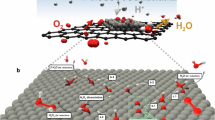
Abstract
Cathodic corrosion is an electrochemical phenomenon that etches metals at moderately negative potentials. Although cathodic corrosion probably occurs by forming a metal-containing anion, such intermediate species have not yet been observed. Here, aiming to resolve this long-standing debate, our work provides such evidence through X-ray absorption spectroscopy. High-energy-resolution X-ray absorption near-edge structure experiments are used to characterize platinum nanoparticles during cathodic corrosion in 10 mol l−1 NaOH. These experiments detect minute chemical changes in the Pt during corrosion that match first-principles simulations of X-ray absorption spectra of surface platinum multilayer hydrides. Thus, this work supports the existence of hydride-like platinum during cathodic corrosion. Notably, these results provide a direct observation of these species under conditions where they are highly unstable and where prominent hydrogen bubble formation interferes with most spectroscopy methods. Therefore, this work identifies the elusive intermediate that underlies cathodic corrosion.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
269,00 € per year
only 22,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data used for this study are available within the Article and its Supplementary Information. Source data are provided with this paper. The data files are also available from the corresponding authors upon request.
References
Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solutions (National Association of Corrosion Engineers, 1974).
Jerkiewicz, G. Applicability of platinum as a counter-electrode material in electrocatalysis research. ACS Catal. 12, 2661–2670 (2022).
Haber, F. The phenomenon of the formation of metallic dust from cathodes. Trans. Am. Electrochem. Soc. 2, 189–196 (1902).
Yanson, A. I. et al. Cathodic corrosion: a quick, clean, and versatile method for the synthesis of metallic nanoparticles. Angew. Chem. Int. Ed. 50, 6346–6350 (2011).
Arulmozhi, N., Hersbach, T. J. P. & Koper, M. T. M. Nanoscale morphological evolution of monocrystalline Pt surfaces during cathodic corrosion. Proc. Natl Acad. Sci. USA 117, 32267–32277 (2020).
Elnagar, M. M., Hermann, J. M., Jacob, T. & Kibler, L. A. An affordable option to Au single crystals through cathodic corrosion of a wire: fabrication, electrochemical behavior, and applications in electrocatalysis and spectroscopy. Electrochim. Acta 372, 137867 (2021).
Yang, Y. et al. Elucidating cathodic corrosion mechanisms with operando electrochemical transmission electron microscopy. J. Am. Chem. Soc. 144, 15698–15708 (2022).
Hersbach, T. J. P. et al. Alkali metal cation effects in structuring Pt, Rh, and Au surfaces through cathodic corrosion. ACS Appl. Mater. Interfaces 10, 39363–39379 (2018).
Bennett, E. et al. A synthetic route for the effective preparation of metal alloy nanoparticles and their use as active electrocatalysts. ACS Catal. 6, 1533–1539 (2016).
Lawrence, M. J., Kolodziej, A. & Rodriguez, P. Controllable synthesis of nanostructured metal oxide and oxyhydroxide materials via electrochemical methods. Curr. Opin. Electrochem. 10, 7–15 (2018).
Yanson, Y. I. & Yanson, A. I. Cathodic corrosion. I. Mechanism of corrosion via formation of metal anions in aqueous medium. Low. Temp. Phys. 39, 304–311 (2013).
Hersbach, T. J. P. & Koper, M. T. M. Cathodic corrosion: 21st century insights into a 19th century phenomenon. Curr. Opin. Electrochem. 26, 100653 (2021).
Friebel, D. et al. Balance of nanostructure and bimetallic interactions in Pt model fuel cell catalysts: in situ XAS and DFT study. J. Am. Chem. Soc. 134, 9664–9671 (2012).
Merte, L. R. et al. Electrochemical oxidation of size-selected Pt nanoparticles studied using in situ high-energy-resolution X-ray absorption spectroscopy. ACS Catal. 2, 2371–2376 (2012).
Teliska, M., O’Grady, W. E. & Ramaker, D. E. Determination of H adsorption sites on Pt/C electrodes in HClO4 from Pt L23 X-ray absorption spectroscopy. J. Phys. Chem. B 108, 2333–2344 (2004).
Hersbach, T. J. P. et al. Base-accelerated degradation of nanosized platinum electrocatalysts. ACS Catal. 11, 9904–9915 (2021).
Bronger, W., Müller, P., Schmitz, D. & Spittank, H. Synthese und Struktur von Na2PtH4, einem ternären Hydrid mit quadratisch planaren PtH42− Baugruppen. Z. Anorg. Allg. Chem. 516, 35–41 (1984).
Bronger, W. & Auffermann, G. High pressure synthesis and structure of Na2PtH6. J. Alloy. Compd. 219, 45–47 (1995).
Karpov, A., Nuss, J., Wedig, U. & Jansen, M. Cs2Pt: a platinide(-II) exhibiting complete charge separation. Angew. Chem. Int. Ed. 42, 4818–4821 (2003).
Arrieta, R. & Brgoch, J. Forming platinide phases under pressure in the Cs–Pt system. J. Phys. Chem. C 126, 2062–2069 (2022).
Teherani, T. H., Peer, W. J., Lagowski, J. J. & Bard, A. J. Electrochemical behavior and standard potential of gold(1−) ion in liquid ammonia. J. Am. Chem. Soc. 100, 7768–7770 (1978).
Tran, N. E. & Lagowski, J. J. Metal ammonia solutions: solutions containing argentide ions. Inorg. Chem. 40, 1067–1068 (2001).
Firman, T. K. & Landis, C. R. Structure and electron counting in ternary transition metal hydrides. J. Am. Chem. Soc. 120, 12650–12656 (1998).
Elnagar, M. M., Jacob, T. & Kibler, L. A. Cathodic corrosion of Au in aqueous methanolic alkali metal hydroxide electrolytes: notable role of water. Electrochem. Sci. Adv. 2, 1–11 (2022).
Hanselman, S., Calle-Vallejo, F. & Koper, M. T. M. Computational description of surface hydride phases on Pt(111) electrodes. J. Chem. Phys. 158, 014703 (2023).
Evazzade, I., Zagalskaya, A. & Alexandrov, V. Revealing elusive intermediates of platinum cathodic corrosion through DFT simulations. J. Phys. Chem. Lett. 13, 3047–3052 (2022).
Bratsch, S. & Lagowski, J. Predicted stabilities of monatomic anions in water and liquid ammonia at 298.15 K. Polyhedron 5, 1763–1770 (1986).
Hersbach, T. J. P., Yanson, A. I. & Koper, M. T. M. Anisotropic etching of platinum electrodes at the onset of cathodic corrosion. Nat. Commun. 7, 12653 (2016).
Hersbach, T. J. P., Mints, V. A., Calle-Vallejo, F., Yanson, A. I. & Koper, M. T. M. Anisotropic etching of rhodium and gold as the onset of nanoparticle formation by cathodic corrosion. Faraday Discuss. 193, 207–222 (2016).
Pantelouris, A., Kueper, G., Hormes, J., Feldmann, C. & Jansen, M. Anionic gold in Cs3AuO and Rb3AuO Established by X-ray absorption spectroscopy. J. Am. Chem. Soc. 117, 11749–11753 (1995).
Vinson, J. Advances in the OCEAN-3 spectroscopy package. Phys. Chem. Chem. Phys. 24, 12787–12803 (2022).
Kirklin, S. et al. The Open Quantum Materials Database (OQMD): assessing the accuracy of DFT formation energies. NPJ Comput. Mater. 1, 15010 (2015).
Ghebouli, M. A. et al. Theoretical prediction of the fundamental properties for the ternary Li2PtH6 and Na2PtH6. J. Solid State Chem. 196, 498–503 (2012).
Kunimatsu, K., Senzaki, T., Samjeské, G., Tsushima, M. & Osawa, M. Hydrogen adsorption and hydrogen evolution reaction on a polycrystalline Pt electrode studied by surface-enhanced infrared absorption spectroscopy. Electrochim. Acta 52, 5715–5724 (2007).
Barber, J., Morin, S. & Conway, B. E. Specificity of the kinetics of H2 evolution to the structure of single-crystal Pt surfaces, and the relation between opd and upd H. J. Electroanal. Chem. 446, 125–138 (1998).
Tan, T. L., Wang, L.-L., Johnson, D. D. & Bai, K. Hydrogen deposition on Pt(111) during electrochemical hydrogen evolution from a first-principles multiadsorption-site study. J. Phys. Chem. C 117, 22696–22704 (2013).
Tan, T. L., Wang, L.-L., Zhang, J., Johnson, D. D. & Bai, K. Platinum nanoparticle during electrochemical hydrogen evolution: adsorbate distribution, active reaction species, and size effect. ACS Catal. 5, 2376–2383 (2015).
McCrum, I. T., Bondue, C. J. & Koper, M. T. M. Hydrogen-induced step-edge roughening of platinum electrode surfaces. J. Phys. Chem. Lett. 10, 6842–6849 (2019).
Ramaker, D. E. & Koningsberger, D. C. The atomic AXAFS and Δμ XANES techniques as applied to heterogeneous catalysis and electrocatalysis. Phys. Chem. Chem. Phys. 12, 5514 (2010).
Jerkiewicz, G. Hydrogen sorption at/in electrodes. Prog. Surf. Sci. 57, 137–186 (1998).
Stoerzinger, K. A. et al. Probing the surface of platinum during the hydrogen evolution reaction in alkaline electrolyte. J. Phys. Chem. B 122, 864–870 (2018).
Chen, X., McCrum, I. T., Schwarz, K. A., Janik, M. J. & Koper, M. T. M. Co-adsorption of cations as the cause of the apparent pH dependence of hydrogen adsorption on a stepped platinum single-crystal electrode. Angew. Chem. Int. Ed. 56, 15025–15029 (2017).
Rizo, R. et al. Investigating the presence of adsorbed species on Pt steps at low potentials. Nat. Commun. 13, 2550 (2022).
Monteiro, M. C. O. et al. Absence of CO2 electroreduction on copper, gold and silver electrodes without metal cations in solution. Nat. Catal. 4, 654–662 (2021).
Osawa, M., Tsushima, M., Mogami, H., Samjeské, G. & Yamakata, A. Structure of water at the electrified platinum−water interface: a study by surface-enhanced infrared absorption spectroscopy. J. Phys. Chem. C 112, 4248–4256 (2008).
Feng, J. et al. Cathodic corrosion of a bulk wire to nonaggregated functional nanocrystals and nanoalloys. ACS Appl. Mater. Interfaces 10, 9532–9540 (2018).
Momma, K. & Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 44, 1272–1276 (2011).
Sokaras, D. et al. A seven-crystal Johann-type hard X-ray spectrometer at the Stanford Synchrotron Radiation Lightsource. Rev. Sci. Instrum. 84, 053102 (2013).
Ravel, B. & Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 12, 537–541 (2005).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Swanson, H. E. & Tatge, E. Standard X-Ray Diffraction Powder Patterns (US Government Printing Office, 1953).
McBride, J. R., Graham, G. W., Peters, C. R. & Weber, W. H. Growth and characterization of reactively sputtered thin‐film platinum oxides. J. Appl. Phys. 69, 1596–1604 (1991).
van Setten, M. J. et al. The PSEUDODOJO: training and grading a 85 element optimized norm-conserving pseudopotential table. Comput. Phys. Commun. 226, 39–54 (2018).
Hamann, D. R. Optimized norm-conserving Vanderbilt pseudopotentials. Phys. Rev. B 88, 1–10 (2013).
Vinson, J. & Shirley, E. L. Fast, efficient, and accurate dielectric screening using a local real-space approach. Phys. Rev. B 103, 1–24 (2021).
Acknowledgements
We acknowledge M.-Y. Wu from MYAnalysis for assisting with transmission electron microscopy measurements. Certain equipment, instruments, software or materials, commercial or non-commercial, are identified in this Article to specify the experimental procedure adequately. Such identification is not intended to imply recommendation or endorsement of any product or service by NIST, nor is it intended to imply that the materials or equipment identified are necessarily the best available for the purpose. Use of the Stanford Synchrotron Radiation Lightsource, SLAC National Accelerator Laboratory, is supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences under contract no. DE-AC02-76SF00515. This work was supported in part by the Netherlands Organization for Scientific Research (NWO) in the framework of the Solar Fuels Graduate Program. The use of supercomputing facilities at SURFsara was sponsored by NWO Physical Sciences, with financial support by NWO. OCEAN simulations were performed using resources of the National Energy Research Scientific Computing Center (NERSC), a US Department of Energy Office of Science User Facility operated under contract no. DE-AC02-05CH11231.
Author information
Authors and Affiliations
Contributions
T.J.P.H., D.S. and M.T.M.K. designed the experimental study. T.J.P.H. conducted and coordinated most of the experimental work, data analysis and data reduction. A.T.G.-E., O.A.P.M. and J.V. performed the OCEAN simulations activities. S.H. and I.T.M. generated the platinum surfaces for first-principles calculations. A.T.G.-E., D.A., J.T.F., T.F.J., A.C.G., P.K., T.K. and D.S. participated in the experimental work. T.H. designed the operando grazing incidence cell. T.J.P.H. led the drafting of the manuscript, with contributions from A.T.G.-E., S.H., J.V., D.S. and M.T.M.K. for editing. D.S. and M.T.M.K. supervised the work and obtained funding and resources. All authors discussed the results and the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Materials thanks Marcel Risch and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–20, discussion and Table 1.
Supplementary Data 1
DFT coordinates used as OCEAN input structure files for Fig. 1a,c.
Supplementary Data 2
DFT coordinates used as OCEAN input structure files for Fig. 1b,d and Supplementary Figs. 8 and 12.
Supplementary Data 3
DFT coordinates used as OCEAN input structure files for Supplementary Fig. 10.
Source data
Source Data Fig. 1
OCEAN spectra and difference spectra for hydrogen-covered platinum surfaces and bulk platinides.
Source Data Fig. 2
Experimental HERFD-XANES spectra, smoothed experimental HERFD-XANES spectra and unsmoothed experimental HERFD-XANES spectra.
Source Data Fig. 3
Smoothed experimental HERFD-XANES difference spectra and rescaled OCEAN difference spectra for hydrogen-covered platinum surfaces and bulk platinides.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hersbach, T.J.P., Garcia-Esparza, A.T., Hanselman, S. et al. Platinum hydride formation during cathodic corrosion in aqueous solutions. Nat. Mater. 24, 574–580 (2025). https://doi.org/10.1038/s41563-024-02080-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-024-02080-y