Abstract
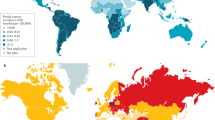
Penile cancer is a rare neoplasm with heterogeneous prevalence influenced by risk factors such as smoking, poor hygiene and human papillomavirus (HPV) infection. Southern Africa, South America and Southeast Asia have the highest incidence of this disease. Penile squamous cell carcinomas (PSCCs) account for the majority of instances of penile cancer, with HPV-related carcinogenesis implicated in up to half of them. Increases in PSCC incidence in industrialized nations parallel the rising high-risk HPV infection rates, particularly HPV-16. Early-stage, localized PSCC is often manageable, but treatment options in advanced disease remain limited, with poor survival outcomes. Emerging evidence suggests that HPV-positive PSCC might exhibit unique therapeutic responses, including increased sensitivity to radiotherapy and chemotherapy, as has been observed in HPV-driven head and neck squamous cell carcinoma. Results of studies in HPV-positive PSCC demonstrate improved responses to chemoradiotherapy and immunotherapy, underscoring the potential for tailored treatments and de-escalation. Additionally, incorporating immunotherapy with radiotherapy in HPV-driven PSCC might provide greater oncological benefits than standard chemotherapy. These observations suggest that treatment strategies for HPV-positive PSCC might benefit from de-escalated chemoradiotherapy regimens or immunotherapy incorporation, potentially optimizing efficacy while minimizing toxic effects. Furthermore, biomarkers such as tumour mutational burden, programmed cell death ligand 1 expression, and genetic alterations could be crucial for predicting treatment response. Comprehensive biomarker assessment and accurate HPV status determination are essential for developing patient-tailored therapeutic strategies. These data provide evidence of the potential benefits of individualized approaches based on tumour biology and biomarker profiles.
Key points
-
Penile cancer is a rare but increasingly diagnosed neoplasm, particularly in regions with high human papillomavirus (HPV) prevalence, and it is substantially influenced by other risk factors such as smoking and poor hygiene.
-
High-risk HPV types, especially HPV-16, are implicated in the majority of penile squamous cell carcinomas (PSCCs), with HPV-related carcinogenesis accounting for up to 50% of instances.
-
Early stages of disease have favourable outcomes, but patients with advanced PSCC with nodal involvement, face limited treatment options and poor survival rates, necessitating innovative therapeutic approaches.
-
Emerging evidence indicates that HPV-positive PSCC might respond more favourably to chemoradiotherapy and immunotherapy than HPV-negative tumours, suggesting the need for tailored treatment strategies.
-
Comprehensive assessment of biomarkers such as tumour mutational burden and programmed cell death 1 ligand 1 expression is crucial for predicting treatment responses and developing personalized therapies for patients with PSCC.
-
De-escalated chemoradiotherapy regimens and the incorporation of immunotherapy into treatment strategies for HPV-positive PSCCs are aimed at enhancing efficacy while reducing toxic effects.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
209,00 € per year
only 17,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Douglawi, A. & Masterson, T. A. Updates on the epidemiology and risk factors for penile cancer. Transl. Androl. Urol. 6, 785–790 (2017).
Moore, T. O. et al. Human papillomavirus, smoking, and cancer. J. Cutan. Med. Surg. 5, 323–328 (2001).
Tsen, H. F., Morgenstern, H., Mack, T. & Peters, R. K. Risk factors for penile cancer: results of a population-based case-control study in Los Angeles County (United States). Cancer Causes Control 12, 267–277 (2001).
Do, H. T. T. et al. The etiologic role of human papillomavirus in penile cancers: a study in Vietnam. Br. J. Cancer 108, 229–233 (2013).
Fu, L. et al. Global pattern and trends in penile cancer incidence: population-based study. JMIR Public Health Surveill. 8, e34874 (2022).
Olesen, T. B. et al. Prevalence of human papillomavirus DNA and p16INK4a in penile cancer and penile intraepithelial neoplasia: a systematic review and meta-analysis. Lancet Oncol. 20, 145–158 (2019).
Wenzel, M. et al. Temporal trends, tumor characteristics and stage-specific survival in penile non-squamous cell carcinoma vs. squamous cell carcinoma. Cancer Causes Control 33, 25–35 (2022).
Xu, L., Dahlstrom, K. R., Lairson, D. R. & Sturgis, E. M. Projected oropharyngeal carcinoma incidence among middle-aged US men. Head Neck 41, 3226–3234 (2019).
Bruni, L. et al. Global and regional estimates of genital human papillomavirus prevalence among men: a systematic review and meta-analysis. Lancet Glob. Health 11, e1345–e1362 (2023).
Vieira, C. B. et al. Profile of patients with penile cancer in the region with the highest worldwide incidence. Sci. Rep. 10, 2965 (2020).
Brouwer, O. R. et al. European Association of Urology-American Society of Clinical Oncology Collaborative Guideline on Penile Cancer: 2023 Update. Eur. Urol. 83, 548–560 (2023).
Tomas, Anita et al. Penile cancer. Nat. Rev. Dis. Primers 7, 11 (2021). This comprehensive primer provides essential insights into the epidemiology, pathology and management strategies for penile cancer, highlighting the need for increased awareness and improved treatment protocols in this rare malignancy.
Schlenker, B. & Schneede, P. The role of human papilloma virus in penile cancer prevention and new therapeutic agents. Eur. Urol. Focus 5, 42–45 (2019). The study emphasizes the association between HPV and penile cancer, advocating for preventive measures and innovative therapeutic approaches to mitigate this risk.
Deng, X. et al. Trends in incidence, mortality, and survival of penile cancer in the United States: a population-based study. Front. Oncol. 12, 891623 (2022).
Djajadiningrat, R. S. et al. Contemporary management of regional nodes in penile cancer — improvement of survival? J. Urol. 191, 68–73 (2014).
Muneer, A. et al. Penile cancer: ESMO–EURACAN Clinical Practice Guideline for diagnosis, treatment and follow-up. ESMO Open 9, 103481 (2024).
Bandini, M., Pederzoli, F. & Necchi, A. Neoadjuvant chemotherapy for lymph node-positive penile cancer: current evidence and knowledge. Curr. Opin. Urol. 30, 218–222 (2020).
Chipollini, J., Necchi, A. & Spiess, P. E. Outcomes for patients with node-positive penile cancer: impact of perioperative systemic therapies and the importance of surgical intervention. Eur. Urol. 74, 241–242 (2018).
Tang, Y., Hu, X., Wu, K. & Li, X. Immune landscape and immunotherapy for penile cancer. Front. Immunol. 13, 1055235 (2022).
Cleary, C. et al. Biological features of human papillomavirus-related head and neck cancers contributing to improved response. Clin. Oncol. 28, 467–474 (2016).
van Kempen, P. M. W. et al. Differences in methylation profiles between HPV-positive and HPV-negative oropharynx squamous cell carcinoma: a systematic review. Epigenetics 9, 194–203 (2014).
McBride, A. A. Human papillomaviruses: diversity, infection and host interactions. Nat. Rev. Microbiol. 20, 95–108 (2022). The complexity of HPVs, their diverse interactions with hosts and their implications for diseases such as penile cancer are discussed in this review, underscoring the importance of understanding HPV biology for effective prevention strategies.
Van Doorslaer, K. Evolution of the papillomaviridae. Virology 445, 11–20 (2013).
Moscicki, A.-B. et al. Updating the natural history of human papillomavirus and anogenital cancers. Vaccine 30, F24–F33 (2012).
de Martel, C., Plummer, M., Vignat, J. & Franceschi, S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int. J. Cancer 141, 664–670 (2017).
Doorbar, J., Egawa, N., Griffin, H., Kranjec, C. & Murakami, I. Human papillomavirus molecular biology and disease association. Rev. Med. Virol. 25, 2–23 (2015).
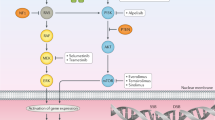
Mittal, S. & Banks, L. Molecular mechanisms underlying human papillomavirus E6 and E7 oncoprotein-induced cell transformation. Mutat. Res. Rev. Mutat. Res. 772, 23–35 (2017).
Nazha, B. et al. Comprehensive genomic profiling of penile squamous cell carcinoma and the impact of human papillomavirus status on immune-checkpoint inhibitor-related biomarkers. Cancer 129, 3884–3893 (2023).
Martinez-Zapien, D. et al. Structure of the E6/E6AP/p53 complex required for HPV-mediated degradation of p53. Nature 529, 541–545 (2016).
Elst, L. et al. Single-cell atlas of penile cancer reveals TP53 mutations as a driver of an aggressive phenotype, irrespective of human papillomavirus status, and provides clues for treatment personalization. Eur. Urol. S0302-2838, 02266–02268 (2024).
DiGiuseppe, S., Bienkowska-Haba, M., Guion, L. G. M., Keiffer, T. R. & Sapp, M. Human papillomavirus major capsid protein L1 remains associated with the incoming viral genome throughout the entry process. J. Virol. 91, e00537–17 (2017).
Ferreira, A. R., Ramalho, A. C., Marques, M. & Ribeiro, D. The interplay between antiviral signalling and carcinogenesis in human papillomavirus infections. Cancers 12, 646 (2020).
Florin, L., Schäfer, F., Sotlar, K., Streeck, R. E. & Sapp, M. Reorganization of nuclear ___domain 10 induced by papillomavirus capsid protein l2. Virology 295, 97–107 (2002).
Someya, M. et al. Radiotherapy for HPV-related cancers: prediction of therapeutic effects based on the mechanism of tumor immunity and the application of immunoradiotherapy. Jpn. J. Radiol. 40, 458–465 (2022).
Qi, S.-Y., Yang, M.-M., Li, C.-Y., Yu, K. & Deng, S.-L. The HPV viral regulatory mechanism of TLRs and the related treatments for HPV-associated cancers. Front. Immunol. 15, 1407649 (2024).
Tang, D., Kang, R., Zeh, H. J. & Lotze, M. T. The multifunctional protein HMGB1: 50 years of discovery. Nat. Rev. Immunol. 23, 824–841 (2023).
Andersen, A. S., Koldjaer Sølling, A. S., Ovesen, T. & Rusan, M. The interplay between HPV and host immunity in head and neck squamous cell carcinoma. Int. J. Cancer 134, 2755–2763 (2014).
Sitz, J. et al. Human papillomavirus E7 oncoprotein targets RNF168 to hijack the host DNA damage response. Proc. Natl Acad. Sci. USA 116, 19552–19562 (2019).
Leeman, J. E. et al. Human papillomavirus 16 promotes microhomology-mediated end-joining. Proc. Natl Acad. Sci. USA 116, 21573–21579 (2019).
Kimple, R. J. et al. Enhanced radiation sensitivity in HPV-positive head and neck cancer. Cancer Res. 73, 4791–4800 (2013).
Fakhry, C. et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J. Natl Cancer Inst. 100, 261–269 (2008).
Ang, K. K. et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl. J. Med. 363, 24–35 (2010).
Riaz, N. et al. Precision radiotherapy: reduction in radiation for oropharyngeal cancer in the 30 ROC trial. J. Natl Cancer Inst. 113, 742–751 (2021).
Lee, N. Y. et al. The 30 ROC trial: precision intra-treatment imaging guiding major radiation reduction in human papillomavirus related oropharyngeal cancer. J. Clin. Oncol. 39, 6019–6019 (2021).
Ferris, R. L. et al. Phase II randomized trial of transoral surgery and low-dose intensity modulated radiation therapy in resectable p16+ locally advanced oropharynx cancer: an ECOG-ACRIN cancer research group trial (E3311). J. Clin. Oncol. 40, 138–149 (2022).
Anderson, G. et al. An updated review on head and neck cancer treatment with radiation therapy. Cancers 13, 4912 (2021).
Lacas, B. et al. Role of radiotherapy fractionation in head and neck cancers (MARCH): an updated meta-analysis. Lancet Oncol. 18, 1221–1237 (2017).
Ferris, R. L. et al. Neoadjuvant nivolumab for patients with resectable HPV-positive and HPV-negative squamous cell carcinomas of the head and neck in the CheckMate 358 trial. J. Immunother. Cancer 9, e002568 (2021).
Rosenberg, A. J. et al. Neoadjuvant nivolumab plus chemotherapy followed by response-adaptive therapy for HPV+ oropharyngeal cancer: OPTIMA II phase 2 open-label nonrandomized controlled trial. JAMA Oncol. 10, 923–931 (2024).
Rakotomalala, A., Escande, A., Furlan, A., Meignan, S. & Lartigau, E. Hypoxia in solid tumors: how low oxygenation impacts the ‘Six Rs’ of radiotherapy. Front. Endocrinol. 12, 742215 (2021).
Lee, N. et al. A strategy of using intra-treatment hypoxia imaging to selectively and safely guide radiation dose deescalation concurrent with chemotherapy for loco-regionally advanced human papillomavirus-related oropharyngeal carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 96, 9–17 (2016).
Lee, N. Y. et al. Hypoxia-directed treatment of human papillomavirus-related oropharyngeal carcinoma. J. Clin. Oncol. 42, 940–950 (2024).
de Vries, H. M. et al. Atezolizumab with or without radiotherapy for advanced squamous cell carcinoma of the penis (The PERICLES Study): a phase II trial. J. Clin. Oncol. 41, 4872–4880 (2023). In this phase II trial, the efficacy of atezolizumab combined with radiotherapy in treating advanced penile squamous cell carcinoma was investigated, potentially paving the way for novel immunotherapeutic strategies in this challenging disease.
Ottenhof, S. R. et al. A prospective study of chemoradiotherapy as primary treatment in patients with locoregionally advanced penile carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 117, 139–147 (2023). In this prospective study, chemoradiotherapy is evaluated as a primary treatment option for locoregionally advanced penile carcinoma, aiming to enhance treatment outcomes and inform clinical practice guidelines.
Bandini, M. et al. Association between human papillomavirus infection and outcome of perioperative nodal radiotherapy for penile carcinoma. Eur. Urol. Oncol. 4, 802–810 (2021). This retrospective study highlights the correlation between HPV status and treatment outcomes in patients undergoing perioperative nodal radiotherapy for penile carcinoma, suggesting that HPV status could be a critical factor in tailoring therapy decisions.
Yuan, Z. et al. The relationship between HPV status and chemoradiotherapy in the locoregional control of penile cancer. World J. Urol. 36, 1431–1440 (2018). In this study, how HPV status influences the effectiveness of chemoradiotherapy in controlling locoregional penile cancer is investigated, providing insights into personalized treatment approaches based on viral status.
Aydin, A. M. et al. Understanding genomics and the immune environment of penile cancer to improve therapy. Nat. Rev. Urol. 17, 555–570 (2020).
Klempner, S. J. et al. Tumor mutational burden as a predictive biomarker for response to immune checkpoint inhibitors: a review of current evidence. Oncologist 25, e147–e159 (2020).
Necchi, A. et al. Genomic profiles and clinical outcomes of penile squamous cell carcinoma with elevated tumor mutational burden. JAMA Netw. Open 6, e2348002 (2023). This research identifies genomic profiles associated with elevated TMB in patients with PSCC, offering potential biomarkers that could guide treatment decisions and prognostic assessments in clinical settings.
Tekin, B. et al. Assessment of PD-L1, TROP2, and nectin-4 expression in penile squamous cell carcinoma. Hum. Pathol. 142, 42–50 (2023).
Grass, G. D. et al. An analysis of nectin-4 (PVRL4) in penile squamous cell carcinoma. Eur. Urol. Open Sci. 49, 1–5 (2023).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT06353906 (2024).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT06104618 (2024).
Joura, E. A. et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N. Engl. J. Med. 372, 711–723 (2015).
Voris, B. R. I., Visintin, C. D. N. & Reis, L. O. HPV vaccination is fundamental for reducing or erradicate penile cancer opinion: YES. Int. Braz. J. Urol. 44, 859–861 (2018).
James, C. D., Morgan, I. M. & Bristol, M. L. The relationship between estrogen-related signaling and human papillomavirus positive cancers. Pathogens 9, 403 (2020).
Monsonego, J. et al. Estrogen and progesterone receptors in cervical human papillomavirus related lesions. Int. J. Cancer 48, 533–539 (1991).
Schiffman, M. et al. Carcinogenic human papillomavirus infection. Nat. Rev. Dis. Primers 2, 16086 (2016).
Alemany, L. et al. Role of human papillomavirus in penile carcinomas worldwide. Eur. Urol. 69, 953–961 (2016).
Udager, A. M. et al. Frequent PD-L1 expression in primary and metastatic penile squamous cell carcinoma: potential opportunities for immunotherapeutic approaches. Ann. Oncol. 27, 1706–1712 (2016).
Ottenhof, S. R. et al. Expression of programmed death ligand 1 in penile cancer is of prognostic value and associated with HPV status. J. Urol. 197, 690–697 (2017).
Ottenhof, S. R. et al. The prognostic value of immune factors in the tumor microenvironment of penile squamous cell carcinoma. Front. Immunol. 9, 1253 (2018).
Sand, F. L., Rasmussen, C. L., Frederiksen, M. H., Andersen, K. K. & Kjaer, S. K. Prognostic significance of HPV and p16 status in men diagnosed with penile cancer: a systematic review and meta-analysis. Cancer Epidemiol. Biomark. Prev. 27, 1123–1132 (2018).
Zargar-Shoshtari, K. et al. Clinical significance of p53 and p16ink4a status in a contemporary North American penile carcinoma cohort. Clin. Genitourin. Cancer 14, 346–351 (2016).
Stankiewicz, E. et al. Alternative HER/PTEN/Akt pathway activation in HPV positive and negative penile carcinomas. PLoS ONE 6, e17517 (2011).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05686226 (2024).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT04708470 (2025).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05639972 (2024).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT06544720 (2024).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT06505551 (2024).
Author information
Authors and Affiliations
Consortia
Contributions
M.L. and G.B. researched data for the article. M.B. and M.L. contributed substantially to the discussion of the content. M.L., F.N. and G.B. wrote the article. M.B., C.D.F., A.S. and P.A.S.J. reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Urology thanks Rui Medeiros, Suengtaek Choi and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Advanced PSCC
-
Extensive (T3–T4), regionally advanced (inguinal or pelvic lymph-node involvement) or metastatic (M1) penile squamous cell carcinoma (PSCC), requiring a multidisciplinary approach owing to its poor prognosis.
- Chemoradiotherapy
-
A combined treatment approach using chemotherapy and radiation therapy, which might be more effective in patients with human papillomavirus (HPV)-positive disease than in those who are HPV negative.
- Human papillomavirus
-
(HPV). A group of viruses, some of which are classified as high-risk owing to their potential to cause cancer, particularly penile squamous cell carcinoma.
- Immunotherapy
-
A treatment strategy that harnesses the immune system to fight cancer, showing promise in human papillomavirus (HPV)-positive penile cancer cases.
- Penile squamous cell carcinoma
-
(PSCC). The most common type of penile cancer, accounting for approximately 95% of instances, often linked to human papillomavirus (HPV) infection.
- Radiosensitivity
-
The susceptibility of cancer cells to damage from radiation therapy, with human papillomavirus (HPV)-positive tumours often exhibiting higher radiosensitivity than their HPV-negative counterparts.
- Tumour mutational burden
-
(TME). A measure of the number of mutations within the DNA of a tumour, which can influence the effectiveness of certain therapies, including immunotherapy.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Longoni, M., Fankhauser, C.D., Negri, F. et al. Treatment strategies in human papillomavirus-related advanced penile cancer. Nat Rev Urol 22, 427–438 (2025). https://doi.org/10.1038/s41585-025-00994-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41585-025-00994-z