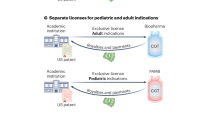
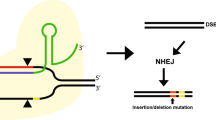
Abstract
Twenty genetic therapies have been approved by the US Food and Drug Administration to date, a number that now includes the first CRISPR genome-editing therapy for sickle cell disease—CASGEVY (exagamglogene autotemcel, Vertex Pharmaceuticals). This extraordinary milestone is widely celebrated owing to the promise for future genome-editing treatments of previously intractable genetic disorders and cancers. At the same time, such genetic therapies are the most expensive drugs on the market, with list prices exceeding US$4 million per patient. Although all approved cell and gene therapies trace their origins to academic or government research institutions, reliance on for-profit pharmaceutical companies for subsequent development and commercialization results in prices that prioritize recouping investments, paying for candidate product failures and meeting investor and shareholder expectations. To increase affordability and access, sustainable discovery-to-market alternatives are needed that address system-wide deficiencies. Here we present recommendations of a multidisciplinary task force assembled to chart such a path. We describe a pricing structure that, once implemented, could reduce per-patient cost tenfold and propose a business model that distributes responsibilities while leveraging diverse funding sources. We also outline how academic licensing provisions, manufacturing innovation and supportive regulations can reduce cost and enable broader patient treatment.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
199,00 € per year
only 3,90 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
No datasets were generated during the course of the study. Input data for the model was taken from cost ranges in the published literature and cited accordingly, or selected for illustrative purposes. The model can be provided on request from M.K.
References
Kohn, D. B., Chen, Y. Y. & Spencer, M. J. Successes and challenges in clinical gene therapy. Gene Ther. 30, 738–746 (2023). This review discusses the therapeutic potential of genetic therapies across modalities as well as key challenges.
Vokinger, K. N., Glaus, C. E. G. & Kesselheim, A. S. Approval and therapeutic value of gene therapies in the US and Europe. Gene Ther. 30, 756–760 (2023).
Katzmann, J. L., Cupido, A. J. & Laufs, U. Gene therapy targeting PCSK9. Metabolites 12, 70 (2022).
Wu, L. L. Verve won trial success with a gene-editing milestone. Why don’t investors like it? Endpoints News (14 November 2023).
Sharma, A. et al. Nomenclature for cellular and genetic therapies: a need for standardization. Transplant. Cell. Ther. 28, 795–801 (2022).
Food and Drug Administration & Center for Biologics Evaluation and Research. Cellular & Gene Therapy Products (FDA, 2023).
American Society of Gene and Cell Therapy & Citeline. Gene, Cell, +RNA Therapy Landscape Report—Q1 2024 Quarterly Data Report (ASGCT & Citeline, 2024).
Wang, J. Y. & Doudna, J. A. CRISPR technology: a decade of genome editing is only the beginning. Science 379, eadd8643 (2023).
Allen, J. et al. Medicaid coverage practices for approved gene and cell therapies: existing barriers and proposed policy solutions. Mol. Ther. Methods Clin. Dev. 29, 513–521 (2023). This paper discusses existing challenges for state Medicaid programs to cover high-priced CGTs and proposes several policy solutions to promote equitable access.
Witkowsky, L., Norstad, M., Glynn, A. R. & Kliegman, M. Towards affordable CRISPR genomic therapies: a task force convened by the Innovative Genomics Institute. Gene Ther. 30, 747–752 (2023). This paper discusses the impetus for launching the Task Force, its structure, membership, operation and decision-making frameworks.
Jensen, K. Orchard abandons promising gene therapy for rare immune disorder. BioPharma Dive (3 June 2021).
Pagliarulo, N. Bluebird, winding down in Europe, withdraws another rare disease gene therapy. BioPharma Dive (21 October 2021).
Peebles, A. Bluebird Bio sees road to revival after gene-therapy misfires. Bloomberg (9 June 2022).
Vertex. Vertex and CRISPR Therapeutics announce authorization of the first CRISPR/Cas9 gene-edited therapy, CASGEVY (exagamglogene autotemcel), by the United Kingdom MHRA for the treatment of sickle cell disease and transfusion-dependent beta thalassemia. Vertex (16 November 2023).
Wong, C. H. et al. The estimated annual financial impact of gene therapy in the United States. Gene Ther. 30, 761–773 (2023).
Klein, D. Cell and gene therapy logistics: meeting the supply chain challenge. Eureka (24 August 2022).
Amar, D. Navigating the complexities of cell and gene therapy supply chain: a CEO’s practical guide. Forbes (28 February 2023).
Papathanasiou, M. M. et al. Autologous CAR T-cell therapies supply chain: challenges and opportunities? Cancer Gene Ther. 27, 799–809 (2020).
Thomson, A. M. et al. Global, regional, and national prevalence and mortality burden of sickle cell disease, 2000–2021: a systematic analysis from the Global Burden of Disease Study 2021. Lancet Haematol. 10, e585–e599 (2023).
Food and Drug Administration. FDA approves first gene therapies to treat patients with sickle cell disease. FDA (8 December 2023).
Doxzen, K. et al. Accelerating Global Access to Gene Therapies: Case Studies from Low- and Middle-Income Countries (World Economic Forum, 2022).
Adair, J. E. et al. Towards access for all: 1st Working Group Report for the Global Gene Therapy Initiative (GGTI). Gene Ther. 30, 216–221 (2021).
Johnson, B. Reducing the costs of blockbuster gene and cell therapies in the Global South. Nat. Biotechnol. 42, 8–12 (2024).
Vokinger, K. N., Avorn, J. & Kesselheim, A. S. Sources of innovation in gene therapies—approaches to achieving affordable prices. N. Engl. J. Med. 388, 292–295 (2023).
Mulcahy, A. W., Schwam, D. & Lovejoy, S. L. International Prescription Drug Price Comparisons: Estimates Using 2022 Data. Report No. RR-A788-3 (RAND Corporation, 2024).
Becker, Z. With 2nd chance at FDA approval, BioMarin preps for hemophilia gene therapy launch in US. Fierce Pharma (29 June 2023).
Lupkin, S. Feds’ contract with Pfizer for Paxlovid has some surprises. NPR (1 February 2022).
Cohrs, R. In new Regeneron deal for Covid drug, White House imposes price limits for first time. STAT News (14 September 2023).
117th Congress (2021-2022). H.R.5376—Inflation Reduction Act of 2022. Public Law 117-169. Congress.gov https://www.congress.gov/bill/117th-congress/house-bill/5376/text (2022). This law includes provisions that enable the US Centers for Medicare and Medicaid Services (CMS) to negotiate prices for selected drugs with pharmaceutical manufacturers.
Fleischut, P. M. & Haas, S. University technology transfer offices: a status report. Biotechnol. Healthc. 2, 48–53 (2005).
California Institute of Technology et al. In the Public Interest: Nine Points to Consider in Licensing University Technology (AUTM, 2007). This accord highlights issues that should be considered by academic institutions to uphold shared core values when negotiating licensing agreements with private entities.
Contreras, J. L. “In the public interest”—university technology transfer and the nine points document—an empirical assessment. UC Irvine Law Rev. 13, 435–512 (2023). This study evaluates the impacts of the Nine Points document and, given mixed adoption among signatories and little change specifically with respect to access to health-related technologies, proposes a reorientation of university policies to better align with public health and access goals.
Menichiello, T. How creative licensing can improve patient access. Cell and Gene (16 November 2023).
Mimura, C. Collaborative innovation to advance global health solutions. Technol. Innov. 16, 99–105 (2014).
Food and Drug Administration & Center for Biologics Evaluation and Research. Guidance for Industry: Manufacturing Changes and Comparability for Human Cellular and Gene Therapy Products—Draft (FDA, 2023).
Kohn, D. B. et al. Autologous ex vivo lentiviral gene therapy for adenosine deaminase deficiency. N. Engl. J. Med. 384, 2002–2013 (2021).
Immune Deficiency Foundation. ADA-SCID gene therapy trials to resume in 2023. Immune Deficiency Foundation (13 October 2022).
Mamcarz, E. et al. Lentiviral gene therapy combined with low-dose busulfan in infants with SCID-X1. N. Engl. J. Med. 380, 1525–1534 (2019).
Cowan, M. J. et al. Lentiviral gene therapy for artemis-deficient SCID. N. Engl. J. Med. 387, 2344–2355 (2022).
Rett Syndrome Research Trust. Rett Syndrome Genetic Medicines Summit 2023, panel discussion. Rett Syndrome Research Trust https://reverserett.org/rett-syndrome-genetic-medicines-summit/#stack-1714423522-887-1 (2023).
Food and Drug Administration, Center for Drug Evaluation and Research & Center for Biologics Evaluation and Research. Guidance for industry: Platform Technology Designation Program for Drug Development—Draft (FDA, 2024).
California Institute for Regenerative Medicine. Open Manufacturing Network for Cell and Gene Therapies. Grant No. INFR5-14719 (CIRM, 2023).
National Institutes of Health Common Fund. Somatic Cell Genome Editing (SCGE). NIH Common Fund https://commonfund.nih.gov/editing (2024).
Food and Drug Administration, Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research & Office of Regulatory Affairs. Guidance for Industry: CGMP for Phase 1 Investigational Drugs (FDA, 2008).
Zu, H. & Gao, D. Non-viral vectors in gene therapy: recent development, challenges, and prospects. AAPS J. 23, 78 (2021).
Foss, D. V. et al. Peptide-mediated delivery of CRISPR enzymes for the efficient editing of primary human lymphocytes. Nat. Biomed. Eng. 7, 647–660 (2023).
Elsallab, M. & Maus, M. V. Expanding access to CAR T cell therapies through local manufacturing. Nat. Biotechnol. https://doi.org/10.1038/s41587-023-01981-8 (2023). This review discusses various models of decentralized manufacturing and outlines key opportunities, challenges and potential solutions.
BioCanRx. BioCanRx invests $10M in promising new cancer immunotherapy research and biomanufacturing to benefit Canadians. Cision (22 September 2020).
Sánchez-Guijo, F. et al. Role of hospital exemption in europe: position paper from the Spanish Advanced Therapy Network (TERAV). Bone Marrow Transplant. 58, 727–728 (2023).
Jayaraman, K. Cut-price CAR-T cell therapies top India’s biotech agenda. Nat. Biotechnol. 37, 1388–1389 (2019).
Center for Drug Evaluation and Research. Distributed Manufacturing and Point-of-Care Manufacturing of Drugs (FDA, 2022).
Moon, S., Mariat, S., Kamae, I. & Pedersen, H. B. Defining the concept of fair pricing for medicines. Brit. Med. J. 368, l4726 (2020).
National Academies of Sciences, Engineering, and Medicine, Health and Medicine Division, Board on Health Care Services & Committee on Ensuring Patient Access to Affordable Drug Therapies. Making Medicines Affordable: A National Imperative. Report No. 24946 (National Academies, 2018).
Novartis. Novartis position on value-based healthcare (Novartis, 2021).
Leschly, N. In pricing our gene therapy, Bluebird weighed value, shared risk, and a lifetime cap. STAT News (26 November 2019).
Yeung, K., Bloudek, L., Ding, Y. & Sullivan, S. D. Value-based pricing of US prescription drugs: estimated savings using reports from the Institute for Clinical and Economic Review. JAMA Health Forum 3, e224631 (2022).
Richardson, M., Rind, D., Beaudoin, F. L., Pearson, S. D. & Campbell, J. D. The fair price for one-time treatments; how can we overcome existing market price distortions? Health Affairs (19 January 2023).
Hyde, R. & Dobrovolny, D. Orphan drug pricing and payer management in the United States: are we approaching the tipping point? Am. Health Drug Benefits 3, 15–23 (2010).
World Health Organization. WHO Guideline on Country Pharmaceutical Pricing Policies, Second Edition (WHO, 2020).
NEWDIGS FoCUS Project. Emerging Market Solutions for Financing and Reimbursement of Durable Cell and Gene Therapies (MIT, 2021).
Slocomb, T., Werner, M., Haack, T., Valluri, S. & Rader, B. New Payment And Financing Models For Curative Regenerative Medicines (Alliance for Regenerative Medicine, 2017).
PwC. Six drug pricing models have emerged to improve product access and affordability. PwC (23 September 2019).
Wouters, O. J., McKee, M. & Luyten, J. Estimated research and development investment needed to bring a new medicine to market, 2009-2018. JAMA 323, 844–853 (2020). This study analyses available data on research and development costs of over 60 therapeutics approved between 2009 and 2018 to estimate the capital needed to bring a new drug to market.
Vieira, M. Research synthesis: costs of pharmaceutical R&D. Geneva Graduate Institute www.knowledgeportalia.org/_files/ugd/356854_e9d75e29c0264bf9b38118fc5f0aeab6.pdf (2020).
DiMasi, J. A., Grabowski, H. G. & Hansen, R. W. Innovation in the pharmaceutical industry: new estimates of R&D costs. J. Health Econ. 47, 20–33 (2016).
Centers for Medicare & Medicaid Services. Medicare Drug Price Negotiation Program: Initial Memorandum, Implementation of Sections 1191–1198 of the Social Security Act for Initial Price Applicability Year 2026, and Solicitation of Comments (CMS, 2023).
Pereira Chilima, T. D., Moncaubeig, F. & Farid, S. S. Estimating capital investment and facility footprint in cell therapy facilities. Biochem. Eng. J. 155, 107439 (2020).
Spink, K. & Steinsapir, A. The long road to affordability: a cost of goods analysis for an autologous CAR-T process. Cell. Gene Ther. Insights 4, 1105–1116 (2018).
Hodgson, V., Lemenze, M., Edwards, E. & Tomtishen, J. Cost-Benefit Analysis of Using the Cellares Cell Shuttle Platform for Autologous CAR-T Cell Therapies (Cellares, 2023).
Lee, J. Cell therapy manufacturing: build or buy? Labcompare (14 February 2023).
Kansteiner, F. Bayer, intent on its cell therapy ambitions, plots $200M plant at overhauled Berkeley campus: report. Fierce Pharma (26 April 2021).
Ten Ham, R. M. T. et al. What does cell therapy manufacturing cost? A framework and methodology to facilitate academic and other small-scale cell therapy manufacturing costings. Cytotherapy 22, 388–397 (2020).
Angelis, A., Polyakov, R., Wouters, O. J., Torreele, E. & McKee, M. High drug prices are not justified by industry’s spending on research and development. Brit. Med. J. 380, e071710 (2023).
Trombetta, B. 22nd annual industry audit: the pharma value picture. Pharm. Exec. 43, 24–29 (2023).
Lopes, A., Noel, R. & Sinclair, A. Cost analysis of vein-to-vein CAR T-cell therapy: automated manufacturing and supply chain. Cell Gene Ther. Insights 6, 487–510 (2020).
Lachaine, J., Jain, M. D., Bibeau, J., Marino, J. P. & Ball, G. PCN191 cost associated with implementation of CAR T-cell therapy for the management of hematologic cancers in Canada. Value Health 24, S55 (2021).
Code of Federal Regulations. Title 21, Sec. 316.31: Scope of Orphan-Drug Exclusive Approval. 21 CFR 316.31 (CFR, 2024).
Suleman, F., Low, M., Moon, S. & Morgan, S. G. New business models for research and development with affordability requirements are needed to achieve fair pricing of medicines. Brit. Med. J. 368, l4408 (2020).
Vieira, M. F., Kimmitt, R., Navarro, D., Bezruki, A. & Moon, S. in Partnerships for Sustainability in Contemporary Global Governance 1st edn (eds Andonova, L. B. et al.) Ch. 5 (Routledge, 2022).
Moon, S., Vieira, M., Alonso Ruiz, A. & Navarro, D. New Business Models for Pharmaceutical Research and Development as a Global Public Good: Considerations for the WHO European Region (WHO, 2022).
Dredge, C. & Scholtes, S. The health care utility model: a novel approach to doing business. NEJM Catalyst (8 July 2021). This commentary outlines the structural and financial pillars of a healthcare utility model that can sustainably manufacture generics.
NEWDIGS FoCUS Project. Are Cell and Gene Therapy programs a better bet? (Tufts Medical Center, 2023).
OECD. Social Impact Investment 2019: The Impact Imperative for Sustainable Development (OECD, 2019).
117th Congress (2021-2022). H.R.3437—LOANS for Biomedical Research Act. Congress.gov www.congress.gov/bill/117th-congress/house-bill/3437/text (2021).
Impact Entrepreneur & Rockefeller Philanthropy Advisors. Building an Impact Economy: A Call to Action for the Philanthropy Sector (Mission Investors, 2019).
Jensen, K. BioMarin secures hemophilia gene therapy coverage in Germany. Biopharma Dive (29 November 2023).
Van Kirk, A.-H. & Maltby, J. EU payment fight could cut 40% from gene therapy sales. Bloomberg (11 May 2023).
Lewin, K. Orchard sets US price tag for rare disease gene therapy Lenmeldy at $4.25M. Endpoints News (20 March 2024).
Waxman Strategies. Nonprofit Pharmaceutical Companies: Background, Challenges, and Policy Options (Waxman, 2019). This report outlines the distinct challenges non-profit pharmaceutical entities face and proposes policy solutions to promote their success.
Ridley, D. B., Grabowski, H. G. & Moe, J. L. Developing drugs for developing countries. Health Aff. 25, 313–324 (2006).
United States Government Accountability Office. Drug Development: FDA’s Priority Review Voucher Programs. Report No. GAO-20-251 (GAO, 2020).
Rare Disease Company Coalition. Impact of the Priority Review Voucher Program on Rare Pediatric Disease Drug Development (Rare Disease Company Coalition, 2024).
Hwang, T. J., Bourgeois, F. T., Franklin, J. M. & Kesselheim, A. S. Impact of the priority review voucher program on drug development for rare pediatric diseases. Health Aff. 38, 313–319 (2019).
Jain, N., Hwang, T., Franklin, J. M. & Kesselheim, A. S. Association of the priority review voucher with neglected tropical disease drug and vaccine development. JAMA 318, 388–389 (2017).
Sinha, M. S., Jain, N., Hwang, T. & Kesselheim, A. S. Expansion of the Priority Review Voucher program under the 21st Century Cures Act: implications for innovation and public health. Am. J. Law Med. 44, 329–341 (2018).
Aerts, C., Barrenho, E., Miraldo, M. & Sicuri, E. The impact of the Priority Review Voucher on research and development for tropical diseases. Pharm. Med. 36, 189–197 (2022).
Ridley, D. B., Ganapathy, P. & Kettler, H. E. US tropical disease Priority Review Vouchers: lessons in promoting drug development and access. Health Aff. 40, 1243–1251 (2021).
Fermaglich, L. J. & Miller, K. L. A comprehensive study of the rare diseases and conditions targeted by orphan drug designations and approvals over the forty years of the Orphan Drug Act. Orphanet J. Rare Dis. 18, 163 (2023).
Innovative Genomics Institute. Making Genetic Therapies Affordable and Accessible (IGI, 2023).
Acknowledgements
We thank the members (Supplementary Table 1) of the Innovative Genomics Institute (IGI) Affordability and Accessibility Task Force, as well as the many individuals who presented to our Task Force, and particularly the patients and patient advocates for their efforts; our donors—the Doris Duke Charitable Foundation, Arnold Ventures and the Armstead-Barnhill Foundation for Sickle Cell Anemia (CureSickleCell.com); and G. Ramit for assistance with figures and graphics.
Author information
Authors and Affiliations
Contributions
M.K. and J.A.D. initiated the task force from which the information presented here originated. M.K. managed and chaired the task force. The following authors each chaired one of four subgroups of the task force: F.D.U. (organizational and funding models), J.H.E. (regulations and manufacturing), R.C.W. (pricing strategies/access) and S.A. (intellectual property management and licensing). M.K. and M.Z. drafted, edited and finalized the manuscript.
Corresponding authors
Ethics declarations
Competing interests
J.A.D. is a co-founder of Caribou Biosciences, Editas Medicine, Scribe Therapeutics, Intellia Therapeutics and Mammoth Biosciences. She is a scientific advisory board member of Vertex Pharmaceuticals, Caribou Biosciences, Intellia Therapeutics, Scribe Therapeutics, Mammoth Biosciences, Algen Biotechnologies, Felix Biosciences, The Column Group and Inari. J.A.D. is Chief Science Advisor to Sixth Street, a Director at Johnson & Johnson, Altos and Tempus, and has research projects sponsored by Apple Tree Partners and Roche. The Regents of the University of California have patents issued and pending for CRISPR technologies on which J.A.D. is listed as an inventor. J.H.E. is a paid advisor to and receives sponsored research funding from Multiply Labs. He serves on its scientific advisory board and holds equity in the company. He is a paid advisor to and serves on the scientific advisory board of Shennon Biotechnologies and holds equity in the company. He receives sponsored research funding from Lonza, for the development of cellular therapy manufacturing devices. His research group received funding from Arsenal Bio. He is named as an inventor on patent application for CRISPR-based gene editing (WO2021183850A1). F.D.U. is a paid advisor to and holds equity in Tune Therapeutics and Cimeio Therapeutics, is a paid advisor to Ionis Pharmaceuticals, a paid consultant to Vertex Pharmaceuticals and receives salary support from Danaher. R.C.W. is a co-founder of EditPep. The other authors declare no competing interests.
Peer review
Peer review information
Nature thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ethical Approval: No experimentation occurred on humans or other animals. Ethical approval was not required.
Supplementary information
Supplementary Table 1
List of the task force members involved in the study.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kliegman, M., Zaghlula, M., Abrahamson, S. et al. A roadmap for affordable genetic medicines. Nature 634, 307–314 (2024). https://doi.org/10.1038/s41586-024-07800-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-024-07800-7
This article is cited by
-
Unlocking patient access to gene therapy: five key practices
Gene Therapy (2025)
-
Tailored therapeutics for cardiomyopathies
Nature Reviews Cardiology (2025)
-
From precision interventions to precision health
Nature Communications (2025)
-
Current Clinical Trials for Treating Elevated Lipoprotein(a)
Current Cardiovascular Risk Reports (2025)
-
The state-of-the-art of N-of-1 therapies and the IRDiRC N-of-1 development roadmap
Nature Reviews Drug Discovery (2025)