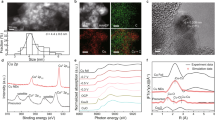
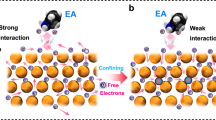
Abstract
Precious metals such as Pt are favoured as catalysts for the hydrogen evolution reaction (HER) due to their excellent catalytic activity. However, the scarcity and high cost of precious metals have prompted researchers to explore cheaper alternatives such as Cu. Nevertheless, Cu shows poor catalytic performance due to weak binding with intermediates. Here the catalytic activity of pure Cu is activated via electroreduction-driven modification of the local structure, achieving a HER catalytic performance superior to commercial Pt/C catalysts for working current densities greater than 100 mA cm−2 in acid electrolyte. Activation involved two steps. First, polycrystalline Cu2O nanoparticles were prepared via pulsed laser ablation, resulting in grain boundaries within the Cu2O particles as observed using electron microscopy. Next, the Cu2O particles were electroreduced to pure Cu, inducing the formation of distorted nanotwins and edge dislocations. These local structures induce high lattice strain and decrease the Cu coordination number, enhancing the interaction between Cu and intermediates—as calculated using density functional theory—leading to the excellent catalytic activity and durability of the catalyst. Our observations show that low-cost pure Cu can be a promising HER catalyst for large-scale industrial applications.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
269,00 € per year
only 22,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data that support the findings of this work are available within the Article and its Supplementary Information. Source data are provided with this paper.
References
Lin, C. et al. In-situ reconstructed Ru atom array on α-MnO2 with enhanced performance for acidic water oxidation. Nat. Catal. 4, 1012–1023 (2021).
Clark, D. et al. Single-step hydrogen production from NH3, CH4, and biogas in stacked proton ceramic reactors. Science 376, 390–393 (2022).
Pang, B. et al. Laser-assisted high-performance PtRu alloy for pH-universal hydrogen evolution. Energy Environ. Sci. 15, 102–108 (2022).
Jiao, Y., Zheng, Y., Jaroniec, M. & Qiao, S. Z. Design of electrocatalysts for oxygen- and hydrogen-involving energy conversion reactions. Chem. Soc. Rev. 44, 2060–2086 (2015).
Rong, C. et al. Electronic structure engineering of single-atom Ru sites via Co–N4 sites for bifunctional pH-universal water splitting. Adv. Mater. 34, 2110103 (2022).
Luo, M. & Guo, S. Strain-controlled electrocatalysis on multimetallic nanomaterials. Nat. Rev. Mater. 2, 17059 (2017).
He, T. et al. Mastering the surface strain of platinum catalysts for efficient electrocatalysis. Nature 598, 76–81 (2021).
Fan, J. et al. Spatially confined PdHx metallenes by tensile strained atomic Ru layers for efficient hydrogen evolution. J. Am. Chem. Soc. 145, 5710–5717 (2023).
Calle-Vallejo, F. et al. Finding optimal surface sites on heterogeneous catalysts by counting nearest neighbors. Science 350, 185–189 (2015).
Wang, L. Boosting activity and stability of metal single-atom catalysts via regulation of coordination number and local composition. J. Am. Chem. Soc. 143, 18854–18858 (2021).
Lei, Z. et al. Coordination modulation of iridium single-atom catalyst maximizing water oxidation activity. Nat. Commun. 13, 24 (2022).
Wu, G. et al. In-plane strain engineering in ultrathin noble metal nanosheets boosts the intrinsic electrocatalytic hydrogen evolution activity. Nat. Commun. 13, 4200 (2022).
Atlan, C. et al. Imaging the strain evolution of a platinum nanoparticle under electrochemical control. Nat. Mater. 22, 754–761 (2023).
Zhao, J. et al. Exploring the strain effect in single particle electrochemistry using Pd nanocrystals. Angew. Chem. Int. Ed. 62, e202304424 (2023).
Huang, D. et al. Conflicting roles of coordination number on catalytic performance of single-atom Pt catalysts. ACS Catal. 11, 5586–5592 (2021).
He, Y. et al. Amorphizing noble metal chalcogenide catalysts at the single-layer limit towards hydrogen production. Nat. Catal. 5, 212–221 (2022).
Li, A. et al. Enhancing the stability of cobalt spinel oxide towards sustainable oxygen evolution in acid. Nat. Catal. 5, 109–118 (2022).
Li, Z. et al. A silver catalyst activated by stacking faults for the hydrogen evolution reaction. Nat. Catal. 2, 1107–1114 (2019).
Kim, S. J. et al. Flat-surface-assisted and self-regulated oxidation resistance of Cu(111). Nature 603, 434–438 (2022).
Chung, K. et al. Non-oxidized bare copper nanoparticles with surface excess electrons in air. Nat. Nanotechnol. 17, 285–291 (2022).
Han, Z. et al. Steering surface reconstruction of copper with electrolyte additives for CO2 electroreduction. Nat. Commun. 13, 3158 (2022).
Stewart, I. E., Ye, S., Chen, Z., Flowers, P. F. & Wiley, B. J. Synthesis of Cu–Ag, Cu–Au, and Cu–Pt core–shell nanowires and their use in transparent conducting films. Chem. Mater. 27, 7788–7794 (2015).
Zhao, J. et al. Achieving high electrocatalytic efficiency on copper: a low-cost alternative to platinum for hydrogen generation in water. ACS Catal. 5, 4115–4120 (2015).
Yu, L. et al. Cu nanowires shelled with NiFe layered double hydroxide nanosheets as bifunctional electrocatalysts for overall water splitting. Energy Environ. Sci. 10, 1820–1827 (2017).
Kang, W. J. et al. Strain-activated copper catalyst for pH-universal hydrogen evolution reaction. Adv. Func. Mater. 32, 2112367 (2022).
Edalati, K. & Horita, Z. J. High-pressure torsion of pure metals: influence of atomic bond parameters and stacking fault energy on grain size and correlation with hardness. Acta Mater. 59, 6831–6836 (2011).
Lu, L., Sui, M. L. & Lu, K. Superplastic extensibility of nanocrystalline copper at room temperature. Science 287, 1463–1465 (2000).
Lu, L., Shen, Y., Chen, X., Qian, L. & Lu, K. Ultrahigh strength and high electrical conductivity in copper. Science 304, 422–426 (2004).
Lu, L., Chen, X., Huang, X. & Lu, K. Revealing the maximum strength in nanotwinned copper. Science 323, 607–610 (2009).
Lu, K., Lu, L. & Suresh, S. Strengthening materials by engineering coherent internal boundaries at the nanoscale. Science 324, 349–352 (2009).
Fernández-Arias, M. et al. Copper nanoparticles obtained by laser ablation in liquids as bactericidal agent for dental applications. Appl. Surf. Sci. 507, 145032 (2020).
Huang, C.-L. et al. Twinning enhances efficiencies of metallic catalysts toward electrolytic water splitting. Adv. Energy Mater. 11, 2101827 (2021).
Song, M. et al. Oriented attachment induces fivefold twins by forming and decomposing high-energy grain boundaries. Science 367, 40–45 (2020).
Johnson, C. L. et al. Effects of elastic anisotropy on strain distributions in decahedral gold nanoparticles. Nat. Mater. 7, 120–124 (2008).
Sun, X. et al. Dislocation-induced stop-and-go kinetics of interfacial transformations. Nature 607, 708–713 (2022).
LaGrow, A. P., Ward, M. R., Lloyd, D. C., Gai, P. L. & Boyes, E. D. Visualizing the Cu/Cu2O interface transition in nanoparticles with environmental scanning transmission electron microscopy. J. Am. Chem. Soc. 139, 179–185 (2017).
Zou, L., Li, J., Zakharov, D., Stach, E. A. & Zhou, G. In situ atomic-scale imaging of the metal/oxide interfacial transformation. Nat. Commun. 8, 307 (2017).
Miao, J., Ercius, P. & Billinge, S. J. L. Atomic electron tomography: 3D structures without crystals. Science 353, aaf2157 (2016).
Zhou, J. et al. Observing crystal nucleation in four dimensions using atomic electron tomography. Nature 570, 500–503 (2019).
Li, Z. et al. Probing the atomically diffuse interfaces in Pd@Pt core–shell nanoparticles in three dimensions. Nat. Commun. 14, 2934 (2023).
Yang, J. et al. Ultrahigh-current-density niobium disulfide catalysts for hydrogen evolution. Nat. Mater. 18, 1309–1314 (2019).
Lagadec, M. F. & Grimaud, A. Water electrolysers with closed and open electrochemical systems. Nat. Mater. 19, 1140–1150 (2020).
Jung, H. et al. Electrochemical fragmentation of Cu2O nanoparticles enhancing selective C–C coupling from CO2 reduction reaction. J. Am. Chem. Soc. 141, 4624–4633 (2019).
Deng, B. et al. The crystal plane is not the key factor for CO2-to-methane electrosynthesis on reconstructed Cu2O microparticles. Angew. Chem. Int. Ed. 61, e202114080 (2022).
Liu, C. et al. Nanoconfinement engineering over hollow multi-shell structured copper towards efficient electrocatalytical C–C coupling. Angew. Chem. Int. Ed. 61, e202113498 (2022).
Wu, Z.-Z. et al. Identification of Cu(100)/Cu(111) interfaces as superior active sites for CO dimerization during CO2 electroreduction. J. Am. Chem. Soc. 144, 259–269 (2022).
Zhu, Q. et al. Hierarchical twinning governed by defective twin boundary in metallic materials. Sci. Adv. 8, eabn8299 (2022).
Wang, J. et al. Small and well-dispersed Cu nanoparticles on carbon nanofibers: self-supported electrode materials for efficient hydrogen evolution reaction. Int. J. Hydrogen Energy 41, 18044–18049 (2016).
Javan, H., Asghari, E., Ashassi-Sorkhabi, H. & Moradi-Haghighi, M. A low-cost platinum-free electrocatalyst based on carbon quantum dots decorated Ni–Cu hierarchical nanocomposites for hydrogen evolution reaction. Int. J. Hydrogen Energy 45, 19324–19334 (2020).
Yao, M. et al. Rational design of self-supported Cu@WC core–shell mesoporous nanowires for pH-universal hydrogen evolution reaction. Appl. Catal. B 280, 656–661 (2019).
Tiwari, A. P., Kim, D., Kim, Y., Prakash, O. & Lee, H. Highly active and stable layered ternary transition metal chalcogenide for hydrogen evolution reaction. Nano Energy 28, 366–372 (2016).
Xue, Y. et al. Self-catalyzed growth of Cu@graphdiyne core–shell nanowires array for high efficient hydrogen evolution cathode. Nano Energy 30, 858–866 (2016).
Li, F. et al. Synthesis of Cu–MoS2/rGO hybrid as non-noble metal electrocatalysts for the hydrogen evolution reaction. J. Power Sources 292, 15–22 (2015).
Cao, M. et al. Facile electrodeposition of Ni–Cu–P dendrite nanotube films with enhanced hydrogen evolution reaction activity and durability. ACS Appl. Mater. Interfaces 10, 35224–35233 (2018).
Kuang, M., Wang, Q., Han, P. & Zheng, G. Cu, Co-embedded N-enriched mesoporous carbon for efficient oxygen reduction and hydrogen evolution reactions. Adv. Energy Mater. 7, 1700193 (2017).
Shen, Y. et al. Nickel–copper alloy encapsulated in graphitic carbon shells as electrocatalysts for hydrogen evolution reaction. Adv. Energy Mater. 8, 1701759 (2018).
Chao, T. et al. Atomically dispersed copper–platinum dual sites alloyed with palladium nanorings catalyze the hydrogen evolution reaction. Angew. Chem. Int. Ed. 56, 16047–16051 (2017).
Yao, Q. et al. Channel-rich RuCu nanosheets for pH-universal overall water splitting electrocatalysis. Angew. Chem. Int. Ed. 58, 13983–13988 (2019).
Liu, Y. et al. A general route to prepare low-ruthenium-content bimetallic electrocatalysts for pH-universal hydrogen evolution reaction by using carbon quantum dots. Angew. Chem. Int. Ed. 59, 1718–1726 (2020).
Li, J., Li, F., Guo, S.-X., Zhang, J. & Ma, J. PdCu@Pd nanocube with Pt-like activity for hydrogen evolution reaction. ACS Appl. Mater. Interfaces 9, 8151–8160 (2017).
Manikandan, A. et al. Graphene-coated copper nanowire networks as a highly stable transparent electrode in harsh environments toward efficient electrocatalytic hydrogen evolution reactions. J. Mater. Chem. A 5, 13320–13328 (2017).
Hou, M. et al. Coralline-like CoP3@Cu as an efficient electrocatalyst for the hydrogen evolution reaction in acidic and alkaline solutions. New J. Chem. 44, 18601–18607 (2020).
Ji, L. et al. One-pot synthesis of porous 1T-phase MoS2 integrated with single-atom Cu doping for enhancing electrocatalytic hydrogen evolution reaction. Appl. Catal. B 251, 87–93 (2019).
Feng, Y. et al. Mechanically processing copper plate into active catalyst for electrochemical hydrogen production. Acta Mater. 237, 118164 (2022).
Ji, L.-P. et al. Epitaxial growth of high-energy copper facets for promoting hydrogen evolution reaction. Small 18, 2107481 (2022).
Ruan, Y.-C. et al. Exposing Cu(100) surface via ion-implantation-induced oxidization and etching for promoting hydrogen evolution reaction. Langmuir 38, 2993–2999 (2022).
Ravel, B. & Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 12, 537–541 (2005).
Yang, Y. et al. Determining the three-dimensional atomic structure of an amorphous solid. Nature 592, 60–64 (2021).
Xu, R. et al. Three-dimensional coordinates of individual atoms in materials revealed by electron tomography. Nat. Mater. 14, 1099–1103 (2015).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Acknowledgements
F.F. gratefully acknowledges funding support from the National Key R&D Program of China (2020YFA0406204), the National Natural Science Foundation of China (no. 52071083) and the Zhuhai Fudan Innovation Institute. Z.L. gratefully acknowledges funding support from the National Natural Science Foundation of China (no. 52401292), the Postdoctoral Innovation Talents Support Program of China (BX2021066) and the China Postdoctoral Science Foundation (no. 2021M700024). Y. Lu gratefully acknowledges funding support from the National Natural Science Foundation of China (nos. 12074016 and 12274009), the Beijing Natural Science Foundation for Outstanding Youth Science Foundation (JQ24009) and the Research and Development Project from the Shanxi-Zheda Institute of Advanced Materials and Chemical Engineering (2022SX-TD001). J.Z. gratefully acknowledges funding support from the National Natural Science Foundation of China (no. 22172003) and strong support from the Electron Microscopy Laboratory at Peking University for the use of aberration-corrected electron microscopes and the High-performance Computing Platform of Peking University. We thank R. Che from Fudan University for charge distribution testing.
Author information
Authors and Affiliations
Contributions
D.S. and F.F. conceived the experiments and supervised the project. Z.L. and Y.F. designed and conducted the experiments. Z.L., H.L., Y. Liu, Y.S. and F.W. performed the experimental data analysis. Z.L., Y. Lu and F.F. wrote the paper. Y.W., Y. Lu and M.S. performed atomic-scale TEM measurements and analysed the data. J.Z. and Z.X. performed the atomic-resolution electron tomography experiments and 3D structural analysis. X.D., M.S. and D.S. contributed the experimental platform. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Materials thanks Mingshang Jin and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–49, Tables 1–3 and Refs. 1–27.
Supplementary Data 1
The atomic coordinates of computational models.
Source data
Source Data Fig. 1
XAS data plotted in Fig. 1e,f.
Source Data Fig. 2
Atomic distance data plotted in Fig. 2c.
Source Data Fig. 3
Radial distribution function and statistical data plotted in Fig. 3d,k.
Source Data Fig. 4
Electrochemical data plotted in Fig. 4a–h.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Z., Wang, Y., Liu, H. et al. Electroreduction-driven distorted nanotwins activate pure Cu for efficient hydrogen evolution. Nat. Mater. 24, 424–432 (2025). https://doi.org/10.1038/s41563-024-02098-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-024-02098-2