Abstract
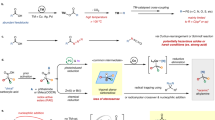
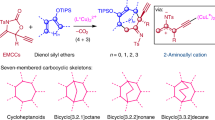
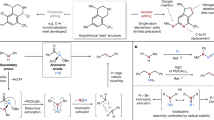
The carbonyl group is one of the most important functional groups in organic chemistry. C=O cleavage and full transfer of the resulting fragments into final products would be extremely attractive and open up new avenues in retrosynthetic planning. In this context, as an N-containing carbonyl compound, the transformations of formamides, wherein C=O cleavage occurring with simultaneous incorporation of ‘O’ and aminomethine moieties to highly functionalized amines, remain a formidable challenge. Here we disclosed a dirhodium/Xantphos or dirhodium–palladium dual catalysed reaction of diazo compounds and allylic substrates in dimethyl formamide, giving various α-aminoketones and cyclopentenone derivatives efficiently featured with extensively reorganized structure, wherein a carbenic carbon was formally inserted into C=O bond and α-group of the carbene was shifted to the residual aminomethine moiety. Mechanistic studies revealed that three or six domino steps are involved in this catalytic process, including epoxidation, dyotropic type rearrangement, allylic alkylation, Claisen rearrangement, isomerization and Nazarov cyclization.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
269,00 € per year
only 22,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data supporting the findings of this study are available within the Article and its Supplementary Information. Crystallographic data for the structures reported in this Article have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers CCDC 2295376 (5a), 2295374 (7d), 2295375 (9f), 2305221 (11e), 2351661 (12p), 2295377 (19), 2350726 (27) and 2305222 (31). These data are available via the Cambridge Crystallographic Data Centre at https://www.ccdc.cam.ac.uk/structures/.
References
Tietze, L. F. Domino reactions in organic synthesis. Chem. Rev. 96, 115–136 (1996).
Lu, L.-Q., Chen, J.-R. & Xiao, W.-J. Development of cascade reactions for the concise construction of diverse heterocyclic architectures. Acc. Chem. Res. 45, 1278–1293 (2012).
Bai, L. & Jiang, X. Catalytic domino reaction: a promising and economic tool in organic synthesis. Chem Catal. 3, 100752 (2023).
Volkov, A., Tinnis, F., Stagbrand, T., Trillo, P. & Adolfsson, H. Chemoselective reduction of carboxamides. Chem. Soc. Rev. 45, 6685–6697 (2016).
Cabrero-Antonino, J. R., Adam, R., Papa, V. & Beller, M. Homogeneous and heterogeneous catalytic reduction of amides and related compounds using molecular hydrogen. Nat. Commun. 11, 3893–3910 (2020).
Huang, P. Direct transformations of amides: tactics and recent progress. Acta Chim. Sinica 76, 357–365 (2018).
Li, G., Ma, S. & Szostak, M. Amide bond activation: the power of resonance. Trends Chem. 2, 914–928 (2020).
Kaiser, D., Bauer, A., Lemmerer, M. & Maulide, N. Amide activation: an emerging tool for chemoselective synthesis. Chem. Soc. Rev. 47, 7899–7925 (2018).
Dander, J. E. & Garg, N. K. Breaking amides using nickel catalysis. ACS Catal. 7, 1413–1423 (2017).
Feng, M., Zhang, H. & Maulide, N. Challenges and breakthroughs in selective amide activation. Angew. Chem. Int. Ed. 61, e20221221 (2022).
Wu, Z., Xu, X., Wang, J. & Dong, G. Carbonyl 1,2-transposition through triflate-mediated α-amination. Science 374, 734–740 (2021).
Shennan, B. D. A., Sánchez-Alonso, S., Rossini, G. & Dixon, D. J. 1,2-Redox transpositions of tertiary amides. J. Am. Chem. Soc. 145, 21745–21751 (2023).
Li, J., Huang, C.-Y. & Li, C.-J. Deoxygenative functionalizations of aldehydes, ketones and carboxylic acids. Angew. Chem. Int. Ed. 61, e202112770 (2022).
Muzart, J. N,N-Dimethylformamide: much more than a solvent. Tetrahedron 65, 8313–8323 (2009).
Ding, S. & Jiao, N. N,N-Dimethylformamide: a multipurpose building block. Angew. Chem. Int. Ed. 51, 9226–9237 (2012).
Bras, J. L. & Muzart, J. Recent uses of N,N-dimethylformamide and N,N-dimethylacetamide as reagents. Molecules 23, 1939–1969 (2018).
Priebbenow, D. L. & Bolm, C. The rhodium-catalysed synthesis of pyrrolidinone substituted (trialkylsilyloxy)acrylic ester. RSC Adv. 3, 10318–10322 (2013).
Li, Q. et al. Rhodium-catalyzed formal C=O bond insertion and sequential acyl 1,4-N-to‑O migratory rearrangement. J. Org. Chem. 87, 16937–16940 (2022).
Nandra, G. S., Pang, P. S., Porter, M. J. & Elliott, J. M. Synthesis of vinylogous carbamates by rhodium(II)-catalyzed olefination of tertiary formamides with a silylated diazoester. Org. Lett. 7, 3453–3455 (2005).
Jung, D. J., Jeon, H. J., Kim, J. H., Kim, Y. & Lee, S.-G. DMF as a source of oxygen and aminomethine: stereoselective 1,2-insertion of rhodium(II) azavinyl carbenes into the C=O bond of formamides for the synthesis of cis-diamino enones. Org. Lett. 16, 2208–2211 (2014).
Goudedranche, S., Besnard, C., Egger, L. & Lacour, J. Synthesis of pyrrolidines and pyrrolizidines with α-pseudoquaternary centers by copper-catalyzed condensation of α-diazodicarbonyl compounds and aryl γ-lactams. Angew. Chem. Int. Ed. 55, 13775–13779 (2016).
Tan, F. et al. Chiral Lewis acid catalyzed reactions of α-diazoester derivatives: construction of dimeric polycyclic compounds. Angew. Chem. Int. Ed. 57, 16176–16179 (2018).
Miura, T., Funakoshi, Y., Tanaka, T. & Murakami, M. Direct production of enaminones from terminal alkynes via rhodium-catalyzed reaction of formamides with N-sulfonyl-1,2,3-triazoles. Org. Lett. 16, 2760–2763 (2014).
Mukherjeea, A. et al. Synthesis of α-amino carbonyl compounds: a brief review. Russ. Chem. Rev. 92, RCR5046 (2023).
Allen, L. A. T., Raclea, R. C., Natho, P. & Parsons, P. J. Recent advances in the synthesis of α-amino ketones. Org. Biomol. Chem. 19, 498–513 (2021).
Fisher, L. E. & Muchowski, J. M. Synthesis of α-aminoaldehydes and α-aminoketones. A review. Org. Prep. Proced. Int. 22, 399–484 (1990).
Wu, W., Chen, C., Peng, J. & Li, Z. Research progress of carbonyl α-position amination. Chin. J. Org. Chem. 43, 2743–2763 (2023).
Padwa, A. Domino reactions of rhodium(II) carbenoids for alkaloid synthesis. Chem. Soc. Rev. 38, 3072–3081 (2009).
Hashimoto, T. & Maruoka, K. Recent advances of catalytic asymmetric 1,3-dipolar cycloadditions. Chem. Rev. 115, 5366–5412 (2015).
Padwa, A. Catalytic decomposition of diazo compounds as a method for generating carbonyl-ylide dipoles. Helv. Chim. Acta 88, 1357–1374 (2005).
Padwa, A. Intramolecular cycloaddition of carbonyl ylides as a strategy for natural product synthesis. Tetrahedron 67, 8057–8072 (2011).
Ning, Y. et al. Rhodium(II) acetate-catalysed cyclization of pyrazol-5-amineand1,3-diketone-2-diazo compounds using N,N-dimethylformamide as a carbon-hydrogen source: access to pyrazolo[3,4-b]pyridines. Adv. Synth. Catal. 361, 3518–3524 (2019).
Ba, D. et al. Rhodium(II)-catalyzed multicomponent assembly of α,α,α-trisubstituted esters via formal insertion of O–C(sp3)–C(sp2) into C–C bonds. Nat. Commun. 11, 4219–4224 (2020).
Trindade, A. F., Coelho, J. A. S., Afonso, C. A. M., Veiros, L. F. & Gois, P. M. P. Fine tuning of dirhodium(II) complexes: exploring the axial modification. ACS Catal. 2, 370–383 (2012).
Hong, B., Shi, L., Li, L., Zhan, S. & Gu, Z. Paddlewheel dirhodium(II) complexes with N-heterocyclic carbene or phosphine ligand: new reactivity and selectivity. Green Synth. Catal. 3, 137–149 (2022).
Wynne, D. C., Olmstead, M. M. & Jessop, P. G. Supercritical and liquid solvent effects on the enantioselectivity of asymmetric cyclopropanation with tetrakis[1-[(4-tert-butylphenyl)-sulfonyl]-(2S)-pyrrolidinecarboxylate]dirhodium(II). J Am Chem Soc 122, 7638–7647 (2000).
Guo, X. & Hu, W. Novel multicomponent reactions via trapping of protic onium ylides with electrophiles. Acc. Chem. Res. 46, 2427–2440 (2013).
Xiao, Q., Zhang, Y. & Wang, J. Diazo compounds and N-tosylhydrazones: novel cross-coupling partners in transition-metal-catalyzed reactions. Acc. Chem. Res. 46, 236–247 (2013).
Xia, Y., Qiu, D. & Wang, J. Transition-metal-catalyzed cross-couplings through carbene migratory insertion. Chem. Rev. 117, 13810–13889 (2017).
Doyle, M. P., McKervey, M. A. & Ye, T. Modern Catalytic Methods for Organic Synthesis with Diazo Compounds (Wiley, 1998).
Davies, H. M. L. & Denton, J. R. Application of donor/acceptor-carbenoids to the synthesis of natural products. Chem. Soc. Rev. 38, 3061–3071 (2009).
Ford, A. et al. Modern organic synthesis with α-diazocarbonyl compounds. Chem. Rev. 115, 9981–10080 (2015).
Liu, Z., Sivaguru, P., Ning, Y., Wu, Y. & Bi, X. Skeletal editing of (hetero)arenes using carbenes. Chem. Eur. J. 29, e202301227 (2023).
Moebius, D. C., Rendina, V. L. & Kingsbury, J. S. Catalysis of diazoalkane–carbonyl homologation. How new developments in hydrazone oxidation enable a carbon insertion strategy for synthesis. Top. Curr. Chem. 346, 111–162 (2014).
Candeias, N. R., Paterna, R. & Gois, P. M. P. Homologation reaction of ketones with diazo compounds. Chem. Rev. 116, 2937–2981 (2016).
Padwa, A. & Hornbuckle, S. F. Ylide formation from the reaction of carbenes and carbenoids with heteroatom lone pairs. Chem. Rev. 91, 263–309 (1991).
Li, M. et al. Copper-catalyzed carbene insertion and ester migration for the synthesis of polysubstituted pyrroles. Chem. Commun. 56, 11050–11053 (2020).
Luo, K. et al. Highly regioselective synthesis of multisubstituted pyrroles via Ag-catalyzed [4+1C]insert cascade. ACS Catal. 10, 3733–3740 (2020).
Jose, J. & Mathew, T. V. Recent advances in the synthesis of 2-cyclopentenones. Tetrahedron 150, 133747–133770 (2024).
Zhou, Z., Dixon, D. D., Jolit, A. & Tius, M. A. The evolution of the total synthesis of rocaglamide. Chem. Eur. J. 22, 15929–15936 (2016).
Lu, B. et al. Dirhodium(II)/Xantphos-catalyzed relay carbene insertion and allylic alkylation process: reaction development and mechanistic insights. J. Am. Chem. Soc. 143, 11799–11810 (2021).
Yang, Y., Lu, B., Xu, G. & Wang, X. Overcoming O–H insertion to para-selective C–H functionalization of free phenols: Rh(II)/Xantphos catalyzed geminal difunctionalization of diazo compounds. ACS Cent. Sci. 8, 581–589 (2022).
Frontier, A. J. & Hernandez, J. J. New twists in Nazarov cyclization chemistry. Acc. Chem. Res. 53, 1822–1832 (2020).
Wenz, D. R. & Alaniz, J. R. d. The Nazarov cyclization: a valuable method to synthesize fully substituted carbon stereocenters. Eur. J. Org. Chem. 2015, 23–37 (2014).
Grandi, M. J. D. Nazarov-like cyclization reactions. Org. Biomol. Chem. 12, 5331–5345 (2014).
Vaidya, T., Eisenberg, R. & Frontier, A. J. Catalytic Nazarov cyclization: the state of the art. ChemCatChem 3, 1531–1548 (2011).
Tornio, A., Neuvonen, P. J., Niemi, M. & Backman, J. T. Role of gemfibrozil as an inhibitor of CYP2C8 and membrane transporters. Expert Opin. Drug Metab. Toxicol. 13, 83–95 (2017).
Berry, J. The role of three-center/four-electron bonds in superelectrophilic dirhodium carbene and nitrene catalytic intermediates. Dalton Trans. 41, 700–703 (2012).
Werlé, C., Goddard, R., Philipps, P., Farès, C. & Fürstner, A. Structures of reactive donor/acceptor and donor/donor rhodium carbenes in the solid state and their implications for catalysis. J. Am. Chem. Soc. 138, 3797–3805 (2016).
Cao, J., Wu, H., Wang, Q. & Zhu, J. C–C bond activation enabled by dyotropic rearrangement of Pd(IV) species. Nat. Chem. 13, 671–676 (2021).
Fernandez, I., Cossio, F. P. & Sierra, M. A. Dyotropic reactions: mechanisms and synthetic applications. Chem. Rev. 109, 6687–6711 (2009).
Bach, R. D. & Domagala, J. M. The effect of Lewis acid and solvent on concerted 1,2-acyl migration. J. Org. Chem. 49, 4181–4188 (1984).
Acknowledgements
We acknowledge financial support from the National Key Research and Development Programme of China (grant no. 2021YFA1500100), the Strategic Priority Research Programme of the Chinese Academy of Sciences (grant nos XDB0610000 and XDB0590000), the National Natural Science Foundation of China (grant nos 92256303, 22171278, 22122104, 22193012 and 21933004), the Shanghai Science and Technology Committee (grant no. 23ZR1482400), the Natural Science Foundation of Ningbo (grant no. 2023J034), the Chinese Academy of Sciences Project for Young Scientists in Basic Research (grant nos YSBR-052 and YSBR-095), Open Research Fund of School of Chemistry and Chemical Engineering, Henan Normal University and the Research Funds of Hangzhou Institute for Advanced Study, University of Chinese Academy of Sciences (2024HIAS-p003). The numerical calculations in this study were carried out on the ORISE Supercomputer.
Author information
Authors and Affiliations
Contributions
K.D. and X.W. conceived the project in collaboration with Y.L., who performed the experimental work and led primary data interpretation and analysis. G.H. and X.-S.X. performed all the computational studies and wrote the section with the calculations.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Rosa Adam, Jianbo Wang, Yanying Zhao and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Tables 1–11 and Figs. 1–18, starting material preparation and experimental procedures, synthetic applications, mechanistic studies and product characterization.
Supplementary Data 1
Crystallographic data for compound 5a; CCDC reference 2295376.
Supplementary Data 2
Crystallographic data for compound 7d; CCDC reference 2295374.
Supplementary Data 3
Crystallographic data for compound 9f; CCDC reference 2295375.
Supplementary Data 4
Crystallographic data for compound 11e; CCDC reference 2305221.
Supplementary Data 5
Crystallographic data for compound 12p; CCDC reference 2351661.
Supplementary Data 6
Crystallographic data for compound 19; CCDC reference 2295377.
Supplementary Data 7
Crystallographic data for compound 27; CCDC reference 2350726.
Supplementary Data 8
Crystallographic data for compound 31a; CCDC reference 2305222.
Supplementary Data 9
Computational details and Cartesian coordinates.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Luo, Y., Huang, G., Ding, K. et al. Oxygen transposition of formamide to α-aminoketone moiety in a carbene-initiated domino reaction. Nat. Chem. (2025). https://doi.org/10.1038/s41557-025-01834-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41557-025-01834-8