Abstract
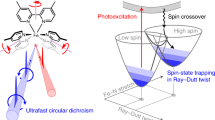
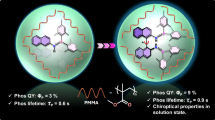
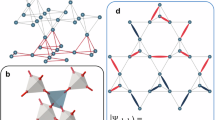
Circularly polarized phosphorescence (CPP) is a spin-forbidden radiative process, and its underlying mechanism is not comprehensively understood, mainly due to the limited examples of efficient triplet emission from small chiral organic molecules with well-defined structures. Here we investigate a pair of chiral enantiomers, R- and S-BBTI, that feature highly distorted spiral ring-locked heteroaromatics with heavy iodine atoms. These chiral molecules are found to exhibit large dissymmetry factors up to 0.013 and emit near-infrared CPP with an efficiency of 4.2% and a lifetime of 119 μs in dimethyl sulfoxide solution excited by ultraviolet irradiation. Their crystals show efficient CPP with 7.0% quantum efficiency and a lifetime of 166 μs. Extensive experimental chiroptical investigations combined with theoretical calculations reveal an efficient spin-flip process that modulates the electron and magnetic transition dipole moments to enhance CPP performance. Moreover, the phosphorescence of R/S-BBTI is oxygen-sensitive and photoactivatable in dimethyl sulfoxide. Therefore, R/S-BBTI can be applied for hypoxia imaging in cells and tumours, expanding the application scope of CPP materials.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
209,00 € per year
only 17,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data that support the findings of this study are reported in the main text and Supplementary Information. The data that support the findings of this study are available from the corresponding author upon reasonable request, and data for all figures can be found via figshare at https://figshare.com/s/2c6dc3e1e94795e4c29b (ref. 48). The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Centre under deposition numbers 2329575, 2329576 and 2329577. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. Source data are provided with this paper.
References
Ye, W. P. et al. Confining isolated chromophores for highly efficient blue phosphorescence. Nat. Mater. 20, 1539–1544 (2021).
Chen, C. et al. Carbazole isomers induce ultralong organic phosphorescence. Nat. Mater. 20, 175–180 (2021).
Chanmungkalakul, S. et al. A descriptor for accurate predictions of host molecules enabling ultralong room-temperature phosphorescence in guest emitters. Angew. Chem. Int. Ed. 61, e202200546 (2022).
Zhao, W., He, Z. & Tang, B. Z. Room-temperature phosphorescence from organic aggregates. Nat. Rev. Mater. 5, 869–885 (2020).
Cai, S. Z., Yao, X. K., Ma, H. L., Shi, H. F. & An, Z. F. Manipulating intermolecular interactions for ultralong organic phosphorescence. Aggregate 4, e320 (2023).
Li, D., Yang, J., Fang, M., Tang, B. Z. & Li, Z. Stimulus-responsive room temperature phosphorescence materials with full-color tunability from pure organic amorphous polymers. Sci. Adv. 8, eabl8392 (2022).
Kabe, R., Notsuka, N., Yoshida, K. & Adachi, C. Afterglow organic light-emitting diode. Adv. Mater. 28, 655–660 (2016).
Miao, Q. et al. Molecular afterglow imaging with bright, biodegradable polymer nanoparticles. Nat. Biotechnol. 35, 1102–1110 (2017).
Dang, Q. et al. Room-temperature phosphorescence resonance energy transfer for construction of near-infrared afterglow imaging agents. Adv. Mater. 32, 2006752 (2020).
Huang, Z., He, Z., Ding, B., Tian, H. & Ma, X. Photoprogrammable circularly polarized phosphorescence switching of chiral helical polyacetylene thin films. Nat. Commun. 13, 7841 (2022).
Li, H. et al. Single-component color-tunable circularly polarized organic afterglow through chiral clusterization. Nat. Commun. 13, 429 (2022).
Gu, L. et al. Circularly polarized organic room temperature phosphorescence from amorphous copolymers. J. Am. Chem. Soc. 143, 18527–18535 (2021).
Chen, W. et al. Long-persistent circularly polarized phosphorescence from chiral organic ionic crystals. Chemistry 24, 17444–17448 (2018).
Ma, C. et al. Insight into chirality on molecular stacking for tunable ultralong organic phosphorescence. J. Mater. Chem. C 6, 10179–10183 (2018).
Liang, X. et al. Organic room-temperature phosphorescence with strong circularly polarized luminescence based on paracyclophanes. Angew. Chem. Int. Ed. 58, 17220–17225 (2019).
Zhi, J., Zhou, Q., Shi, H., An, Z. & Huang, W. Organic room temperature phosphorescence materials for biomedical applications. Chem. Asian J. 15, 947–957 (2020).
Nie, F. & Yan, D. P. Brightening room-temperature phosphorescence in solution. Sci. China Chem. 66, 611–612 (2023).
Wang, X. J., Ma, S., Zhao, B. & Deng, J. P. Frontiers in circularly polarized phosphorescent materials. Adv. Funct. Mater. 33, 2214364 (2023).
Khan, A. et al. Intramolecular-locked high efficiency ultrapure violet-blue (CIE-y <0.046) thermally activated delayed fluorescence emitters exhibiting amplified spontaneous emission. Adv. Funct. Mater. 31, 2009488 (2021).
Xiong, J. B. et al. Evidence for aggregation-induced emission from free rotation restriction of double bond at excited state. Org. Lett. 20, 373–376 (2018).
Aizawa, N. et al. Delayed fluorescence from inverted singlet and triplet excited states. Nature 609, 502–506 (2022).
Yuan, Y. X. et al. Enhanced DNA sensing and chiroptical performance by restriction of double-bond rotation of aie cis-tetraphenylethylene macrocycle diammoniums. Org. Lett. 22, 1836–1840 (2020).
Yuan, Y. X. et al. Fluorescent TPE macrocycle relayed light-harvesting system for bright customized-color circularly polarized luminescence. J. Am. Chem. Soc. 144, 5389–5399 (2022).
Xiong, J.-B. et al. The fixed propeller-like conformation of tetraphenylethylene that reveals aggregation-induced emission effect, chiral recognition, and enhanced chiroptical property. J. Am. Chem. Soc. 138, 11469–11472 (2016).
Peng, Q., Ma, H. L. & Shuai, Z. G. Theory of long-lived room-temperature phosphorescence in organic aggregates. Acc. Chem. Res. 54, 940–949 (2021).
Zhao, W. et al. Rational molecular design for achieving persistent and efficient pure organic room-temperature phosphorescence. Chem 1, 592–602 (2016).
Ams, M. R., Trapp, N., Schwab, A., Milic, J. V. & Diederich, F. Chalcogen bonding “2S-2N squares” versus competing interactions: exploring the recognition properties of sulfur. Chemistry 25, 323–333 (2019).
Frisch, M. J. et al. Gaussian 16 Rev. C.01 (Gaussian, 2016).
Lu, T. & Chen, F. Multiwfn: a multifunctional wavefunction analyzer. J. Comput. Chem. 33, 580–592 (2012).
Lu, T. & Chen, Q. A simple method of identifying π orbitals for non-planar systems and a protocol of studying π electronic structure. Theor. Chem. Acc. 139, 25 (2020).
Neese, F. Software update: the ORCA program system, version 4.0. WIREs Comput. Mol. Sci. 8, e1327 (2017).
Wang, S.-F. et al. Polyatomic molecules with emission quantum yields >20% enable efficient organic light-emitting diodes in the NIR(II) window. Nat. Photon. 16, 843–850 (2022).
Humphrey, W., Dalke, A. & Schulten, K. VMD: visual molecular dynamics. J. Mol. Graph. 14, 33–38 (1996).
Zhang, K. et al. Novel Deep Red thermally activated delayed fluorescence molecule with aggregation-induced emission enhancement: theoretical design and experimental validation. J. Phys. Chem. Lett. 13, 4711–4720 (2022).
Shuai, Z. Thermal vibration correlation function formalism for molecular excited state decay rates. Chin. J. Chem. 38, 1223–1232 (2020).
Aidas, K. et al. The Dalton quantum chemistry program system. Wiley Interdiscip. Rev. Comput. Mol. Sci. 4, 269–284 (2014).
Chen, Z. et al. Pivotal role of transition density in circularly polarized luminescence. Chem. Sci. 14, 6022–6031 (2023).
Wan, S. & Lu, W. Reversible photoactivated phosphorescence of gold(I) arylethynyl complexes in aerated DMSO solutions and gels. Angew. Chem. Int. Ed. 56, 1784–1788 (2017).
Jin, Y. et al. Aggregation-induced barrier to oxygen—a new AIE mechanism for metal clusters with phosphorescence. Natl Sci. Rev. 9, nwab216 (2022).
Gomes, A., Fernandes, E. & Lima, J. L. Fluorescence probes used for detection of reactive oxygen species. J. Biochem. Biophys. Methods 65, 45–80 (2005).
Feng, G. X., Zhang, G. Q. & Ding, D. Design of superior phototheranostic agents guided by Jablonski diagrams. Chem. Soc. Rev. 49, 8179–8234 (2020).
Pham, T. C., Nguyen, V.-N., Choi, Y., Lee, S. & Yoon, J. Recent strategies to develop innovative photosensitizers for enhanced photodynamic therapy. Chem. Rev. 121, 13454–13619 (2021).
Nichols, J. W. & Bae, Y. H. EPR: evidence and fallacy. J. Control. Release 190, 451–464 (2014).
Frawley, A. T., Pal, R. & Parker, D. Very bright, enantiopure europium(III) complexes allow time-gated chiral contrast imaging. Chem. Commun. 52, 13349–13352 (2016).
Stachelek, P., MacKenzie, L., Parker, D. & Pal, R. Circularly polarised luminescence laser scanning confocal microscopy to study live cell chiral molecular interactions. Nat. Commun. 13, 553 (2022).
Gavanji, S., Bakhtari, A., Famurewa, A. C. & Othman, E. M. Cytotoxic activity of herbal medicines as assessed in vitro: a review. Chem. Biodivers. 20, e202201098 (2023).
Ma, H. et al. Ultrastable near-infrared aggregation-induced emission nanoparticles as a fluorescent probe: long-term tumor monitoring and lipid droplet tracking. CCS Chem. 3, 1569–1606 (2021).
Liu, D. et al. Highly efficient circularly polarized near-infrared phosphorescence in both solution and aggregate. figshare https://figshare.com/s/2c6dc3e1e94795e4c29b (2024).
Acknowledgements
We thank the Materials Characterization and Preparation Center, The Chinese University of Hong Kong, Shenzhen, for materials characterization, and we are grateful to X. Li and G. Liu for their help with high-resolution mass and high-performance liquid chromatography measurements. This work was supported by the National Key Research and Development Program of China (2023YFB3810001 (to Z.Z.)); the National Natural Science Foundation of China grant (52273197 (to Z.Z.), 52333007 (to B.Z.T.), 52250410355 (to P.A.), 22305204 (to D.L.) and 52303382 (to Z.Q.)); the Shenzhen Key Laboratory of Functional Aggregate Materials (ZDSYS 20211021111400001 (to B.Z.T., Z.Z. and Z.Q.)); the Science, Technology, and Innovation Commission of Shenzhen Municipality (JCYJ 2021324134613038 (to Z.Z.), JCYJ20220530143805012 (to Z.Q.), KQTD 20210811090142053 (to B.Z.T.), GJHZ 20210705141810031 (to Z.Z.) and JCYJ20220818103007014 (to B.Z.T.)); and the Innovation and Technology Commission (ITCCNERC14SC01 (to B.Z.T.)).
Author information
Authors and Affiliations
Contributions
Z.Q., Z.Z. and B.Z.T. supervised the project. D.L. designed and synthesized the molecules and carried out the measurements of the photophysical characteristics. W.-J.W. performed the cell and animal experiments. D.L., Z.Y. and B.W. performed theoretical calculations. K.W., L.Z., Y.X., S.C., B.W., Q.W. and P.A. contributed to the discussions. D.L., P.A., Z.Q. and Z.Z. contributed to the manuscript writing. All authors discussed the progress of the research and reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Photonics thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Discussion, Figs. 1–51 and Tables 1–7.
Supplementary Video 1
The process of writing ‘AIE’ with a UV pen.
Crystallographic Data 1
CCDC 2329575 (R-BBT).
Crystallographic Data 2
CCDC 2329576 (R-BBTI).
Crystallographic Data 3
CCDC 2329577 (S-BBTI).
Source data
Source Data Fig. 2
Photophysical properties in crystalline state.
Source Data Fig. 3
Photoluminescent and chiroptical properties of R/S-BBTI in solution.
Source Data Fig. 4
CPP mechanistic investigations.
Source Data Fig. 5
Photoactivated performance of R/S-BBTI in DMSO solution (10−5 M).
Source Data Fig. 6
Dynamic light scattering data of S-BBTI-based nanoparticles.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, D., Wang, WJ., Alam, P. et al. Highly efficient circularly polarized near-infrared phosphorescence in both solution and aggregate. Nat. Photon. 18, 1276–1284 (2024). https://doi.org/10.1038/s41566-024-01538-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41566-024-01538-4
This article is cited by
-
Design of circularly polarized phosphorescence materials guided by transfer learning
Nature Communications (2025)