Abstract
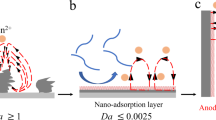
Zn/MnO2 batteries, driven by a dual deposition reaction, are a prominent avenue for achieving high energy density in aqueous systems. Introducing an initially dual-electrode-free (anode/cathode) configuration can further boost energy density to over 200 Wh kg−1, but with limited cycle life due to the poor reversibility of Zn/MnO2 deposition and stripping. Drawing inspiration from soft templating strategies in material synthesis, here we apply this approach to electrodeposition and stripping by designing an in situ formed liquid crystal interphase. This concept is achieved by incorporating just 0.1 mM of surfactant molecules into the electrolyte, which induces favourable c-axis orientations in depositing both hexagonal Zn and MnO2. This enhancement subsequently increases the deposition/stripping reversibility and promotes the cycle life of the dual-electrode-free battery, achieving 80% capacity retention after ~950 cycles. This liquid crystal interphase chemistry also holds great promise for regulating deposition in other crystal systems, opening an exciting research direction for next-generation high-energy-density and long-duration energy storage based on aqueous chemistries.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
118,99 € per year
only 9,92 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings of this study are available within this article and its Supplementary Information.
References
Li, W., Dahn, J. R. & Wainwright, D. S. Rechargeable lithium batteries with aqueous electrolytes. Science 264, 1115–1118 (1994).
Liang, Y. & Yao, Y. Designing modern aqueous batteries. Nat. Rev. Mater. 8, 109–122 (2023).
Parker, J. F. et al. Rechargeable nickel–3D zinc batteries: an energy-dense, safer alternative to lithium-ion. Science 356, 415–418 (2017).
Chen, W. et al. A manganese–hydrogen battery with potential for grid-scale energy storage. Nat. Energy 3, 428–435 (2018).
Zhong, C. et al. Decoupling electrolytes towards stable and high-energy rechargeable aqueous zinc–manganese dioxide batteries. Nat. Energy 5, 440–449 (2020).
Chao, D. et al. An electrolytic Zn–MnO2 battery for high-voltage and scalable energy storage. Angew. Chem. 131, 7905–7910 (2019).
Li, G. et al. Membrane-free Zn/MnO2 flow battery for large-scale energy storage. Adv. Energy Mater. 10, 1902085 (2020).
Ming, F. et al. Co-solvent electrolyte engineering for stable anode-free zinc metal batteries. JACS 144, 7160–7170 (2022).
Louli, A. J. et al. Diagnosing and correcting anode-free cell failure via electrolyte and morphological analysis. Nat. Energy 5, 693–702 (2020).
Li, Y. et al. Interfacial engineering to achieve an energy density of over 200 Wh kg−1 in sodium batteries. Nat. Energy 7, 511–519 (2022).
Xiao, X. et al. Ultrahigh-loading manganese-based electrode for aqueous battery via polymorph tuning. Adv. Mater. 35, 2211555 (2023).
Zheng, J. et al. Reversible epitaxial electrodeposition of metals in battery anodes. Science 366, 645–648 (2019).
Pileni, M.-P. The role of soft colloidal templates in controlling the size and shape of inorganic nanocrystals. Nat. Mater. 2, 145–150 (2003).
Xiao, J. & Qi, L. Surfactant-assisted, shape-controlled synthesis of gold nanocrystals. Nanoscale 3, 1383–1396 (2011).
Tiddy, G. J. Surfactant-water liquid crystal phases. Phys. Rep. 57, 1–46 (1980).
Wang, D. et al. Insight on organic molecules in aqueous Zn-ion batteries with an emphasis on the Zn anode regulation. Adv. Energy Mater. 12, 2102707 (2022).
Guan, K. et al. Anti-corrosion for reversible zinc anode via a hydrophobic interface in aqueous zinc batteries. Adv. Energy Mater. 12, 2103557 (2022).
Bayaguud, A., Luo, X., Fu, Y. & Zhu, C. Cationic surfactant-type electrolyte additive enables three-dimensional dendrite-free zinc anode for stable zinc-ion batteries. ACS Energy Lett. 5, 3012–3020 (2020).
Zhao, F. et al. Trace amounts of fluorinated surfactant additives enable high performance zinc-ion batteries. Energy Storage Mater. 53, 638–645 (2022).
Lin, Y. et al. Dendrite-free Zn anode enabled by anionic surfactant-induced horizontal growth for highly-stable aqueous Zn-ion pouch cells. Energy Environ. Sci. 16, 687–697 (2023).
Kato, T. et al. Transport of ions and electrons in nanostructured liquid crystals. Nat. Rev. Mater. 2, 17001 (2017).
Li, Y., Yu, Z., Huang, J., Wang, Y. & Xia, Y. Constructing solid electrolyte interphase for aqueous zinc batteries. Angew. Chem. Int. Ed. 62, e202309957 (2023).
Zeng, X. et al. Toward a reversible Mn4+/Mn2+ redox reaction and dendrite-free Zn anode in near-neutral aqueous Zn/MnO2 batteries via salt anion chemistry. Adv. Energy Mater. 10, 1904163 (2020).
Yang, H. et al. Protocol in evaluating capacity of Zn–Mn aqueous batteries: a clue of pH. Adv. Mater. 35, 2300053 (2023).
Chen, H. et al. Reunderstanding the reaction mechanism of aqueous Zn–Mn batteries with sulfate electrolytes: role of the zinc sulfate hydroxide. Adv. Mater. 34, 2109092 (2022).
Yu, X. et al. Ten concerns of Zn metal anode for rechargeable aqueous zinc batteries. Joule 7, 1145–1175 (2023).
Shi, F. et al. Strong texturing of lithium metal in batteries. Proc. Natl Acad. Sci. USA 114, 12138–12143 (2017).
Yuan, D. et al. Anion texturing towards dendrite-free Zn anode for aqueous rechargeable batteries. Angew. Chem. 133, 7289–7295 (2021).
Tiberg, F., Brinck, J. & Grant, L. Adsorption and surface-induced self-assembly of surfactants at the solid–aqueous interface. Curr. Opin. Colloid Interface Sci. 4, 411–419 (1999).
Wang, N. et al. Zincophobic electrolyte achieves highly reversible zinc-ion batteries. Adv. Funct. Mater. 33, 2300795 (2023).
Jin, S. et al. Production of fast-charge Zn-based aqueous batteries via interfacial adsorption of ion-oligomer complexes. Nat. Commun. 13, 2283 (2022).
Thieghi, L. T., Longo, L. S. Jr, Licence, P. & Alves, S. Effect of dicationic ionic liquids on lyotropic liquid crystals formed by a binary system composed of Triton-X 100 and water. Mol. Cryst. Liq. Cryst. 657, 95–101 (2017).
Deng, Y. et al. Nanomicellar electrolyte to control release ions and reconstruct hydrogen bonding network for ultrastable high-energy-density Zn–Mn battery. JACS 145, 20109–20120 (2023).
Zhang, Z. et al. Capturing the swelling of solid-electrolyte interphase in lithium metal batteries. Science 375, 66–70 (2022).
Cui, X., Mao, S., Liu, M., Yuan, H. & Du, Y. Mechanism of surfactant micelle formation. Langmuir 24, 10771–10775 (2008).
Wang, Y. et al. Sulfolane-containing aqueous electrolyte solutions for producing efficient ampere-hour-level zinc metal battery pouch cells. Nat. Commun. 14, 1828 (2023).
Sutherland, E., Mercer, S. M., Everist, M. & Leaist, D. G. Diffusion in solutions of micelles. What does dynamic light scattering measure? J. Chem. Eng. Data 54, 272–278 (2009).
Ahir, S., Petrov, P. & Terentjev, E. Rheology at the phase transition boundary: 2. hexagonal phase of Triton X100 surfactant solution. Langmuir 18, 9140–9148 (2002).
Wang, X. et al. Characterization of lipid-based lyotropic liquid crystal and effects of guest molecules on its microstructure: a systematic review. AAPS PharmSciTech 19, 2023–2040 (2018).
Weiss, V., Thiruvengadathan, R. & Regev, O. Preparation and characterization of a carbon nanotube−lyotropic liquid crystal composite. Langmuir 22, 854–856 (2006).
Oyafuso, M. H. et al. Development and in vitro evaluation of lyotropic liquid crystals for the controlled release of dexamethasone. Polymers 9, 330 (2017).
Carey, C. R. et al. Imaging and absolute extinction cross-section measurements of nanorods and nanowires through polarization modulation microscopy. J. Phys. Chem. C. 114, 16029–16036 (2010).
Yoshio, M., Mukai, T., Ohno, H. & Kato, T. One-dimensional ion transport in self-organized columnar ionic liquids. JACS 126, 994–995 (2004).
Lin, Z. et al. Scalable solution-phase epitaxial growth of symmetry-mismatched heterostructures on two-dimensional crystal soft template. Sci. Adv. 2, e1600993 (2016).
Kum, H. et al. Epitaxial growth and layer-transfer techniques for heterogeneous integration of materials for electronic and photonic devices. Nat. Electron. 2, 439–450 (2019).
Hong, S. et al. Efficient scalable hydrothermal synthesis of MnO2 with controlled polymorphs and morphologies for enhanced battery cathodes. ACS Energy Lett. 8, 1744–1751 (2023).
Hao, Z. et al. Metal anodes with ultrahigh reversibility enabled by the closest packing crystallography for sustainable batteries. Adv. Mater. 35, 2209985 (2023).
Chen, C.-H., Postlethwaite, T. A., Hutchison, J. E., Samulski, E. T. & Murray, R. W. Electrochemical measurements of anisotropic diffusion in thin lyotropic liquid crystal films using interdigitated array electrodes. J. Phys. Chem. 99, 8804–8811 (1995).
Sun, W. et al. A rechargeable zinc-air battery based on zinc peroxide chemistry. Science 371, 46–51 (2021).
Zhang, Y. et al. Nonionic surfactant-assisted in situ generation of stable passivation protective layer for highly stable aqueous Zn metal anodes. Nano Lett. 22, 8574–8583 (2022).
Ghavami, R. K. & Rafiei, Z. Performance improvements of alkaline batteries by studying the effects of different kinds of surfactant and different derivatives of benzene on the electrochemical properties of electrolytic zinc. J. Power Sources 162, 893–899 (2006).
Wang, F. et al. Production of gas-releasing electrolyte-replenishing Ah-scale zinc metal pouch cells with aqueous gel electrolyte. Nat. Commun. 14, 4211 (2023).
Ye, X. et al. Unraveling the deposition/dissolution chemistry of MnO2 for high-energy aqueous batteries. Energy Environ. Sci. 16, 1016–1023 (2023).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Monkhorst, H. J. & Pack, J. D. Special points for Brillouin-zone integrations. Phys. Rev. B 13, 5188–5192 (1976).
Carlson, E. Z., Chueh, W. C., Mefford, J. T. & Bajdich, M. Selectivity of electrochemical ion insertion into manganese dioxide polymorphs. ACS Appl. Mater. Interfaces 15, 1513–1524 (2023).
Li, W., Tchelepi, H. A., Ju, Y. & Tartakovsky, D. M. Stability-guided strategies to mitigate dendritic growth in lithium-metal batteries. J. Electrochem. Soc. 169, 060536 (2022).
Li, W., Tchelepi, H. A. & Tartakovsky, D. M. Screening of electrolyte-anode buffers to suppress lithium dendrite growth in all-solid-state batteries. J. Electrochem. Soc. 170, 050510 (2023).
Acknowledgements
This work is supported by the Aqueous Battery Consortium, an energy innovation hub under the US Department of Energy, Office of Basic Energy Sciences, Division of Materials Science and Engineering. We also acknowledge the use and support of the Stanford Nano Shared Facilities and the Stanford Nanofabrication Facility and the use of the computer time allocation m2997 at the National Energy Research Scientific Computing Center, a DOE Office of Science User Facility supported by the Office of Science of the US Department of Energy under contract number DE-AC02-05CH11231. Y.C. acknowledges cryo-EM support from the US Department of Energy, Office of Basic Energy Sciences, Division of Materials Science and Engineering under contract DE-AC02-76SF00515. M.B. acknowledges support by the US Department of Energy, Office of Science, Office of Basic Energy Sciences, Chemical Sciences, Geosciences and Biosciences Division, Catalysis Science Program, to the SUNCAT Center for Interface Science and Catalysis.
Author information
Authors and Affiliations
Contributions
Y.L. and Y.C. (corresponding author) conceived the project and designed the experiments. Y.L. performed electrochemical measurements and soft matter characterizations. Y.L. and X.X. performed SEM and XRD experiments. E.Z.C. and M.B. carried out DFT calculations. W.L. carried out two-dimensional computation. Y.C. carried out cryo-(S)TEM experiments. Y.Y. performed XPS experiments. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Energy thanks Yi-Chun Lu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Table 1, Notes 1–3, Figs. 1–36 and References.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Y., Zheng, X., Carlson, E.Z. et al. In situ formation of liquid crystal interphase in electrolytes with soft templating effects for aqueous dual-electrode-free batteries. Nat Energy 9, 1350–1359 (2024). https://doi.org/10.1038/s41560-024-01638-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-024-01638-z
This article is cited by
-
Critical Bimetallic Phosphide Layer Enables Fast Electron Transfer and Extra Energy Supply for Flexible Quasi-Solid-State Zinc Batteries
Nano-Micro Letters (2025)
-
Building interphases for electrode-free batteries
Nature Energy (2024)