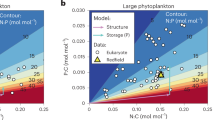
Abstract
The elemental stoichiometry of carbon (C), nitrogen (N) and phosphorus (P) regulates marine biogeochemical cycles and underpins the Redfield ratio paradigm. However, its global variability and response to environmental change remain poorly constrained. Here we compile a global dataset of 56,031 plankton (particulate) and 388,515 seawater (dissolved) samples from 1971 to 2020, spanning surface to 1,000 m depth, to assess spatial and temporal dynamics in marine C:N:P ratios. We show that planktonic C:P and N:P, and oceanic C:N and C:P ratios, consistently exceed Redfield ratio throughout the study period, indicating widespread deviation from canonical stoichiometry. Planktonic C:N and N:P ratios rose markedly in the late twentieth century, followed by a decline, suggesting a progressive alleviation of P limitation, probably driven by increased anthropogenic P inputs. Depth-resolved patterns show decreasing oceanic C:N and C:P, and increasing N:P ratios with depth, attributable to differential remineralization and microbial nutrient cycling. Our findings highlight dynamic, non-static stoichiometric patterns over decadal scales, offering critical observational constraints for refining the representation of elemental cycling in biogeochemical models and improving projections of marine ecosystem responses to global change.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
269,00 € per year
only 22,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The datasets generated and analysed during the current study are available via figshare at https://doi.org/10.6084/m9.figshare.27282792 (ref. 60).
References
Sterner, R. W. & Elser, J. J. Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere (Princeton Univ. Press, 2002).
Redfield, A. C. The influence of organisms on the composition of seawater. Sea 2, 26–77 (1963).
Penuelas, J. et al. Human-induced nitrogen-phosphorus imbalances alter natural and managed ecosystems across the globe. Nat. Commun. 4, 2934 (2013).
Elser, J. et al. Nutritional constraints in terrestrial and freshwater food webs. Nature 408, 578–580 (2000).
Redfield, A. C. in James Johnstone Memorial Volume (ed. Daniel, R. J.) 176–192 (Univ. Press Liverpool, 1934).
Gruber, N. & Deutsch, C. Redfield’s evolving legacy. Nat. Geosci. 7, 853–855 (2014).
Lipizer, M., Cossarini, G., Falconi, C., Solidoro, C. & Umani, S. F. Impact of different forcing factors on N:P balance in a semi-enclosed bay: the Gulf of Trieste (North Adriatic Sea). Cont. Shelf Res. 31, 1651–1662 (2011).
Redfield, A. C. The biological control of chemical factors in the environment. Am. Sci. 46, 205–221 (1958).
Martiny, A. C. et al. Strong latitudinal patterns in the elemental ratios of marine plankton and organic matter. Nat. Geosci. 6, 279–283 (2013).
Falkowski, P. Rationalizing elemental ratios in unicellular algae. J. Phycol. 36, 3–6 (2000).
Gerace, S. et al. Depth variance of organic matter respiration stoichiometry in the subtropical north atlantic and the implications for the global oxygen cycle. Glob. Biogeochem. Cycles 37, e2023GB007814 (2023).
Gerace, S. D. et al. Observed declines in upper ocean phosphate-to-nitrate availability. Proc. Natl Acad. Sci. USA 122, e2411835122 (2025).
Sterner, R., Elser, J. J., Fee, E., Guildford, S. J. & Chrzanowski, T. H. The light:nutrient ratio in lakes: the balance of energy and materials affects ecosystem structure and process. Am. Nat. 150, 663–684 (1998).
Hutchins, D. & Capone, D. The marine nitrogen cycle: new developments and global change. Nat. Rev. Microbiol. 20, 401–414 (2022).
Gruber, N. Consistent patterns of nitrogen fixation identified in the ocean. Nature 566, 191–193 (2019).
Moreno, A., Hagstrom, G., Primeau, F., Levin, S. & Martiny, A. Marine phytoplankton stoichiometry mediates nonlinear interactions between nutrient supply, temperature, and atmospheric CO2. Biogeosciences 15, 2761–2779 (2018).
Pan, Y. et al. Enhanced atmospheric phosphorus deposition in Asia and Europe in the past two decades. Atmos. Ocean. Sci. Lett. 14, 100051 (2021).
Ackerman, D., Millet, D. B. & Chen, X. Global estimates of inorganic nitrogen deposition across four decades. Glob. Biogeochem. Cycles 33, 100–107 (2019).
Friedlingstein, P. et al. Global carbon budget 2022. Earth Syst. Sci. Data 14, 4811–4900 (2022).
van de Waal, D. B., Verschoor, A. M., Verspagen, J. M. H., van Donk, E. & Huisman, J. Climate-driven changes in the ecological stoichiometry of aquatic ecosystems. Front. Ecol. Environ. 8, 145–152 (2010).
Hutchins, D., Mulholland, M. & Feixue, F. Nutrient cycles and marine microbes in a CO2-enriched ocean. Oceanography 22, 128–145 (2009).
Guignard, M. et al. Impacts of nitrogen and phosphorus: from genomes to natural ecosystems and agriculture. Front. Ecol. Evol. https://doi.org/10.3389/fevo.2017.00070 (2017).
Ayo, B. et al. Imbalanced nutrient recycling in a warmer ocean driven by differential response of extracellular enzymatic activities. Glob. Chang. Biol. 23, 4084–4093 (2017).
Toseland, A. et al. The impact of temperature on marine phytoplankton resource allocation and metabolism. Nat. Clim. Chang. 3, 979–984 (2013).
Alewell, C. et al. Global phosphorus shortage will be aggravated by soil erosion. Nat. Commun. 11, 4546 (2020).
Matsumoto, K., Tanioka, T. & Rickaby, R. Linkages between dynamic phytoplankton C:N:P and the ocean carbon cycle under climate change. Oceanography 33, 44–52 (2020).
Tanaka, T. et al. Availability of phosphate for phytoplankton and bacteria and of glucose for bacteria at different pCO2 levels in a mesocosm study. Biogeosciences 5, 669–678 (2008).
Paul, C., Matthiessen, B. & Sommer, U. Warming, but not enhanced CO2 concentration, quantitatively and qualitatively affects phytoplankton biomass. Mar. Ecol. Prog. Ser. 528, 39–51 (2015).
Bellerby, R. et al. Marine ecosystem community carbon an nutrient uptake stoichiometry under varying ocean acidification during the PeECE III experiment. Biogeosciences 5, 1517–1527 (2008).
Deutsch, C. & Weber, T. Nutrient ratios as a tracer and driver of ocean biogeochemistry. Ann. Rev. Mar. Sci. 4, 113–141 (2012).
Islam, M. et al. C:N:P stoichiometry of particulate and dissolved organic matter in river waters and changes during decomposition. J. Ecol. Environ. 43, 4 (2019).
Louis, J., Bressac, M., Pedrotti, M. L. & Guieu, C. Dissolved inorganic nitrogen and phosphorus dynamics in seawater following an artificial Saharan dust deposition event. Front. Mar. Sci. 2, 27 (2015).
Ward, B. B. et al. Denitrification as the dominant nitrogen loss process in the Arabian Sea. Nature 461, 78–81 (2009).
Fakhraee, M., Planavsky, N. & Reinhard, C. The role of environmental factors in the long-term evolution of the marine biological pump. Nat. Geosci. 13, 812–816 (2020).
Tanioka, T. & Matsumoto, K. A meta-analysis on environmental drivers of marine phytoplankton C:N:P. Biogeosciences 17, 2939–2954 (2020).
Weber, T., Deutsch, C., Weber, T. S. & Deutsch, C. Ocean nutrient ratios governed by plankton biogeography. Nature 467, 550–554 (2010).
Liefer, J. et al. The macromolecular basis of phytoplankton C:N:P under nitrogen starvation. Front. Microbiol. 10, 763 (2019).
Guo, L. et al. Acceleration of phosphorus weathering under warm climates. Sci. Adv. 10, eadm7773 (2024).
Seitzinger, S. P. et al. Global river nutrient export: a scenario analysis of past and future trends. Glob. Biogeochem. Cycles 24, GB0A08 (2010).
Lapointe, B. E. et al. Nutrient content and stoichiometry of pelagic Sargassum reflects increasing nitrogen availability in the Atlantic Basin. Nat. Commun. 12, 3060 (2021).
Wang, M. et al. The great Atlantic Sargassum belt. Science 365, 83–87 (2019).
Kwiatkowski, L., Aumont, O., Bopp, L. & Ciais, P. The impact of variable phytoplankton stoichiometry on projections of primary production, food quality, and carbon uptake in the global ocean. Glob. Biogeochem. Cycles 32, 516–528 (2018).
Inomura, K., Deutsch, C., Jahn, O., Dutkiewicz, S. & Follows, M. Global patterns in marine organic matter stoichiometry driven by phytoplankton ecophysiology. Nat. Geosci. 15, 1034–1040 (2022).
Moore, C. et al. Processes and patterns of oceanic nutrient limitation. Nat. Geosci. 6, 701–710 (2013).
Armin, G. & Inomura, K. Modeled temperature dependencies of macromolecular allocation and elemental stoichiometry in phytoplankton. Comput. Struct. Biotechnol. J. 19, 5421–5427 (2021).
Liefer, J. et al. Latitudinal patterns in ocean C:N:P reflect phytoplankton acclimation and macromolecular composition. Proc. Natl Acad. Sci. USA 121, e2404460121 (2024).
Sterner, R. W. et al. Scale-dependent carbon:nitrogen:phosphorus seston stoichiometry in marine and freshwaters. Limnol. Oceanogr. 53, 1169–1180 (2008).
Frigstad, H. et al. Seasonal variation in marine C:N:P stoichiometry: can the composition of seston explain stable Redfield ratios? Biogeosciences 8, 2917–2933 (2011).
Berry, D. L. et al. Shifts in Cyanobacterial Strain Dominance during the Onset of Harmful Algal Blooms in Florida Bay, USA. Microb. Ecol. 70, 361–371 (2015).
Martiny, A., Vrugt, J. & Lomas, M. Concentrations and ratios of particulate organic carbon, nitrogen, and phosphorus in the global ocean. Sci. Data 1, 140048 (2014).
Tanioka, T. et al. Global patterns and predictors of C:N:P in marine ecosystems. Commun. Earth Environ. 3, 271 (2022).
Hutchins, D. A. & Tagliabue, A. Feedbacks between phytoplankton and nutrient cycles in a warming ocean. Nat. Geosci. 17, 495–502 (2024).
Conway, T. M., Horner, T. J., Plancherel, Y. & González, A. G. A decade of progress in understanding cycles of trace elements and their isotopes in the oceans. Chem. Geol. 580, 120381 (2021).
Dietze, H., Oschlies, A. & Kähler, P. Concentrations of Organic Carbon, Nitrogen and Phosphorus on Vertical Profiles in Waters of the Tropical North Atlantic in March 2002 (PANGAEA, 2008).
Lauvset, S. et al. An updated version of the global interior ocean biogeochemical data product, GLODAPv2.2021. Earth Syst. Sci. Data 13, 5565–5589 (2021).
Conover, W. J. in Practical Nonparametric Statistics (ed. Wiley, B. II) Ch. 3 (Wiley & Sons, 1999).
Wilcoxon, F. Individual comparisons by ranking methods. Biom. Bull. 1, 80–83 (1945).
Mann, H. B. & Whitney, D. R. On a test of whether one of two random variables is stochastically larger than the other. Ann. Stat. 18, 50–60 (1947).
Toms, J. & Lesperance, M. Piecewise regression: a tool for identifying ecological thresholds. Ecology 84, 2034–2041 (2003).
Liu, J. et al. Database title. figshare https://doi.org/10.6084/m9.figshare.27282792 (2025).
Reay, D. S., Dentener, F., Smith, P., Grace, J. & Feely, R. A. Global nitrogen deposition and carbon sinks. Nat. Geosci. 1, 430–437 (2008).
Graber, J., Amthor, J., Dahlman, R., Drell, D. & Weatherwax, S. Carbon Cycling and Biosequestration Integrating Biology and Climate Through Systems Science Report from the March 2008 Workshop (US Department of Energy Office of Science, 2008).
Galloway, J. N. et al. Nitrogen cycles: past, present, and future. Biogeochemistry 70, 153–226 (2004).
Zhang, X., Ward, B. B. & Sigman, D. M. Global nitrogen cycle: critical enzymes, organisms, and processes for nitrogen budgets and dynamics. Chem. Rev. 120, 5308–5351 (2020).
Wang, R. et al. Significant contribution of combustion-related emissions to the atmospheric phosphorus budget. Nat. Geosci. 8, 48–54 (2015).
Acknowledgements
J.L. is funded by the National Natural Science Foundation of China (grant no. 42207107) and the Horizon Europe Framework Programme (grant no. 101205485). K.I. is supported by a grant from the Simons Foundation (grant no. LS-ECIAMEE-00001549). Ji.C. is granted by the National Natural Science Foundation of China (grant nos. 32471685 and 42361144886) and Shaanxi Province Natural Science Foundation for Distinguished Young Scholar (grant no. 2024JC-JCQN-32).
Author information
Authors and Affiliations
Contributions
J.L. conceived the study. J.L., H.W. and J.M. designed the methodology, conducted the investigation and generated the visualizations. J.L. drafted the paper. Ji.C. and J.P. supervised the project. J.L., H.W., J.M., J.P., M.D.-B., A.C.M., G.Z., D.A.H., K.I., M.W.L., M.F., A.P., T.J.K., C.A.D., N.P., B.L., Yo.Z., Ya.L., J.Z., Yi.Z., S.S., Yo.L., W.Z., Ju.C. and Ji.C. reviewed and edited the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Geoscience thanks Dag Hessen, Helena Klip and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: James Super, in collaboration with the Nature Geoscience team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 2 Trends in the planktonic concentrations (a) and stoichiometries (b) of carbon (C), nitrogen (N) and phosphorus (P) at different seawater depths.
Δ represents the amount of change in the planktonic C, N, and P concentrations at different seawater depths relative to that of the surface water. Predictor variables (C, N, P) plus lowercase 0 represent the mean value of the predictor variable at the seawater surface. Predictor variables (C:N, C:P, N:P) plus lowercase 0 represent the median value of the predictor variable at the seawater surface. The solid lines and shading area represent the mean for ΔC, ΔN, and ΔP or median for ΔC:N, ΔC:P, and ΔN:P and 95% confidence intervals of the predictor variables, respectively. Predictor variables divided by depth represent the change coefficient of the predictor variables with increasing seawater depth in the epipelagic and mesopelagic zones. ***, p < 0.001.
Extended Data Fig. 3 Temporal trends in planktonic (a, n = 28135) and oceanic (b, n = 177487) ecological stoichiometry of the Pacific Ocean.
The boxplot represents the distribution of data for all years from 1970 to 2020. For the boxplot, the straight line in the center represents the median, or 2nd quartile (Q2), the top edge of the box represents the 3rd quartile (Q3), and the bottom edge of the box represents the 1st quartile (Q1). The black and blue dashed lines represent the Redfield ratio and the ecological stoichiometric ratio established in this study, respectively. The error bars represent the mean and standard deviation of the stoichiometric ratios for each year. The significance of the non-zero coefficients in the segmented model fitting is evaluated through a two-tailed t-test. If p > 0.05, it indicates that there is no obvious trend of change in the stoichiometric ratio over time. The shaded area of the fitted line represents the 95% confidence interval of the predicted values. The shaded area at the segmentation point represents the uncertainty of the time breakpoints, which is derived from the standard deviation of the breakpoint estimates across iterations based on resampling. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Extended Data Fig. 4 Temporal trends in planktonic (a, n = 23788) and oceanic (b, n = 105541) ecological stoichiometry of the Atlantic Ocean.
The boxplot represents the distribution of data for all years from 1970 to 2020. For the boxplot, the straight line in the center represents the median, or 2nd quartile (Q2), the top edge of the box represents the 3rd quartile (Q3), and the bottom edge of the box represents the 1st quartile (Q1). The black and blue dashed lines represent the Redfield ratio and the ecological stoichiometric ratio established in this study, respectively. The error bars represent the mean and standard deviation of the stoichiometric ratios for each year. The significance of the non-zero coefficients in the segmented model fitting is evaluated through a two-tailed t-test. If p > 0.05, it indicates that there is no obvious trend of change in the stoichiometric ratio over time. The shaded area of the fitted line represents the 95% confidence interval of the predicted values. The shaded area at the segmentation point represents the uncertainty of the time breakpoints, which is derived from the standard deviation of the breakpoint estimates across iterations based on resampling. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Extended Data Fig. 5 Temporal trends in planktonic (a, n = 3612) and oceanic (b, n = 45558) ecological stoichiometry of the Indian Ocean.
The boxplot represents the distribution of data for all years from 1970 to 2020. For the boxplot, the straight line in the center represents the median, or 2nd quartile (Q2), the top edge of the box represents the 3rd quartile (Q3), and the bottom edge of the box represents the 1st quartile (Q1). The black and blue dashed lines represent the Redfield ratio and the ecological stoichiometric ratio established in this study, respectively. The error bars represent the mean and standard deviation of the stoichiometric ratios for each year. The significance of the non-zero coefficients in the segmented model fitting is evaluated through a two-tailed t-test. If p > 0.05, it indicates that there is no obvious trend of change in the stoichiometric ratio over time. The shaded area of the fitted line represents the 95% confidence interval of the predicted values. The shaded area at the segmentation point represents the uncertainty of the time breakpoints, which is derived from the standard deviation of the breakpoint estimates across iterations based on resampling. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Extended Data Fig. 6 Temporal trends in planktonic (a, n = 296) and oceanic (b, n = 59929) ecological stoichiometry of the Arctic Ocean.
The boxplot represents the distribution of data for all years from 1970 to 2020. For the boxplot, the straight line in the center represents the median, or 2nd quartile (Q2), the top edge of the box represents the 3rd quartile (Q3), and the bottom edge of the box represents the 1st quartile (Q1). The black and blue dashed lines represent the Redfield ratio and the ecological stoichiometric ratio established in this study, respectively. The error bars represent the mean and standard deviation of the stoichiometric ratios for each year. The significance of the non-zero coefficients in the segmented model fitting is evaluated through a two-tailed t-test. If p > 0.05, it indicates that there is no obvious trend of change in the stoichiometric ratio over time. The shaded area of the fitted line represents the 95% confidence interval of the predicted values. The shaded area at the segmentation point represents the uncertainty of the time breakpoints, which is derived from the standard deviation of the breakpoint estimates across iterations based on resampling. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Supplementary information
Supplementary Information
Supplementary methods, Figs. 1–14 and Tables 1–3.
Source data
Source Data 1
Statistical source data (marine ecological stoichiometry data).
Source Data 2
Statistical source data (global carbon, nitrogen and phosphorus flux data).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, J., Wang, H., Mou, J. et al. Global-scale shifts in marine ecological stoichiometry over the past 50 years. Nat. Geosci. (2025). https://doi.org/10.1038/s41561-025-01735-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41561-025-01735-y