Abstract
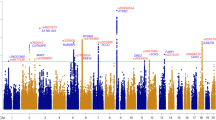
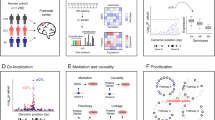
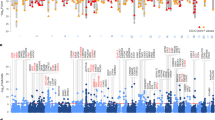
Deciphering the genetic architecture of depression is pivotal for characterizing the associated pathophysiological processes and development of new therapeutics. Here we conducted a cross-ancestry genome-wide meta-analysis on depression (416,437 cases and 1,308,758 controls) and identified 287 risk loci, of which 49 are new. Variant-level fine mapping prioritized potential causal variants and functional genomic analysis identified variants that regulate the binding of transcription factors. We validated that 80% of the identified functional variants are regulatory variants, and expression quantitative trait loci analysis uncovered the potential target genes regulated by the prioritized risk variants. Gene-level analysis, including transcriptome and proteome-wide association studies, colocalization and Mendelian randomization-based analyses, prioritized potential causal genes and drug targets. Gene prioritization analyses highlighted likely causal genes, including TMEM106B, CTNND1, AREL1 and so on. Pathway analysis indicated significant enrichment of depression risk genes in synapse-related pathways. Finally, knockdown of Tmem106b in mice resulted in depression-like behaviours, supporting the involvement of Tmem106b in depression. Our study identified new risk loci, likely causal variants and genes for depression, providing important insights into the genetic architecture of depression and potential therapeutic targets.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
118,99 € per year
only 9,92 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
Genome-wide summary statistics of MVP were obtained from dbGaP via application (accession no. phs001672.v1.p.1), summary statistics of FinnGen (publicly available) were downloaded from FinnGen website (https://www.finngen.fi/en), summary statistics of Howard et al. (UKB + PGC) were publicly available and downloaded from https://doi.org/10.7488/ds/2458, summary statistics of the AGDS were from B.L.M., summary statistics of Giannakopoulou et al. were downloaded from PGC website (https://pgc.unc.edu/) and summary statistics of Sakaue et al. were publicly available and downloaded from BioBank Japan (BBJ) (https://pheweb.jp/downloads). The genome-wide summary statistics of 23andMe were obtained under a data transfer agreement. The genome-wide summary statistics (not including 23andMe and AGDS) will be made publicly available (at https://doi.org/10.6084/m9.figshare.24521968.v1 (ref. 97)) once the article has been published. The full GWAS summary statistics for the 23andMe discovery dataset will be made available through 23andMe to qualified researchers under an agreement with 23andMe that protects the privacy of the 23andMe participants. Please visit https://research.23andme.com/collaborate/#dataset-access/ for more information and to apply to access the data. GWAS summary statistics for the AGDS dataset will be available upon reasonable request (please contact B.L.M. at Brittany.mitchell@qimrberghofer). The SNP–expression weights of PsychENCODE used in this study were downloaded from http://resource.psychencode.org/. The processed protein weight files were downloaded from https://www.synapse.org/ (Synapse ID: syn9884314 and syn23245237). The PsychENCODE cis-eQTL data were downloaded from the SMR website (https://yanglab.westlake.edu.cn/data/SMR/PsychENCODE_cis_eqtl_HCP100_summary.tar.gz). The gene expression data used for MAGMA were from the GTEx consortium, GTEx_Analysis_2017-06-05_v8_RNASeQCv1.1.9. All GO terms (including cellular components, biological processes and molecular functions) and Kyoto Encyclopedia of Genes and Genomes pathway gene sets were downloaded from the MSigDB database (https://www.gsea-msigdb.org/gsea/msigdb/human/collections.jsp#C5, v2022.1.Hs). The gene list and feature files were downloaded from the PoPS GitHub page (https://github.com/FinucaneLab/pops) to calculate the PoPS score of all candidate genes. Source data are provided with this paper.
Code availability
GWAS meta-analysis was used an inverse-variance-based fixed-effects meta-analysis implemented in PLINK (v1.90, https://www.cog-genomics.org/plink/). FUMA v1.3.7 (https://fuma.ctglab.nl/home) was used to define the risk loci, with the default parameters. LDSC (https://github.com/bulik/ldsc) was used to estimate the SNP-based heritability and pairwise genetic correlations between the GWASs. FIMO was used to compare the derived binding motifs with the publicly available PWM and search the best-matched motifs. MESuSiE (https://github.com/borangao/meSuSie) was used for statistical fine mapping. The TwoSampleMR R package was used to perform two-sample MR analysis (v0.5.6, https://mrcieu.github.io/TwoSampleMR/). Other custom codes is available via Zenodo at https://doi.org/10.5281/zenodo.13856052 (ref. 98).
References
Malhi, G. S. & Mann, J. J. Depression. Lancet 392, 2299–2312 (2018).
Friedrich, M. J. Depression is the leading cause of disability around the world. JAMA 317, 1517 (2017).
Levey, D. F. et al. Bi-ancestral depression GWAS in the Million Veteran Program and meta-analysis in >1.2 million individuals highlight new therapeutic directions. Nat. Neurosci. 24, 954–963 (2021).
Wray, N. R. et al. Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat. Genet. 50, 668–681 (2018).
Howard, D. M. et al. Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat. Neurosci. 22, 343–352 (2019).
Hyde, C. L. et al. Identification of 15 genetic loci associated with risk of major depression in individuals of European descent. Nat. Genet. 48, 1031–1036 (2016).
CONVERGE Consortium. Sparse whole-genome sequencing identifies two loci for major depressive disorder. Nature 523, 588–591 (2015).
Howard, D. M. et al. Genome-wide association study of depression phenotypes in UK Biobank identifies variants in excitatory synaptic pathways. Nat. Commun. 9, 1470 (2018).
Mitchell, B. L. et al. The Australian Genetics of Depression Study: new risk loci and dissecting heterogeneity between subtypes. Biol. Psychiatry 92, 227–235 (2021).
Giannakopoulou, O. et al. The Genetic Architecture of Depression in individuals of East Asian ancestry: a genome-wide association study. JAMA Psychiatry 78, 1258–1269 (2021).
Sakaue, S. et al. A cross-population atlas of genetic associations for 220 human phenotypes. Nat. Genet. 53, 1415–1424 (2021).
Als, T. D. et al. Depression pathophysiology, risk prediction of recurrence and comorbid psychiatric disorders using genome-wide analyses. Nat. Med. 29, 1832–1844 (2023).
Meng, X. et al. Multi-ancestry genome-wide association study of major depression aids locus discovery, fine mapping, gene prioritization and causal inference. Nat. Genet. https://doi.org/10.1038/s41588-023-01596-4 (2024).
Speed, D., Holmes, J. & Balding, D. J. Evaluating and improving heritability models using summary statistics. Nat. Genet. 52, 458–462 (2020).
Bulik-Sullivan, B. K. et al. LD score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat. Genet. 47, 291–295 (2015).
Brown, B. C., Asian Genetic Epidemiology Network Type 2 Diabetes Consortium, Ye, C. J., Price, A. L. & Zaitlen, N. Transethnic genetic-correlation estimates from summary statistics. Am. J. Hum. Genet. 99, 76–88 (2016).
Huo, Y., Li, S., Liu, J., Li, X. & Luo, X. J. Functional genomics reveal gene regulatory mechanisms underlying schizophrenia risk. Nat. Commun. 10, 670 (2019).
Li, S. et al. Regulatory mechanisms of major depressive disorder risk variants. Mol. Psychiatry 25, 1926–1945 (2020).
Whitington, T. et al. Gene regulatory mechanisms underpinning prostate cancer susceptibility. Nat. Genet. 48, 387–397 (2016).
Gao, B. & Zhou, X. MESuSiE enables scalable and powerful multi-ancestry fine-mapping of causal variants in genome-wide association studies. Nat. Genet. 56, 170–179 (2024).
Qi, T. et al. Genetic control of RNA splicing and its distinct role in complex trait variation. Nat. Genet. 54, 1355–1363 (2022).
Gandal, M. J. et al. Transcriptome-wide isoform-level dysregulation in ASD, schizophrenia, and bipolar disorder. Science https://doi.org/10.1126/science.aat8127 (2019).
Gusev, A. et al. Integrative approaches for large-scale transcriptome-wide association studies. Nat. Genet. 48, 245–252 (2016).
Wingo, A. P. et al. Integrating human brain proteomes with genome-wide association data implicates new proteins in Alzheimer’s disease pathogenesis. Nat. Genet. 53, 143–146 (2021).
Willer, C. J., Li, Y. & Abecasis, G. R. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics 26, 2190–2191 (2010).
Deng, Y. T. et al. Identifying causal genes for depression via integration of the proteome and transcriptome from brain and blood. Mol. Psychiatry 27, 2849–2857 (2022).
Li, W. et al. Genome-wide meta-analysis, functional genomics and integrative analyses implicate new risk genes and therapeutic targets for anxiety disorders. Nat. Hum. Behav. 8, 361–379 (2024).
Giambartolomei, C. et al. Bayesian test for colocalisation between pairs of genetic association studies using summary statistics. PLoS Genet. 10, e1004383 (2014).
Giambartolomei, C. et al. A Bayesian framework for multiple trait colocalization from summary association statistics. Bioinformatics 34, 2538–2545 (2018).
Gaziano, L. et al. Actionable druggable genome-wide Mendelian randomization identifies repurposing opportunities for COVID-19. Nat. Med. 27, 668–676 (2021).
Koopmans, F. et al. SynGO: an evidence-based, expert-curated knowledge base for the synapse. Neuron 103, 217–234 e4 (2019).
Tartt, A. N., Mariani, M. B., Hen, R., Mann, J. J. & Boldrini, M. Dysregulation of adult hippocampal neuroplasticity in major depression: pathogenesis and therapeutic implications. Mol. Psychiatry 27, 2689–2699 (2022).
Krishnan, V. & Nestler, E. J. The molecular neurobiology of depression. Nature 455, 894–902 (2008).
Abe-Higuchi, N. et al. Hippocampal sirtuin 1 signaling mediates depression-like behavior. Biol. Psychiatry 80, 815–826 (2016).
Cui, L. et al. Major depressive disorder: hypothesis, mechanism, prevention and treatment. Signal Transduct. Target. Ther. 9, 30 (2024).
Lein, E. S. et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature 445, 168–176 (2007).
Dall’Aglio, L., Lewis, C. M. & Pain, O. Delineating the genetic component of gene expression in major depression. Biol. Psychiatry 89, 627–636 (2021).
Noh, K. et al. Negr1 controls adult hippocampal neurogenesis and affective behaviors. Mol. Psychiatry 24, 1189–1205 (2019).
Szczurkowska, J. et al. NEGR1 and FGFR2 cooperatively regulate cortical development and core behaviours related to autism disorders in mice. Brain 141, 2772–2794 (2018).
Carboni, L. et al. Depression-associated gene Negr1–Fgfr2 pathway is altered by antidepressant treatment. Cells 9, 1818 (2020).
Ji, W. et al. CNTNAP2 is significantly associated with schizophrenia and major depression in the Han Chinese population. Psychiatry Res. 207, 225–228 (2013).
Zhang, R. X. et al. EphB2 in the medial prefrontal cortex regulates vulnerability to stress. Neuropsychopharmacology 41, 2541–2556 (2016).
Grunwald, I. C. et al. Kinase-independent requirement of EphB2 receptors in hippocampal synaptic plasticity. Neuron 32, 1027–1040 (2001).
Calabrese, F., Riva, M. A. & Molteni, R. Synaptic alterations associated with depression and schizophrenia: potential as a therapeutic target. Expert Opin. Ther. Targets 20, 1195–1207 (2016).
Holmes, S. E. et al. Lower synaptic density is associated with depression severity and network alterations. Nat. Commun. 10, 1529 (2019).
Price, R. B. & Duman, R. Neuroplasticity in cognitive and psychological mechanisms of depression: an integrative model. Mol. Psychiatry 25, 530–543 (2020).
Duman, R. S., Aghajanian, G. K., Sanacora, G. & Krystal, J. H. Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat. Med. 22, 238–249 (2016).
Duman, R. S. & Aghajanian, G. K. Synaptic dysfunction in depression: potential therapeutic targets. Science 338, 68–72 (2012).
Shonesy, B. C. et al. CaMKII regulates diacylglycerol lipase-alpha and striatal endocannabinoid signaling. Nat. Neurosci. 16, 456–463 (2013).
Ogasawara, D. et al. Rapid and profound rewiring of brain lipid signaling networks by acute diacylglycerol lipase inhibition. Proc. Natl Acad. Sci. USA 113, 26–33 (2016).
Bisogno, T. et al. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J. Cell Biol. 163, 463–468 (2003).
Jenniches, I. et al. Anxiety, stress, and fear response in mice with reduced endocannabinoid levels. Biol. Psychiatry 79, 858–868 (2016).
Liu, X. et al. Plasma lipidomics reveals potential lipid markers of major depressive disorder. Anal. Bioanal. Chem. 408, 6497–6507 (2016).
Hill, M. N., Miller, G. E., Carrier, E. J., Gorzalka, B. B. & Hillard, C. J. Circulating endocannabinoids and N-acyl ethanolamines are differentially regulated in major depression and following exposure to social stress. Psychoneuroendocrinology 34, 1257–1262 (2009).
Iranpour, S. & Sabour, S. Inverse association between caffeine intake and depressive symptoms in US adults: data from National Health and Nutrition Examination Survey (NHANES) 2005-2006. Psychiatry Res. 271, 732–739 (2019).
Woolf, B. et al. Appraising the causal relationship between plasma caffeine levels and neuropsychiatric disorders through Mendelian randomization. BMC Med. 21, 296 (2023).
Harvey, M., Belleau, P. & Barden, N. Gene interactions in depression: pathways out of darkness. Trends Genet. 23, 547–556 (2007).
Vereczkei, A. et al. Association of purinergic receptor P2RX7 gene polymorphisms with depression symptoms. Prog. Neuropsychopharmacol. Biol. Psychiatry 92, 207–216 (2019).
Hodes, G. E., Kana, V., Menard, C., Merad, M. & Russo, S. J. Neuroimmune mechanisms of depression. Nat. Neurosci. 18, 1386–1393 (2015).
Wohleb, E. S., Franklin, T., Iwata, M. & Duman, R. S. Integrating neuroimmune systems in the neurobiology of depression. Nat. Rev. Neurosci. 17, 497–511 (2016).
Boucher, A. A. et al. Resilience and reduced c-Fos expression in P2X7 receptor knockout mice exposed to repeated forced swim test. Neuroscience 189, 170–177 (2011).
Basso, A. M. et al. Behavioral profile of P2X7 receptor knockout mice in animal models of depression and anxiety: relevance for neuropsychiatric disorders. Behav. Brain Res. 198, 83–90 (2009).
Deussing, J. M. & Arzt, E. P2X7 receptor: a potential therapeutic target for depression? Trends Mol. Med. 24, 736–747 (2018).
Groettrup, M. et al. A role for the proteasome regulator PA28α in antigen presentation. Nature 381, 166–168 (1996).
Wong, M. L., Dong, C., Maestre-Mesa, J. & Licinio, J. Polymorphisms in inflammation-related genes are associated with susceptibility to major depression and antidepressant response. Mol. Psychiatry 13, 800–812 (2008).
Liu, J. et al. Genome-wide Mendelian randomization identifies actionable novel drug targets for psychiatric disorders. Neuropsychopharmacology 48, 270–280 (2023).
Li, X. et al. Common variants on 6q16.2, 12q24.31 and 16p13.3 are associated with major depressive disorder. Neuropsychopharmacology 43, 2146–2153 (2018).
FinnGen (documentation of R5 release). FinnGen https://finngen.gitbook.io/documentation/ (2020).
Gelernter, J. et al. Genome-wide association study of post-traumatic stress disorder reexperiencing symptoms in >165,000 US veterans. Nat. Neurosci. 22, 1394–1401 (2019).
Byrne, E. M. et al. Cohort profile: the Australian Genetics of Depression Study. BMJ Open 10, e032580 (2020).
Evangelou, E. & Ioannidis, J. P. A. Meta-analysis methods for genome-wide association studies and beyond. Nat. Rev. Genet. 14, 379–389 (2013).
Purcell, S. et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81, 559–575 (2007).
Watanabe, K., Taskesen, E., van Bochoven, A. & Posthuma, D. Functional mapping and annotation of genetic associations with FUMA. Nat. Commun. 8, 1826 (2017).
Bulik-Sullivan, B. et al. An atlas of genetic correlations across human diseases and traits. Nat. Genet. 47, 1236–1241 (2015).
Grant, C. E., Bailey, T. L. & Noble, W. S. FIMO: scanning for occurrences of a given motif. Bioinformatics 27, 1017–1018 (2011).
Arnold, M., Raffler, J., Pfeufer, A., Suhre, K. & Kastenmuller, G. SNiPA: an interactive, genetic variant-centered annotation browser. Bioinformatics 31, 1334–1336 (2015).
Wang, G., Sarkar, A., Carbonetto, P. & Stephens, M. A simple new approach to variable selection in regression, with application to genetic fine mapping. J. R. Stat. Soc. Ser. B 82, 1273–1300 (2020).
Abecasis, G. R. et al. A map of human genome variation from population-scale sequencing. Nature 467, 1061–1073 (2010).
Wang, D. et al. Comprehensive functional genomic resource and integrative model for the human brain. Science 362, eaat8464 (2018).
Gandal, M. J. et al. Transcriptome-wide isoform-level dysregulation in ASD, schizophrenia, and bipolar disorder. Science 362, eaat8127 (2018).
Vialle, R. A., de Paiva Lopes, K., Bennett, D. A., Crary, J. F. & Raj, T. Integrating whole-genome sequencing with multi-omic data reveals the impact of structural variants on gene regulation in the human brain. Nat. Neurosci. 25, 504–514 (2022).
Hemani, G. et al. The MR-Base platform supports systematic causal inference across the human phenome. eLife 7, e34408 (2018).
Mendez, D. et al. ChEMBL: towards direct deposition of bioassay data. Nucleic Acids Res. 47, D930–D940 (2019).
Yang, Z., Yang, J., Liu, D. & Yu, W. Mendelian randomization analysis identified genes pleiotropically associated with central corneal thickness. BMC Genomics 22, 517 (2021).
Wang, F. et al. Mendelian randomization analysis identified genes potentially pleiotropically associated with periodontitis. Saudi J. Biol. Sci. 28, 4089–4095 (2021).
Burgess, S., Butterworth, A. & Thompson, S. G. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet. Epidemiol. 37, 658–665 (2013).
Zhu, Z. et al. Integration of summary data from GWAS and eQTL studies predicts complex trait gene targets. Nat. Genet. 48, 481–487 (2016).
de Leeuw, C. A., Mooij, J. M., Heskes, T. & Posthuma, D. MAGMA: generalized gene-set analysis of GWAS data. PLoS Comput. Biol. 11, e1004219 (2015).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005).
Liu, J. et al. Genome-wide association study followed by trans-ancestry meta-analysis identify 17 new risk loci for schizophrenia. BMC Med. 19, 177 (2021).
Bryois, J. et al. Genetic identification of cell types underlying brain complex traits yields insights into the etiology of Parkinson’s disease. Nat. Genet. 52, 482–493 (2020).
Weeks, E. M. et al. Leveraging polygenic enrichments of gene features to predict genes underlying complex traits and diseases. Nat. Genet. https://doi.org/10.1038/s41588-023-01443-6 (2020).
Li, Y. et al. Regulatory variant rs2535629 in ITIH3 intron confers schizophrenia risk by regulating CTCF binding and SFMBT1 expression. Adv. Sci. 9, e2104786 (2022).
Wang, J. et al. Functional variant rs2270363 on 16p13.3 confers schizophrenia risk by regulating NMRAL1. Brain 145, 2569–2585 (2022).
Aurnhammer, C. et al. Universal real-time PCR for the detection and quantification of adeno-associated virus serotype 2-derived inverted terminal repeat sequences. Hum. Gene Ther. Methods 23, 18–28 (2011).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25, 402–408 (2001).
Dang, X. Genome-wide summary statistics from a cross-ancestry meta-analysis of depression. figshare https://doi.org/10.6084/m9.figshare.24521968.v1 (2025).
Dang, X. & Luo, X. Codes for ‘Cross-ancestry genome-wide association study and systems-level integrative analyses implicate new risk genes and therapeutic targets for depression’. Zenodo https://doi.org/10.5281/zenodo.13856052 (2024).
Acknowledgements
This study was equally supported by the startup funds from Southeast University (RF1028623032 to X.-J.L.) and the Special Project for Social Development of Yunnan Province (nos. 202203AC100007 and 202305AH340006). This study was also supported by the National Natural Science Foundation of China (nos. U2102205, 82271570, 82260276, 82301690, 82130042, 82471552, 82230046 and 81920108018), the Talent and Fundamental Research Project of Yunnan Province (nos. 202301AY070001-299, 202105AC160004 and 202301AT070037), the China Science and Technology Innovation 2030 – Major Project (nos. 2022ZD0211701 and 2021ZD0200700), and the SEU Innovation Capability Enhancement Plan for Doctoral Students (CXJH_SEU 25233). We thank Q. Li and Z. Ding for their technical assistance.
Author information
Authors and Affiliations
Contributions
X.-J.L. conceived, designed and supervised the whole study. X.D. performed all the analyses, including meta-analysis, TWAS, PWAS, colocalization, fine mapping, SMR and so on. Y.L., J.W. and S.L. conducted the behavioural tests. R.C. performed reporter gene assays. X.-J.L., Z.T., Y.Z., Y. Yue, B.L.M., Y.-G.Y., M.L., Z.L., Y. Yuan, T.L. and Z.Z. contributed to this work in study design, data interpretation, paper writing and revision. X.D. drafted the paper. X.-J.L. oversaw the project and finalized the paper. All authors revised the paper critically and approved the final version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Human Behaviour thanks Jonathan Coleman and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary methods and Figs. 1–18.
Supplementary Tables 1–23
Supplementary Tables 1–23.
Supplementary Data 1
Statistical source data for Supplementary Fig. 6.
Source data
Source Data Figs. 3, 4 and 8
Statistical source data for Figs. 3, 4 and 8.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Y., Dang, X., Chen, R. et al. Cross-ancestry genome-wide association study and systems-level integrative analyses implicate new risk genes and therapeutic targets for depression. Nat Hum Behav 9, 806–823 (2025). https://doi.org/10.1038/s41562-024-02073-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41562-024-02073-6
This article is cited by
-
Cross-ancestry genome-wide association study identifies new susceptibility genes for preeclampsia
BMC Pregnancy and Childbirth (2025)
-
Elevated NEGR1 in brain induces anxiety or depression-like phenotypes and synaptic dysfunction
Molecular Psychiatry (2025)