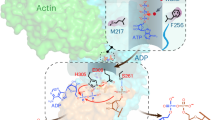
Abstract
AMPylation is a post-translational modification in which AMP is added to the amino acid side chains of proteins1,2. Here we show that, with ATP as the ligand and actin as the host activator, the effector protein LnaB of Legionella pneumophila exhibits AMPylase activity towards the phosphoryl group of phosphoribose on PRR42-Ub that is generated by the SidE family of effectors, and deubiquitinases DupA and DupB in an E1- and E2-independent ubiquitination process3,4,5,6,7. The product of LnaB is further hydrolysed by an ADP-ribosylhydrolase, MavL, to Ub, thereby preventing the accumulation of PRR42-Ub and ADPRR42-Ub and protecting canonical ubiquitination in host cells. LnaB represents a large family of AMPylases that adopt a common structural fold, distinct from those of the previously known AMPylases, and LnaB homologues are found in more than 20 species of bacterial pathogens. Moreover, LnaB also exhibits robust phosphoryl-AMPylase activity towards phosphorylated residues and produces unique ADPylation modifications in proteins. During infection, LnaB AMPylates the conserved phosphorylated tyrosine residues in the activation loop of the Src family of kinases8,9, which dampens downstream phosphorylation signalling in the host. Structural studies reveal the actin-dependent activation and catalytic mechanisms of the LnaB family of AMPylases. This study identifies, to our knowledge, an unprecedented molecular regulation mechanism in bacterial pathogenesis and protein phosphorylation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
199,00 € per year
only 3,90 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The structural factors and coordinates of the MavL–Ub complex (PDB ID: 8XEP) have been deposited into the PDB database (https://www.rcsb.org/). The cryo-EM density maps and structural models of the LHLS–actin complexes have been deposited into the EMDB database (https://www.ebi.ac.uk/emdb/) and the PDB with the following accession numbers: wild-type LHLS–actin–AMP complex, EMD-36454 and 8JO3; LHLS(H287A)–actin–ATP complex, EMD-36455 and 8JO4. The structures of the ADPR-bound PARG (ref. 13) from Thermomonospora curvata (PDB ID: 3SIG) and the Fic domains from H. pylori (PDB ID: 2F6S) and N. meningitidis28 (PDB ID: 3S6A), which were used in the structural analyses in this study, were obtained from the PDB. The predicted structures of LnaB homologues, including LHBA (https://alphafold.ebi.ac.uk/entry/A0A0Q9YZ20) and LHEI (https://alphafold.ebi.ac.uk/entry/C5BCE7), were obtained from the AlphaFold protein structure database (https://alphafold.ebi.ac.uk/).
Code availability
No custom code or mathematical algorithm was used or developed for the analysis of data reported in this study.
Change history
23 July 2024
A Correction to this paper has been published: https://doi.org/10.1038/s41586-024-07847-6
References
Gulen, B. & Itzen, A. Revisiting AMPylation through the lens of Fic enzymes. Trends Microbiol. 30, 350–363 (2022).
Casey, A. K. & Orth, K. Enzymes involved in AMPylation and deAMPylation. Chem. Rev. 118, 1199–1215 (2018).
Qiu, J. et al. Ubiquitination independent of E1 and E2 enzymes by bacterial effectors. Nature 533, 120–124 (2016).
Bhogaraju, S. et al. Phosphoribosylation of ubiquitin promotes serine ubiquitination and impairs conventional ubiquitination. Cell 167, 1636–1649 (2016).
Kotewicz, K. M. et al. A single Legionella effector catalyzes a multistep ubiquitination pathway to rearrange tubular endoplasmic reticulum for replication. Cell Host Microbe 21, 169–181 (2017).
Wan, M. et al. Deubiquitination of phosphoribosyl-ubiquitin conjugates by phosphodiesterase-___domain-containing Legionella effectors. Proc. Natl Acad. Sci. USA 116, 23518–23526 (2019).
Shin, D. et al. Regulation of phosphoribosyl-linked serine ubiquitination by deubiquitinases DupA and DupB. Mol. Cell 77, 164–179 (2020).
Shen, K. et al. The Src family kinase Fgr is a transforming oncoprotein that functions independently of SH3–SH2 ___domain regulation. Sci. Signal. 11, eaat5916 (2018).
Kovacs, M. et al. The Src family kinases Hck, Fgr, and Lyn are critical for the generation of the in vivo inflammatory environment without a direct role in leukocyte recruitment. J. Exp. Med. 211, 1993–2011 (2014).
Hubber, A. & Roy, C. R. Modulation of host cell function by Legionella pneumophila type IV effectors. Annu. Rev. Cell Dev. Biol. 26, 261–283 (2010).
Komander, D. & Rape, M. The ubiquitin code. Annu. Rev. Biochem. 81, 203–229 (2012).
Voth, K. et al. Structural and functional characterization of Legionella pneumophila effector MavL. Biomolecules 11, 1802 (2021).
Slade, D. et al. The structure and catalytic mechanism of a poly(ADP-ribose) glycohydrolase. Nature 477, 616–620 (2011).
Yan, F. et al. Threonine ADP-ribosylation of ubiquitin by a bacterial effector family blocks host ubiquitination. Mol. Cell 78, 641–652 (2020).
Tezcan-Merdol, D. et al. Actin is ADP-ribosylated by the Salmonella enterica virulence-associated protein SpvB. Mol. Microbiol. 39, 606–619 (2001).
Beck, C., Robert, I., Reina-San-Martin, B., Schreiber, V. & Dantzer, F. Poly(ADP-ribose) polymerases in double-strand break repair: focus on PARP1, PARP2 and PARP3. Exp. Cell. Res. 329, 18–25 (2014).
Leung, A. K. Poly(ADP-ribose): an organizer of cellular architecture. J. Cell Biol. 205, 613–619 (2014).
Fu, H. et al. Poly(ADP-ribosylation) of P-TEFb by PARP1 disrupts phase separation to inhibit global transcription after DNA damage. Nat. Cell Biol. 24, 513–525 (2022).
Guo, Z., Stephenson, R., Qiu, J., Zheng, S. & Luo, Z. Q. A Legionella effector modulates host cytoskeletal structure by inhibiting actin polymerization. Microbes Infect. 16, 225–236 (2014).
Qiu, J. & Luo, Z. Q. Hijacking of the host ubiquitin network by Legionella pneumophila. Front. Cell. Infect. Microbiol. 7, 487 (2017).
Price, C. T., Al-Quadan, T., Santic, M., Rosenshine, I. & Abu Kwaik, Y. Host proteasomal degradation generates amino acids essential for intracellular bacterial growth. Science 334, 1553–1557 (2011).
Bence, N. F., Sampat, R. M. & Kopito, R. R. Impairment of the ubiquitin-proteasome system by protein aggregation. Science 292, 1552–1555 (2001).
Roberts, J. Z., Crawford, N. & Longley, D. B. The role of ubiquitination in apoptosis and necroptosis. Cell Death Differ. 29, 272–284 (2022).
Ge, J. et al. A Legionella type IV effector activates the NF-κB pathway by phosphorylating the IκB family of inhibitors. Proc. Natl Acad. Sci. USA 106, 13725–13730 (2009).
Kinch, L. N., Yarbrough, M. L., Orth, K. & Grishin, N. V. Fido, a novel AMPylation ___domain common to fic, doc, and AvrB. PLoS One 4, e5818 (2009).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
van Kempen, M. et al. Fast and accurate protein structure search with Foldseek. Nat. Biotechnol. 42, 243–246 (2024).
Engel, P. et al. Adenylylation control by intra- or intermolecular active-site obstruction in Fic proteins. Nature 482, 107–110 (2012).
Pearson, J. D., Lee, J. K., Bacani, J. T., Lai, R. & Ingham, R. J. NPM-ALK: the prototypic member of a family of oncogenic fusion tyrosine kinases. J. Signal Transduct. 2012, 123253 (2012).
Zhao, Z., Tan, Z., Diltz, C. D., You, M. & Fischer, E. H. Activation of mitogen-activated protein (MAP) kinase pathway by pervanadate, a potent inhibitor of tyrosine phosphatases. J. Biol. Chem. 271, 22251–22255 (1996).
D’Ambrosio, D. et al. Recruitment and activation of PTP1C in negative regulation of antigen receptor signaling by Fc gamma RIIB1. Science 268, 293–297 (1995).
Kamat, P. K., Rai, S., Swarnkar, S., Shukla, R. & Nath, C. Molecular and cellular mechanism of okadaic acid (OKA)-induced neurotoxicity: a novel tool for Alzheimer’s disease therapeutic application. Mol. Neurobiol. 50, 852–865 (2014).
Ballatore, C., Lee, V. M. & Trojanowski, J. Q. Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat. Rev. Neurosci. 8, 663–672 (2007).
Prickett, T. D. et al. TAB4 stimulates TAK1–TAB1 phosphorylation and binds polyubiquitin to direct signaling to NF-κB. J. Biol. Chem. 283, 19245–19254 (2008).
Wetzel, D. M., Rhodes, E. L., Li, S., McMahon-Pratt, D. & Koleske, A. J. The Src kinases Hck, Fgr and Lyn activate Arg to facilitate IgG-mediated phagocytosis and Leishmania infection. J. Cell Sci. 129, 3130–3143 (2016).
Guo, X. et al. UBLCP1 is a 26S proteasome phosphatase that regulates nuclear proteasome activity. Proc. Natl Acad. Sci. USA 108, 18649–18654 (2011).
Hopfner, D. et al. Monoclonal anti-AMP antibodies are sensitive and valuable tools for detecting patterns of AMPylation. iScience 23, 101800 (2020).
Kabsch, W. XDS. Acta Crystallogr. D 66, 125–132 (2010).
Potterton, L. et al. CCP4i2: the new graphical user interface to the CCP4 program suite. Acta Crystallogr. D 74, 68–84 (2018).
Liebschner, D. et al. Macromolecular structure determination using X-rays, neutrons and electrons: recent developments in Phenix. Acta Crystallogr. D 75, 861–877 (2019).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D 66, 486–501 (2010).
Williams, C. J. et al. MolProbity: more and better reference data for improved all-atom structure validation. Protein Sci. 27, 293–315 (2018).
Mirdita, M. et al. ColabFold: making protein folding accessible to all. Nat. Methods 19, 679–682 (2022).
Katoh, K. & Standley, D. M. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 30, 772–780 (2013).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Zivanov, J. et al. New tools for automated high-resolution cryo-EM structure determination in RELION-3. eLife 7, e42166 (2018).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Jamali, K. et al. Automated model building and protein identification in cryo-EM maps. Nature 628, 450–457 (2024).
Sanchez-Garcia, R. et al. DeepEMhancer: a deep learning solution for cryo-EM volume post-processing. Commun. Biol. 4, 874 (2021).
Sun, J. et al. The tuberculosis necrotizing toxin kills macrophages by hydrolyzing NAD. Nat. Struct. Mol. Biol. 22, 672–678 (2015).
Acknowledgements
We thank the core facility of the Life Sciences Institute at Zhejiang University for equipment support; the staff at the beamline BL17U1 of the SSRF and the cryo-EM centre of Zhejiang University for assistance with diffraction and cryo-EM data collection; A. Itzen for providing the anti-AMP antibody; P. Jiang, Y. Yu, X.-H. Feng, C. Ji and P. Xu for assistance with reagents; and Y. Lu and Z.-Q. Luo for providing A. carinii and Legionella strains. This work was supported by grants from the NSFC (81925024 and U23A20163 to Y. Zhu; 21974002 and 22174003 to X.L.), the National Science and Technology Major Project (2017YFA0503900 to Y. Zhu) and the Fundamental Research Funds for the Central Universities to Y. Zhu and Y. Zhou. Y. Zhu and Y. Zhou were supported by the National High-level Talents Special Support Program of China.
Author information
Authors and Affiliations
Contributions
Y. Zhu conceived and supervised the study. T. Wang, X.S., J.T., X.Z., Y. Zhou and Y. Zhu designed experiments. T. Wang determined the activities and functions of LnaB and its homologues. X.S. and J.T. determined the activity and structure of MavL. X.Z. and X.W. determined cryo-EM structures. W.X. and X.L. performed mass spectrometric analyses. T. Wu, Y.R., B.Y., Y.X., M.Y. and G.F. assisted with assays. K.Y. and J.T. analysed LnaB homologues. T. Wang, X.S., J.T., K.Y., W.X., X.L. and Y. Zhu prepared the figures and a draft of the methods. Y. Zhu and Y. Zhou wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
T. Wang, Y. Zhou and Y. Zhu have filed a patent application related to this work. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature thanks Elizabeth Hartland and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
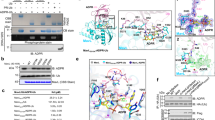
Extended Data Fig. 1 Interactions between MavL and Ub.
a, Native PAGE analyses of Ub, ADPRR42-Ub and PRR42-Ub after treatments by the cell lysates of the wild-type and sidE-deleted strains of L. pneumophila. b, In vitro ARH assays of MavL toward ADPRR42-Ub. The ADP-ribosyl hydrolysation of ADPRR42-Ub was examined by the mobility shifts on native PAGE gels and Coomassie blue staining. c, Interactions of MavL with polyUb and Ub. d, 2mFo-DFc and mFo-DFc electron density maps of Ub in the MavL–Ub complex. The 2mFo-DFc map is shown as blue meshes and contoured at 1.0 σ. The mFo-DFc map is shown as meshes coloured in green (positive) and red (negative) and contoured at 3.0 σ. e,f, Detailed interactions of MavL with Ub in the MavL–Ub complex structure. The mFo-DFc electron density map of the interacting residues (f) was calculated with the residues and loop regions removed and is shown as meshes coloured in green (positive) and red (negative) and contoured at 3.0 σ. g, Structural superimposition of MavL with Thermomonospora curvata PARG13 (PDB ID: 3SIG). The ADPR ligand in the PARG structure is represented as sticks in cyan. The catalytic pocket of MavL is highlighted within the dashed box. h, Close-up view of the ADPR-binding pockets of MavL and PARG. The catalytic residues of MavL and PARG are shown as sticks. i, Interactions of the catalytic pocket of MavL with the ADPR ligand that was modelled from PARG, according to the structural superimposition of MavL and PARG (g). j,k, Mutagenetic analyses of the Ub-interacting residues of MavL on its interactions with Ub (j) and its ARH activity (k). All uncropped and unprocessed scans of blots and gels are listed in Supplementary Fig. 1.
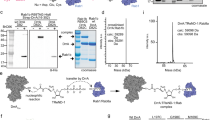
Extended Data Fig. 2 Hydrolysis of ADPRR42-Ub by MavL.
a, Mass spectrometric analysis of Ub, ADPRR42-Ub and the MavL-produced Ub from ADPRR42-Ub. The peak of the MavL product overlapped with that of Ub. b, In vitro ARH assays of MavL toward ADPR–actin. The ADP-ribosyl hydrolysation of ADPRR42-Ub and ADPR–actin was examined by SDS–PAGE and immunoblotting with the anti-ADPR antibody. c,d, In vitro ARH assays of MavL toward ADPR–PARP1 (c) and ADPR–CycT1 (d). Recombinant auto-ADP-ribosylated PARP1 protein (ADPR–PARP1), and ADPR–CycT1 that was immunoprecipitated from H2O2-treated 293T cells were incubated with MavL. *, nonspecific band (c). e, Interactions of the inactive D323A mutant of MavL with ADP-ribosylated Ub and polyUb. Ub and polyUb immunoprecipitated from 293T cells were ADP-ribosylated by the SdeA mART ___domain and then incubated with the FLAG-tagged D323A mutant that was also expressed and immunoprecipitated from 293T cells. The interactions were examined by immunoprecipitation with the anti-HA antibody. f, MavL had no effects on the total ADP-ribosylation level of proteins in cells. The total ADP-ribosylation level in the cells was examined by immunoblotting with an anti-ADPR antibody. A PARP1 inhibitor significantly reduced the total ADP-ribosylation level in cells. g, MavL is not a NADase and had no effects on the NAD level in cells. Plasmids containing MavL or the NADase toxin TNT50 of Mycobacterium tuberculosis were transfected into 293T cells. The total amount of NAD/NADH in the cells was examined with a NAD/NADH detection kit and BioTek Microplate Reader. Data represent the mean ± SD of four replicates, shown as black dots (n = 4). The P values were calculated by one-way, two-tailed ANOVA. A post hoc Dunnett’s multiple comparisons test was further conducted to compare differences between the two groups in GraphPad Prism 8.0. NS, not significant P > 0.05, **P < 0.01, ****P < 0.0001. h, Schematic diagram of the ARH activity of MavL. i, Detection of ADPRR42-Ub in the L. pneumophila-infected cells. The cell lysates of U937 cells infected with the wild-type Lp02 strain or the ΔmavL strain were subjected to 14% SDS–PAGE and immunoblotting with the anti-ADPR and anti-Ub antibodies.
Extended Data Fig. 3 ATP is the ligand and actin is the host activator of LnaB.
a, Representative screening results of the effectors responsible for producing ADPRR42-Ub from PRR42-Ub. PRR42-Ub was treated with the cell lysates of 293T cells transfected with the plasmids encoding the indicated effectors and analysed by SDS–PAGE. b, Other representative screening results of the effectors responsible for producing ADPRR42-Ub from PRR42-Ub. c, Discrimination of PRR42-Ub and ADPRR42-Ub by 13% phosphate-affinity Phos-tag SDS–PAGE. d, GFP-LnaB immunoprecipitated from 293T cells was incubated with PRR42-Ub with the ligand mixture of AMP, ADP, ATP and NAD. Effects of depletion of each ligand in the reactions were examined by immunoblotting. e, Immunoblotting analysis of the treatment of PRR42-Ub by recombinant GST–LnaB protein that was purified from E. coli. f, GST and GST-tagged LnaB purified from E. coli were incubated with 293T cell lysates. After pull-down, GST or GST-tagged LnaB reacted with PRR42-Ub in the presence of ATP. ADPRR42-Ub was examined by immunoblotting with the anti-ADPR antibody. g, PRR42-Ub was incubated with recombinant LnaB protein purified from E. coli and the proteinase K-treated or untreated 293T cell lysates. The samples were analysed by SDS–PAGE. h, Recombinant GST–LnaB protein purified from E. coli was incubated with the 293T cell lysates. After GST pull-down, the samples were analysed by SDS–PAGE and silver staining. The band of actin on the gel is highlighted with an arrow. i, List of the proteins identified by mass spectrometry analysis of the protein band bound by LnaB on SDS–PAGE in (h). j, Interactions of the LnaB fragments with endogenous actin in 293T cells. k, The activities of the LnaB fragments. PRR42-Ub was treated by the cell lysates of 293T cells expressing GFP-tagged LnaB fragments. l, LnaB and LHLS inhibit yeast growth. LnaB HE/AA, the double mutant of H305A and E309A of LnaB; LHLS HE/AA, the double mutant of H287A and E291A of LHLS.
Extended Data Fig. 4 Mutagenetic and mass spectrometric analysis of LnaB AMPylase activity.
a, GST pull-down assay of the interactions of the LnaB HE/AA mutant and G-actin. b, The AMPylase activities of the LnaB mutants H305A, E309A, H305A/E309A and D312A. The recombinant LnaB mutant proteins purified from E. coli were incubated with PRR42-Ub, actin and ATP. PRR42-Ub and ADPRR42-Ub were examined by 13% Phos-tag SDS–PAGE. c, The AMPylase activities of the LnaB mutants E156A, E276A, E281A and Y348A. d, Mass spectrometric analyses of the R42-containing peptide (EGIPPDQQRLIFAGK) in PRR42-Ub and the LnaB-produced ADPRR42-Ub. e, Determination of the phosphoribosylation site on PRR42-Ub that was generated by SdeA in vitro and used as the substrate of LnaB by ETD MS/MS. The MS/MS spectrum of the phosphoribosylated peptide EGIPPDQQRPRLIFAGK is shown as indicated. f, Schematic diagram of the activities of LnaB and MavL. g, The reversal of the SdeA-catalysed noncanonical ubiquitination by LnaB and MavL. *, a nonspecific band of the crosslinked PR-Ub. h, Localization of LnaB on the Legionella-containing vacuoles during infection. U937 cells were infected with the Lp02 and Lp03 strains that were transformed with the pJB908 plasmid containing the LnaB gene with an N-terminal HA tag for 3 h and examined by immunofluorescence with Zeiss LSM710 confocal microscope. The scale bar represents 5 μm. i,j, Double deletion of mavL and lnaB genes inhibited the auto-ubiquitination of TRAF6 (i) and degradation of IκBα (j) during infection. k, Effects of the gene deletion of mavL and lnaB on the ubiquitination of RIPK1 in 293T cells infected with the indicated L. pneumophila Lp02 strains. Ubiquitination of RIPK1 in the cells was analysed by immunoblotting after immunoprecipitation. l, Effects of the gene deletion of mavL and lnaB on the ubiquitination of RIPK3 in U937 cells infected with the indicated Lp02 strains. *, the heavy chain of antibody.
Extended Data Fig. 5 AMPylase activities of LnaB family members.
a, Recombinant GST-tagged LnaB and its truncated mutant (residues 1–327), which were purified from E. coli, were incubated with the Acanthamoeba castellanii cell lysate. The GST pull-down assays were analysed by SDS–PAGE and immunoblotting with an anti-α-actin antibody recognizing the amoebic actin. b, The actin-contained cell lysates of A. castellanii amoebae activated the AMPylase activity of LnaB. c, The intracellular growth of the L. pneumophila strains in amoebae and macrophage cells. Acanthamoeba castellanii and U937 cells were infected with the indicated L. pneumophila strains. Experiments were performed in triplicate and repeated three times with similar results. error bars represent s.d. (n = 3). d, Multiple-sequence alignment of the residues around the two conserved catalytic consensuses of LnaB family members. 40 homologous sequences were selected for analysis. e,f, Interactions of the fragments of LnaB (e,f), LHLS (e), LHEI (e) and LHBA proteins (f), respectively, with endogenous actin in transfected 293T cells. g,h, The actin-activated AMPylase activities of the LnaB homologues. PRR42-Ub was incubated by recombinant LnaB, LHLS (g), LHEI (g) and LHBA proteins (h), respectively, with actin and ATP. *, the degraded fragments of GST-LHEI. i, The AlphaFold2-predicted structure of LnaB. The prediction quality is indicated by the pLDDT (predicted Local Distance Difference Test) scores ranging from blue for high confidence to red for low confidence. The catalytic ___domain of LnaB is highlighted by a black dashed box. j, Sequence coverage scores of LnaB in the structure prediction. k, The pLDDT scores of the five AlphaFold2-predicted LnaB models. l, Comparison of the AlphaFold2-predicted structures of the catalytic domains of LHLS, LHBA and LHEI with that of LnaB.
Extended Data Fig. 6 Cryo-EM data processing, and the cryo-EM structure of the LHLS(H287A) mutant in complex with actin and ATP.
a, Cryo-EM data processing flowchart and local resolution distributions of the reconstructions of the wild-type LHLS–actin–AMP (left) and LHLS(H287A)–actin–ATP (right) complexes. Representative 2D class averages of the particles obtained from both datasets are shown as indicated. The orientation distributions of the particles are calculated in cryoSPARC. The number of particles for each viewing angle is shown in the heat map. The FSC curves of the final reconstructions are displayed as indicated. The average resolutions of wild-type LHLS–actin-AMP and LHLS(H287A)–actin–ATP complexes were estimated at 2.7 Å and 3.0 Å, respectively, using an FSC threshold of 0.143. b,c, Cryo-EM density maps of the wild-type LHLS–actin–AMP (b) and LHLS(H287A)–actin–ATP (c) complexes at contour levels 0.82 and 0.47. d,e, Densities of AMP in the wild-type LHLS–actin–AMP complex (d) and of ATP in the LHLS(H287A)–actin–ATP complex (e) in experimental (contour level, AMP: 0.76, ATP: 0.508) and deepEMhancer (contour level, AMP: 0.0157, ATP: 0.0377) sharpened maps. The magnesium ion was shown as a sphere in green (e). f, Cryo-EM structure of the LHLS(H287A)–actin–ATP complex. ATP is shown as sticks in grey. g, Structural comparison of the wild-type LHLS structure (cyan) determined by cryo-EM with the AlphaFold2-predicted structures of LHLS (light purple) and LnaB (grey). The C-terminal flexible regions of LHLS and LnaB are not included in structural comparison. h, Detailed interactions of ATP with the catalytic pocket of LHLS in the LHLS(H287A)–actin–ATP complex. Hydrogen bonds are indicated as blue dashed lines.
Extended Data Fig. 7 Mass spectrometric analyses of the AMPylation of pY-containing and pT-containing peptides by LnaB.
a, MS/MS analysis of the 9-amino acid tyrosine-phosphorylated peptide that was used as the substrate of LnaB in Fig. 5c. b, MS detection of the 9-amino acid tyrosine-phosphorylated peptide and the LnaB-produced ADP-Y peptide after AMPylation. Extracted ion chromatograms of the protonated peptides are shown with peak intensities, which indicate the relative amounts of the pY-containing (m/z = 559.72) and the ADP-Y-containing (m/z = 724.25) peptides. The modified tyrosine was labelled as YADP in the peptide. c, MS/MS analysis of the 13-amino acid tyrosine-phosphorylated peptide derived from the FcγRII-B1 receptor that was used as the substrate of LnaB. d, MS/MS analysis of the LnaB-produced ADP-Y peptide of FcγRII-B1. e, MS detection of the 13-amino acid tyrosine-phosphorylated peptide of FcγRII-B1 and the LnaB-modified ADP-Y peptide of FcγRII-B1. f, MS/MS analysis of the 12-amino acid threonine-phosphorylated peptide SLPpTPPTREPKK of Tau, which was used as the substrate of LnaB in Fig. 5e. g, MS detection of the 12-amino acid threonine-phosphorylated peptide of Tau and the 12-amino acid ADP-T peptide after modification by LnaB.
Extended Data Fig. 8 Mass spectrometric analyses of the AMPylation of threonine-phosphorylated and serine-phosphorylated peptides by LnaB.
a, MS/MS analysis of the 10-amino acid threonine-phosphorylated peptide APKpTPPSSGE of Tau, which was used as the substrate of LnaB. b, MS detection of the 10-amino acid threonine-phosphorylated peptide of Tau and the 10-amino acid ADP-T peptide after modification by LnaB. Extracted ion chromatograms of the protonated peptides are shown with peak intensities, which indicate the relative amounts of the pT-containing (m/z = 525.73) and the ADP-T (m/z = 690.26) peptides. c, MS/MS analysis of AMPylation on the 10-amino acid threonine-phosphorylated peptide of Tau by LnaB. d, MS/MS analysis of the 8-amino acid serine-phosphorylated peptide NNKGpSAAW of TAK1, which was used as the substrate of LnaB in Fig. 5f. e, MS detection of the 8-amino acid serine-phosphorylated peptide of TAK1 and the 8-amino acid ADP-S peptide after modification by LnaB. f, AMPylation of the okadaic acid- stimulated 293T cell lysates by FLAG-tagged LHEI proteins immunoprecipitated from the LHEI-overexpressed cells. The samples were analysed by SDS–PAGE and immunoblotting. *, nonspecific bands. g, Screening of AMPylation of various kinases by LnaB during infection. 293T cells expressing NPM-ALK, GFP-TBK1, 3×FLAG-IKK or 3×FLAG-AKT1 were infected with the wild-type Lp02 or ΔlnaB strain of L. pneumophila for 6 h. After immunoprecipitation, AMPylation was analysed by immunoblotting. h, MS/MS analysis of the activation loop peptide LIKDDEpYNPCQGSK of Fgr during infection of the L. pneumophila ΔlnaB strain.
Extended Data Fig. 9 Phosphoryl AMPylation of Fgr and Src by LnaB during infection.
a, 293T cells expressing C- terminal FLAG-tagged Fgr or Src were infected with the wild-type Lp02 or ΔlnaB strain of L. pneumophila. After immunoprecipitation with the anti-FLAG antibody, AMPylation of the kinases were analysed by SDS–PAGE and immunoblotting. b, AMPylation of an amoebic Fgr homologue by LnaB. 293T cells were co-transfected with the LnaB variants and the FLAG-tagged Fgr homologue from A. castellanii. AMPylation of the amoebic Fgr homologue was analysed by immunoblotting after anti-FLAG immunoprecipitation. c, AMPylation of endogenous Src by LnaB during infection. 293T cells were infected with the indicated L. pneumophila strains. AMPylation of Src was analysed by SDS–PAGE and western blotting after immunoprecipitation with the anti-Src antibody. d, 293T cells expressing the C-terminal HA-tagged Fgr variants were infected with the wild-type Lp02 stain of L. pneumophila. AMPylation of Fgr variants was analysed by western blotting after immunoprecipitation. e, The phosphorylation of Y416 and AMPylation of Src in 293T cells transfected with the LnaB variants were analysed by western blotting with an antibody specific for pY416 and anti-AMP antibody, respectively. f, Mass spectrometric analyses of the Y416-containing peptide (LIEDNEYTARQGAK) in p-Src and the LnaB-produced ADP-Src. g, MS/MS analysis of the AMPylation site on Src during infection. The fragment ions b7, b8, b10, b13 and y8, y10, y11, y12, y13 have a mass increase of 409 Da, corresponding to the addition of one ADP group, whereas the fragment ions b2 to b5 and y6, y7 do not exhibit this mass shift, confirming LnaB-catalysed AMPylation on pY416 of Src. h, Phosphorylation of endogenous Fgr in infected cells. U937 cells were infected with the indicated L. pneumophila strains at an MOI of 20 for 1 and 2 h. After immunoprecipitation with the anti-Fgr antibody, phosphorylation of Y412 of Fgr was analysed by western blotting with an antibody specific for pY412 (48984S, CST). i, 293T cells expressing the C-terminal HA-tagged Fgr Y523F were infected with the indicated L. pneumophila strains. Phosphorylation of Y412 and AMPylation of Fgr were analysed by western blotting with an anti-pY412 antibody (70926S, CST) and anti-AMP antibody, respectively.
Supplementary information
Supplementary Figure 1
Uncropped and unprocessed scans of blots and gels of Figures and Extended Data Figures.
Supplementary Table 1
The LnaB homologues and genomic analyses of the LnaB homologue-encoded bacterial strains. The locus of the 162 LnaB homologues in the bacterial genomes are listed as indicated. LnaB is highlighted in red in the list. The homologues of MavL and SidEs are also examined in the bacterial strains. The signal peptides of the type 3, 4 and 6 secretion systems in the LnaB homologues’ sequences were predicted using th program DeepT3 v2.0, the T4SEfinder web server (https://tool2-mml.sjtu.edu.cn/T4SEfinder_TAPE/), Bastion6 web server (https://bastion6.erc.monash.edu/), and DeepSecE web server (https://tool2-mml.sjtu.edu.cn/DeepSecEdb/), respectively. Type 3, 4 and 6 secretion systems in the LnaB homologue-containing bacterial strains are analysed, according to the conserve type 3 secretion system components SctC, SctJ, and ATPase SctN4, type 4 secretion system components VirD4 and VirB45-7, and type 6 secretion system components TssB and TssC, respectively.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, T., Song, X., Tan, J. et al. Legionella effector LnaB is a phosphoryl-AMPylase that impairs phosphosignalling. Nature 631, 393–401 (2024). https://doi.org/10.1038/s41586-024-07573-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-024-07573-z
This article is cited by
-
Modulation of host ATP levels by secreted bacterial effectors
Nature Communications (2025)
-
Legionella pneumophila evades host-autophagic clearance using phosphoribosyl-polyubiquitin chains
Nature Communications (2024)
-
Global atlas of predicted functional domains in Legionella pneumophila Dot/Icm translocated effectors
Molecular Systems Biology (2024)