Compounds (Ellis Horwood, 1976).
Hudlicky, M. & Pavlath, A. E. Chemistry of Organic Fluorine Compounds II: A Critical Review (American Chemical Society, 1995).
Liu, J. et al. Synthesis of N-trifluoromethyl amides from carboxylic acids. Chem 7, 2245–2255 (2021).
Scattolin, T., Bouayad-Gervais, S. & Schoenebeck, F. Straightforward access to N-trifluoromethyl amides, carbamates, thiocarbamates and ureas. Nature 573, 102–107 (2019).
Arlow, S. I. & Hartwig, J. F. Synthesisof aryldifluoroamides by copper-catalyzed cross-coupling. Angew. Chem. Int. Ed. 55, 4567–4572 (2016).
Berndt, U. et al. Synthesis of a [18F]fluorobenzothiazole as potential amyloid imaging agent. J. Label. Compd. Radiopharm. 51, 137–145 (2008).
Clark, R. D. et al. Synthesis and evaluation of ureido- and vinylureidopenicillins as inhibitors of intraruminal lactic acid production. J. Med. Chem. 24, 1250–1253 (1981).
McCrae, J. C., Morrison, E. E., MacIntyre, I. M., Dear, J. W. & Webb, D. J. Long-term adverse effects of paracetamol—a review. Br. J. Clin. Pharmacol. 84, 2218–2230 (2018).
Venkov, A. P. & Lukanov, L. K. New modification of the intramolecular α-amidoalkylation for the synthesis of 2-acyl-1,2,3,4-tetrahydroisoquinolines. Synthesis 1989, 59–61 (1989).
Acknowledgements
We thank funding support from the National Natural Science Foundation of China (grant numbers 82373839 and 22301315), and the Hundred Talents Programme of Sun Yat-sen University.
Author information
Authors and Affiliations
Contributions
M.T. and J.Q. carried out and analysed the experiment. L.D., D.M.W. and X.Z. wrote the paper. J.L., the head of the project, conceived and supervised the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Scott Bagley and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–42, Tables 1–8, procedural details and synthesis and characterization data (NMR spectra, HRMS data, high-performance liquid chromatography spectra and X-ray crystallographic data).
Supplementary Data
Crystallographic data for compound 20, CCDC reference 2330509.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tao, M., Qian, J., Deng, L. et al. Preparation, separation and storage of N-monofluoromethyl amides and carbamates. Nat. Chem. 17, 532–540 (2025). https://doi.org/10.1038/s41557-025-01767-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-025-01767-2