Abstract
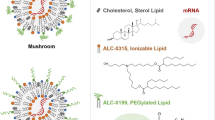
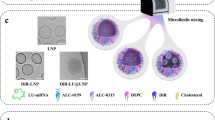
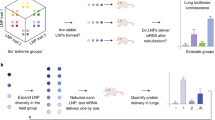
Messenger RNA (mRNA) therapeutics are a promising strategy to combat diverse diseases. Traditional lipid nanoparticle (LNP) formulations for mRNA delivery contain poly(ethylene) glycol (PEG), a polymer widely used in drug delivery carriers but that recently has been associated with efficacy and immunogenicity concerns. Here we report poly(carboxybetaine) (PCB) lipids as surrogates for PEG-lipids used in mRNA formulations. In vitro studies with immortalized and primary cells show that PCB-containing LNPs have higher mRNA transfection efficiency than PEG-containing LNPs across different formulations. Moreover, primary cell engineering and in vivo immunization studies in mice further demonstrate greater therapeutic efficacy of PCB-containing LNPs over their PEG counterparts. Mechanistic assays show that this improvement is attributed to enhanced endosomal escape of PCB-containing LNPs. These formulations exhibit a safe immunotoxicity profile and effectively mitigate the accelerated blood clearance effect that has been observed for PEG-containing LNPs, enabling repeated administrations without efficacy loss. Overall, these findings highlight PCB-containing LNPs as a potent and safe mRNA delivery platform for clinical applications.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
269,00 € per year
only 22,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data in this study are available within the Article and its Supplementary Information. The raw and analysed datasets generated during the study are available for research purposes from the corresponding author on request. Source data are provided with this paper.
References
Cullis, P. R. & Hope, M. J. Lipid nanoparticle systems for enabling gene therapies. Mol. Ther. 25, 1467–1475 (2017).
Mullard, A. COVID-19 vaccine success enables a bolder vision for mRNA cancer vaccines, says BioNTech CEO. Nat. Rev. Drug Discov. 20, 500–501 (2021).
Hou, X., Zaks, T., Langer, R. & Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 6, 1078–1094 (2021).
Akinc, A. et al. The Onpattro story and the clinical translation of nanomedicines containing nucleic acid-based drugs. Nat. Nanotechnol. 14, 1084–1087 (2019).
Hassett, K. J. et al. Optimization of lipid nanoparticles for intramuscular administration of mRNA vaccines. Mol. Ther. Nucleic Acids 15, 1–11 (2019).
Schoenmaker, L. et al. mRNA-lipid nanoparticle COVID-19 vaccines: structure and stability. Int. J. Pharm. 601, 120586 (2021).
Zhang, P., Sun, F., Liu, S. & Jiang, S. Anti-PEG antibodies in the clinic: current issues and beyond PEGylation. J. Control. Release 244, 184–193 (2016).
Kumar, V. et al. Shielding of lipid nanoparticles for siRNA delivery: impact on physicochemical properties, cytokine induction, and efficacy. Mol. Ther. Nucleic Acids 3, e210 (2014).
Yang, Q. et al. Analysis of pre-existing IgG and IgM antibodies against polyethylene glycol (PEG) in the general population. Anal. Chem. 88, 11804–11812 (2016).
Besin, G. et al. Accelerated blood clearance of lipid nanoparticles entails a biphasic humoral response of B-1 followed by B-2 lymphocytes to distinct antigenic moieties. Immunohorizons 3, 282–293 (2019).
Sanchez, A. et al. Substituting poly(ethylene glycol) lipids with poly(2-ethyl-2-oxazoline) lipids improves lipid nanoparticle repeat dosing. Adv. Health. Mater. 13, e2304033 (2024).
Ju, Y. et al. Anti-PEG antibodies boosted in humans by SARS-CoV-2 lipid nanoparticle mRNA vaccine. ACS Nano 16, 11769–11780 (2022).
Vrieze, J. Suspicions grow that nanoparticles in Pfizer’s COVID-19 vaccine trigger rare allergic reactions. Science https://www.science.org/content/article/suspicions-grow-nanoparticles-pfizer-s-covid-19-vaccine-trigger-rare-allergic-reactions (21 December 2020).
Kang, D. D. et al. Engineering LNPs with polysarcosine lipids for mRNA delivery. Bioact. Mater. 37, 86–93 (2024).
Nogueira, S. S. et al. Polysarcosine-functionalized lipid nanoparticles for therapeutic mRNA delivery. ACS Appl. Nano Mater. 3, 10634–10645 (2020).
Cao, Z., Zhang, L. & Jiang, S. Superhydrophilic zwitterionic polymers stabilize liposomes. Langmuir 28, 11625–11632 (2012).
Li, Y. et al. Enhanced endosomal/lysosomal escape by distearoyl phosphoethanolamine-polycarboxybetaine lipid for systemic delivery of siRNA. J. Control. Release 176, 104–114 (2014).
Li, Q. et al. Zwitterionic biomaterials. Chem. Rev. 122, 17073–17154 (2022).
Li, B. et al. Zwitterionic nanocages overcome the efficacy loss of biologic drugs. Adv. Mater. 30, e1705728 (2018).
Keefe, A. J. & Jiang, S. Poly(zwitterionic)protein conjugates offer increased stability without sacrificing binding affinity or bioactivity. Nat. Chem. 4, 59–63 (2011).
Li, B. et al. Revealing the immunogenic risk of polymers. Angew. Chem. Int. Ed. 57, 13873–13876 (2018).
Wang, X. et al. Preparation of selective organ-targeting (SORT) lipid nanoparticles (LNPs) using multiple technical methods for tissue-specific mRNA delivery. Nat. Protoc. 18, 265–291 (2023).
Dong, Y. et al. Lipopeptide nanoparticles for potent and selective siRNA delivery in rodents and nonhuman primates. Proc. Natl Acad. Sci. USA 111, 3955–3960 (2014).
Kumar, A. R. K., Shou, Y., Chan, B., L, K. & Tay, A. Materials for improving immune cell transfection. Adv. Mater. 33, e2007421 (2021).
Patel, S. et al. Beyond CAR T cells: other cell-based immunotherapeutic strategies against cancer. Front. Oncol. 9, 196 (2019).
Weber, E. W., Maus, M. V. & Mackall, C. L. The emerging landscape of immune cell therapies. Cell 181, 46–62 (2020).
Jahan, F. et al. Using the Jurkat reporter T cell line for evaluating the functionality of novel chimeric antigen receptors. Front. Mol. Med. 3, 1070384 (2023).
Chang, Y. et al. CAR-neutrophil mediated delivery of tumor-microenvironment responsive nanodrugs for glioblastoma chemo-immunotherapy. Nat. Commun. 14, 2266 (2023).
Ren, J. et al. Multiplex genome editing to generate universal CAR T cells resistant to PD1 inhibition. Clin. Cancer Res. 23, 2255–2266 (2017).
Barbier, A. J., Jiang, A. Y., Zhang, P., Wooster, R. & Anderson, D. G. The clinical progress of mRNA vaccines and immunotherapies. Nat. Biotechnol. 40, 840–854 (2022).
Kreiter, S. et al. Increased antigen presentation efficiency by coupling antigens to MHC class I trafficking signals. J. Immunol. 180, 309–318 (2008).
Yanez Arteta, M. et al. Successful reprogramming of cellular protein production through mRNA delivered by functionalized lipid nanoparticles. Proc. Natl Acad. Sci. USA 115, E3351–E3360 (2018).
Aburai, K., Hatanaka, K., Takano, S., Fujii, S. & Sakurai, K. Characterizing an siRNA-containing lipid-nanoparticle prepared by a microfluidic reactor: small-angle X-ray scattering and cryotransmission electron microscopic studies. Langmuir 36, 12545–12554 (2020).
Patel, S. et al. Naturally-occurring cholesterol analogues in lipid nanoparticles induce polymorphic shape and enhance intracellular delivery of mRNA. Nat. Commun. 11, 983 (2020).
Cornebise, M. et al. Discovery of a novel amino lipid that improves lipid nanoparticle performance through specific interactions with mRNA. Adv. Funct. Mater. 32, 2106727 (2022).
Leung, A. K. et al. Lipid nanoparticles containing siRNA synthesized by microfluidic mixing exhibit an electron-dense nanostructured core. J. Phys. Chem. C 116, 18440–18450 (2012).
Cheng, M. H. Y. et al. Induction of bleb structures in lipid nanoparticle formulations of mRNA leads to improved transfection potency. Adv. Mater. 35, e2303370 (2023).
Dammes, N. et al. Conformation-sensitive targeting of lipid nanoparticles for RNA therapeutics. Nat. Nanotechnol. 16, 1030–1038 (2021).
Sedic, M. et al. Safety evaluation of lipid nanoparticle-formulated modified mRNA in the Sprague-Dawley rat and cynomolgus monkey. Vet. Pathol. 55, 341–354 (2018).
Vlatkovic, I. Non-immunotherapy application of LNP-mRNA: maximizing efficacy and safety. Biomedicines 9, 530 (2021).
Liang, F. et al. Efficient targeting and activation of antigen-presenting cells in vivo after modified mRNA vaccine administration in rhesus macaques. Mol. Ther. 25, 2635–2647 (2017).
Chaudhary, N. et al. Amine headgroups in ionizable lipids drive immune responses to lipid nanoparticles by binding to the receptors TLR4 and CD1d. Nat. Biomed. Eng 8, 1483–1498 (2024).
Schlich, M. et al. Cytosolic delivery of nucleic acids: the case of ionizable lipid nanoparticles. Bioeng Transl. Med. 6, e10213 (2021).
Bruininks, B. M., Souza, P. C., Ingolfsson, H. & Marrink, S. J. A molecular view on the escape of lipoplexed DNA from the endosome. eLife 9, e52012 (2020).
Liu, S. & Jiang, S. Zwitterionic polymer-protein conjugates reduce polymer-specific antibody response. Nano Today 11, 285–291 (2016).
Kochenderfer, J. N. et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood 116, 4099–4102 (2010).
Henderson, J. M. et al. Cap 1 messenger RNA synthesis with co-transcriptional CleanCap® analog by in vitro transcription. Curr. Protoc. 1, e39 (2021).
Nance, K. D. & Meier, J. L. Modifications in an emergency: the role of N1-methylpseudouridine in COVID-19 vaccines. ACS Cent. Sci. 7, 748–756 (2021).
Hopkins, J. B., Gillilan, R. E. & Skou, S. BioXTAS RAW: improvements to a free open-source program for small-angle X-ray scattering data reduction and analysis. J. Appl. Crystallogr. 50, 1545–1553 (2017).
Luozhong, S. et al. Phosphatidylserine lipid nanoparticles promote systemic RNA delivery to secondary lymphoid organs. Nano Lett. 22, 8304–8311 (2022).
Nelson, J. et al. Impact of mRNA chemistry and manufacturing process on innate immune activation. Sci. Adv. 6, eaaz6893 (2020).
Baiersdorfer, M. et al. A facile method for the removal of dsRNA contaminant from in vitro-transcribed mRNA. Mol. Ther. Nucleic Acids 15, 26–35 (2019).
Acknowledgements
We acknowledge financial support from the National Institute of Allergy and Infectious Diseases (NIAID R01AI178125-01), the National Cancer Institute (NCI U54 CA272688), Robert Langer ’70 Family and Friends Professorship and Cornell NEXT Nano Initiative. Animal IVIS images were acquired through the Cornell Institute of Biotechnology’s Imaging Facility under support of the National Institutes of Health (NIH S10OD025049). The cryo-EM analysis was performed at the State University of New York College for Environmental Science and Forestry Analytical & Technical Services facility. S.W. performed the cryo-EM analysis and is supported by NIH (R35 GM141908). M.G. and S. Lipkin provided guidance and support in the hPBMC experiments and are supported by NCI U54 CA272688 and NIAID R01AI178125-01. BioSAXS experiments were conducted at the Center for High-Energy X-ray Sciences (CHEXS) at Cornell University, which is supported by the National Science Foundation (BIO, ENG and MPS Directorates) under award no. DMR-1829070, and the Macromolecular Diffraction at Cornell High Energy Synchrotron Source (MacCHESS) facility at Cornell University, which is supported by the National Science Foundation (NSF DMR-1829070), and the Macromolecular Diffraction at CHESS (MacCHESS) facility at Cornell University, which is supported by award NIH (1-P30-GM124166-01A1), and by New York State’s Empire State Development Corporation (NYSTAR). We also acknowledge generous assistance from R. Gillilan on the data collection of BioSAXS experiments. Finally, this work also made use of the Cornell University NMR Facility, which is supported, in part, by the NSF through MRI award CHE-1531632.
Author information
Authors and Affiliations
Contributions
S. Luozhong and S.J. conceptualized and designed this study. S. Luozhong, P.L. and Z.Y. performed the chemical synthesis and characterization. S. Luozhong, R. Li, E.D., Y.C. and K.M. performed the in vitro and in vivo experiments. S. Luozhong, R. Li, Y.H., Z.C., M.C., C.M., D.B., P.Z. and Y.M. performed the molecular biology experiments and biological assays. S. Luozhong, R. Li, P.L., Y.C., C.M., A.K. and R. Lai performed the LNP characterizations. S.W. performed the cyro-EM imaging. S. Luozhong, M.G. and S. Lipkin performed the hPBMC experiments. R. Li supervised and performed the experiments during the peer review process. S. Luozhong and S.J. wrote the paper, and all authors discussed and commented on it.
Corresponding author
Ethics declarations
Competing interests
S. Luozhong, P.L., Z.Y. and S.J. are authors of a patent application related to this work (PCT/US2021/064639) filed by Cornell University in the United States. S. Luozhong, R. Li, P.L. and S.J. are authors of a patent related to this work (PCT/US2024/048135) filed by Cornell University in the United States. The other authors declare no competing interests.
Peer review
Peer review information
Nature Materials thanks Camilla Foged, Liangfang Zhang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–38, Tables 1–3 and Methods.
Supplementary Data
Source data for the Supplementary Figures.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Luozhong, S., Liu, P., Li, R. et al. Poly(carboxybetaine) lipids enhance mRNA therapeutics efficacy and reduce their immunogenicity. Nat. Mater. (2025). https://doi.org/10.1038/s41563-025-02240-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41563-025-02240-8
This article is cited by
-
Zwitterionic polymers as PEG replacements for improved mRNA delivery
Nature Reviews Bioengineering (2025)