Abstract
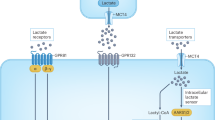
l-lactate modifies proteins through lactylation1, but how this process occurs is unclear. Here we identify the alanyl-tRNA synthetases AARS1 and AARS2 (AARS1/2) as intracellular l-lactate sensors required for l-lactate to stimulate the lysine lactylome in cells. AARS1/2 and the evolutionarily conserved Escherichia coli orthologue AlaRS bind to l-lactate with micromolar affinity and they directly catalyse l-lactate for ATP-dependent lactylation on the lysine acceptor end. In response to l-lactate, AARS2 associates with cyclic GMP–AMP synthase (cGAS) and mediates its lactylation and inactivation in cells and in mice. By establishing a genetic code expansion orthogonal system for lactyl-lysine incorporation, we demonstrate that the presence of a lactyl moiety at a specific cGAS amino-terminal site abolishes cGAS liquid-like phase separation and DNA sensing in vitro and in vivo. A lactyl mimetic knock-in inhibits cGAS, whereas a lactyl-resistant knock-in protects mice against innate immune evasion induced through high levels of l-lactate. MCT1 blockade inhibits cGAS lactylation in stressed mice and restores innate immune surveillance, which in turn antagonizes viral replication. Thus, AARS1/2 are conserved intracellular l-lactate sensors and have an essential role as lactyltransferases. Moreover, a chemical reaction process of lactylation targets and inactivates cGAS.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
199,00 € per year
only 3,90 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The genomics and transcriptomics data reported in this paper have been deposited into the Genome Sequence Archive in the National Genomics Data Center, China National Center for Bioinformation (accession numbers HRA007223 and HRA007096). MS data have been deposited into the OMIX, China National Center for Bioinformation/Beijing Institute of Genomics, Chinese Academy of Sciences (accession numbers OMIX006151, OMIX006152 and OMIX006153). Gene sets for gene set enrichment analysis were obtained from the MSigDB database (v.3.0; https://www.gsea-msigdb.org/gsea/downloads.jsp, September 2010 release). Full versions of all blots are provided in Supplementary Fig. 1. The gating strategy for the flow cytometry experiments are provided as Supplementary information. All information supporting the conclusions are provided with the paper. Source data are provided with this paper.
References
Zhang, D. et al. Metabolic regulation of gene expression by histone lactylation. Nature 574, 575–580 (2019).
Kraut, J. A. & Madias, N. E. Lactic acidosis. N. Engl. J. Med. 371, 2309–2319 (2014).
Certo, M., Tsai, C. H., Pucino, V., Ho, P. C. & Mauro, C. Lactate modulation of immune responses in inflammatory versus tumour microenvironments. Nat. Rev. Immunol. 21, 151–161 (2021).
Felmlee, M. A., Jones, R. S., Rodriguez-Cruz, V., Follman, K. E. & Morris, M. E. Monocarboxylate transporters (SLC16): function, regulation, and role in health and disease. Pharmacol. Rev. 72, 466–485 (2020).
Wang, N. et al. Structural basis of human monocarboxylate transporter 1 inhibition by anti-cancer drug candidates. Cell 184, 370–383.e13 (2021).
Chen, Y. et al. Metabolic regulation of homologous recombination repair by MRE11 lactylation. Cell 187, 294–311.e21 (2024).
Moreno-Yruela, C. et al. Class I histone deacetylases (HDAC1–3) are histone lysine delactylases. Sci. Adv. 8, eabi6696 (2022).
Colegio, O. R. et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature 513, 559–563 (2014).
Faubert, B. et al. Lactate metabolism in human lung tumors. Cell 171, 358–371.e9 (2017).
Ablasser, A. & Chen, Z. J. cGAS in action: expanding roles in immunity and inflammation. Science 363, eaat8657 (2019).
Ishikawa, H., Ma, Z. & Barber, G. N. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature 461, 788–792 (2009).
Wu, J. et al. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science 339, 826–830 (2013).
Sun, L., Wu, J., Du, F., Chen, X. & Chen, Z. J. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 339, 786–791 (2013).
Gao, P. et al. Cyclic [G(2′,5′)pA(3′,5′)p] is the metazoan second messenger produced by DNA-activated cyclic GMP-AMP synthase. Cell 153, 1094–1107 (2013).
Ablasser, A. et al. cGAS produces a 2′-5′-linked cyclic dinucleotide second messenger that activates STING. Nature 498, 380–384 (2013).
Diner, E. J. et al. The innate immune DNA sensor cGAS produces a noncanonical cyclic dinucleotide that activates human STING. Cell Rep. 3, 1355–1561 (2013).
Zhang, X. et al. Cyclic GMP-AMP containing mixed phosphodiester linkages is an endogenous high-affinity ligand for STING. Mol. Cell 51, 226–235 (2013).
Dai, J. et al. Acetylation blocks cGAS activity and inhibits self-DNA-induced autoimmunity. Cell 176, 1447–1460.e14 (2019).
Li, T. et al. Phosphorylation and chromatin tethering prevent cGAS activation during mitosis. Science 371, eabc5386 (2021).
Barnett, K. C. et al. Phosphoinositide interactions position cGAS at the plasma membrane to ensure efficient distinction between self- and viral DNA. Cell 176, 1432–1446.e11 (2019).
Brooks, G. A. The science and translation of lactate shuttle theory. Cell Metab. 27, 757–785 (2018).
Ibba, M. & Soll, D. Aminoacyl-tRNA synthesis. Annu. Rev. Biochem. 69, 617–650 (2000).
Soderberg, O. et al. Characterizing proteins and their interactions in cells and tissues using the in situ proximity ligation assay. Methods 45, 227–232 (2008).
de la Torre, D. & Chin, J. W. Reprogramming the genetic code. Nat. Rev. Genet. 22, 169–184 (2021).
Chin, J. W. Expanding and reprogramming the genetic code of cells and animals. Annu. Rev. Biochem. 83, 379–408 (2014).
Chin, J. W. Expanding and reprogramming the genetic code. Nature 550, 53–60 (2017).
Neumann, H., Peak-Chew, S. Y. & Chin, J. W. Genetically encoding Nε-acetyllysine in recombinant proteins. Nat. Chem. Biol. 4, 232–234 (2008).
Gao, D. et al. Activation of cyclic GMP-AMP synthase by self-DNA causes autoimmune diseases. Proc. Natl Acad. Sci. USA 112, E5699–E5705 (2015).
Gluck, S. et al. Innate immune sensing of cytosolic chromatin fragments through cGAS promotes senescence. Nat. Cell Biol. 19, 1061–1070 (2017).
Dou, Z. et al. Cytoplasmic chromatin triggers inflammation in senescence and cancer. Nature 550, 402–406 (2017).
Yu, C. H. et al. TDP-43 triggers mitochondrial DNA release via mPTP to activate cGAS/STING in ALS. Cell 183, 636–649.e18 (2020).
Sprenger, H. G. et al. Cellular pyrimidine imbalance triggers mitochondrial DNA-dependent innate immunity. Nat. Metab. 3, 636–650 (2021).
Mackenzie, K. J. et al. cGAS surveillance of micronuclei links genome instability to innate immunity. Nature 548, 461–465 (2017).
Harding, S. M. et al. Mitotic progression following DNA damage enables pattern recognition within micronuclei. Nature 548, 466–470 (2017).
Stetson, D. B., Ko, J. S., Heidmann, T. & Medzhitov, R. Trex1 prevents cell-intrinsic initiation of autoimmunity. Cell 134, 587–598 (2008).
Gray, E. E., Treuting, P. M., Woodward, J. J. & Stetson, D. B. Cutting edge: cGAS is required for lethal autoimmune disease in the Trex1-deficient mouse model of Aicardi–Goutieres syndrome. J. Immunol. 195, 1939–1943 (2015).
Bourin, M., Petit-Demouliere, B., Dhonnchadha, B. N. & Hascoet, M. Animal models of anxiety in mice. Fundam. Clin. Pharmacol. 21, 567–574 (2007).
Steimer, T. Animal models of anxiety disorders in rats and mice: some conceptual issues. Dialogues Clin. Neurosci. 13, 495–506 (2011).
Choudhary, C., Weinert, B. T., Nishida, Y., Verdin, E. & Mann, M. The growing landscape of lysine acetylation links metabolism and cell signalling. Nat. Rev. Mol. Cell Biol. 15, 536–550 (2014).
Varner, E. L. et al. Quantification of lactoyl-CoA (lactyl-CoA) by liquid chromatography mass spectrometry in mammalian cells and tissues. Open Biol. 10, 200187 (2020).
Kim, S. C. et al. A clean, more efficient method for in-solution digestion of protein mixtures without detergent or urea. J. Proteome Res. 5, 3446–3452 (2006).
Chen, Y. et al. Quantitative acetylome analysis reveals the roles of SIRT1 in regulating diverse substrates and cellular pathways. Mol. Cell. Proteomics 11, 1048–1062 (2012).
Acknowledgements
We are grateful to Hailan Hu for valuable recommendation about mice models. The current work was supported by the Chinese National Natural Science Funds (31925013, U20A20393 and W2411011), a special programme from the Ministery of Science and Technology of China (2021YFA1101000), a Key R&D Program of Zhejiang Province (2024C03142) and the Joint Project of Pinnacle Disciplinary Group, the Second Affiliated Hospital of Chongqing Medical. We acknowledge support from the platform of Life Sciences Institute and the Zhejiang University School of Medicine.
Author information
Authors and Affiliations
Contributions
L. Zhang, H. Li and R.L. designed the experiments and analysed the data. H. Li, C.L., L. Zhou, Y.R. and Q.Y. performed the experiments. B.Y. performed the MS analysis. H.R. performed the structural modelling. H.H., H. Lu, H.S. and S.L. provide valuable discussion. L. Zhang and H. Li wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Ileana Cristea, Pengda Liu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 MCT1-mediated L-lactate uptake inhibits innate immunity by suppressing cGAS activity.
a, Upper: volcano plots (ggplot2) of the RNA-seq analysis of Peripheral Blood Mononuclear Cells (PBMCs) from Normal (Nor.), High-Lac (H-lac) and Lac-Acidosis (LA) patients. The significantly up- or down- regulated genes are reported as red or blue dots, respectively. Left: differential expression analysis in the LA versus the H-Lac groups (edgeR, P < 0.05 and log2 (FC) > 1). Middle and right: differential expression analysis in the H-Lac and LA groups compared to the Nor. groups (edgeR, P < 0.0001 and log2 (FC) > 2), respectively. Lower: Gene Ontology (GO) analysis of the top 10 enriched pathways are listed. Up- or down- regulated pathways are reported as red or blue strips, respectively. Pathways related to immune response are highlighted in brown. Functional enrichment analysis using DAVID functional annotation tool with the Gene Ontology biological process term annotations. P values were derived from DAVID v.6.8 database (https://david.ncifcrf.gov/), which uses a modified one-sided Fisher’s exact test with Benjamini correction. b, Peritoneal macrophages (PM) from wild-type mice pre-treated with control PBS, L-lactate (Lac, 25 mM) or sodium L-lactate (NaLac, 25 mM) were infected for 12 h with mock (PBS), HCMV, HSV-1 (MOI (multiplicity of infection), 10). respectively, or transfected with HT-DNA or cGAMP for 12 h. ELISA of cellular cGAMP (upper) and IFN-β (lower) were shown. c, Immunoblot (IB) analysis of monomeric (Mono) and dimeric (Dimer) IRF3 (top blot; native PAGE) and total lysates (bottom blot; denaturing gel) of primary peritoneal macrophages pre-treated with control PBS, L-lactate (25 mM) or NaLac (25 mM) for 12 h, followed by infection with HSV-1 (left) or HCMV (right) for indicated time points. d, Immunoblot (IB) analysis of monomeric (Mono) and dimeric (Dimer) IRF3 (top blot; native PAGE) and total lysate (bottom blot; denaturing gel) of primary peritoneal macrophages pre-treated for 12 h with PBS, L-lactate (25 mM) or NaLac (25 mM), followed by transfection with HT-DNA (2.5 ng/ul) or cGAMP (100 nM) for 12 h. e, Measurement of intracellular L-lactate in PBMCs, BMDMs, CT26 and HCT116 cells. PBMCs and BMDMs were pre-treated with control DMSO, AR-C155858 (100 nM), Oxamate (50 mM) or AZD3965 (100 nM) for 4 h, followed by incubation for 24 h with NaCl (25 mM) or NaLac (25 mM). Two-way ANOVA with Dunnett’s test. f, ELISA of cGAMP in PBMCs and BMDMs treated as in e followed by mock infection (PBS) or infection for 12 h with HSV-1(MOI, 10). g, qPCR analysis of Ifnb1 mRNA (left), HSV-1-specific genomic DNA (gDNA) (middle) and plaque assay of HSV-1 titers (right) in PBMCs and BMDMs treated as in f. Data are representative of at least three independent experiments (b-g). Mean ± s.d., statistical analysis was performed using two-tailed Student’s t-test (b, e-g). ND, not detected. Supplementary Fig. 1 shows full gels.
Extended Data Fig. 2 Myeloid MCT1 mediates the exogenous L-lactate-induced cGAS inactivation and the lactylated cGAS are enzymatically much less active.
a, ELISA of cGAMP (left), qPCR analysis of Ifnb1 mRNA, and plaque assay of the HSV-1 titer (right) in Lyz2-Cre−Mct1f/f and Lyz2-Cre+Mct1f/f peritoneal macrophages pre-treated for 24 h with NaCl (25 mM) or NaLac (25 mM), followed by infection with mock (PBS) or HSV-1 (MOI, 10) for 12 h. b, Immunoblot (IB) analysis of monomeric (Mono) and dimeric (Dimer) IRF3 (top blot; native PAGE) and total lysates (bottom blot; denaturing gel) of Lyz2-Cre−Mct1f/f and Lyz2-Cre+Mct1f/f peritoneal macrophages pre-treated with (+) or without (−) L-lactate (25 mM) for 12 h, followed by mock infected (PBS) or infection for 12 h with HSV-1 (+). c, Lyz2-Cre−Mct1f/f and Lyz2-Cre+Mct1f/f mice (n = 5) were pre-educated with sodium chloride (NaCl) (1 g/kg; i.p.) or sodium L-lactate (NaLac) (1 g/kg; i.p.) every day for 10 days, followed by HSV-1 infection for 24 h. d, ELISA of cGAMP (left) and IFN-β (right) in serum from mice as in c. e, ELISA of cGAMP (left), qPCR analysis of Ifnb1 mRNA (right) in the lung, spleen and liver of Lyz2-Cre−Mct1f/f and Lyz2-Cre+Mct1f/f mice (n = 5 animals per group) as in c. f, Plaque assay of HSV-1 titer in lung, liver, and spleen of infected mice as in c. g, qPCR analysis of HSV-1-specific genomic DNA (gDNA) in the lung, spleen and liver of mice as in c. h, Immunoblot of HSV-1 (UL46) in lung, liver, and spleen of infected mice as in c. i, Survival of mice (n = 10 animals per group) as in c at various days after infection with HSV-1. j, ELISA of the in vitro cGAMP synthesis (left) by incubating Recombinant (Rec.) human (h-) or mouse (m-) cGAS, ATP (2 mM), GTP (2 mM), HT-DNA (0.01 mg/ml) with mock (PBS), NaCl (2 mM), NaLac (2 mM) and lysate of HEK293T cells as indicated in the left scheme, cell lysate for the 5th and 7th reactions were preheated at 95 °C for 5 min to denature proteins (left). Immunoblot (IB) of pan-Klac and the cGAS input were shown (right). k, Measurement of intracellular L-lactate in Lyz2-Cre−Mct1f/f and Lyz2-Cre+Mct1f/f peritoneal macrophages after incubation with NaCl (25 mM) or NaLac (25 mM) for indicate time points. l, m, ELISA of the in vitro cGAMP synthesis as in j using h- or m-cGAS purified from HEK293T (l) or RAW264.7 cells (m) pre-treated with PBS, L-lactate (25 mM), or NaLac (25 mM) for 24 h. Scheme of purification (left), immunoblot (IB) verification of the Flag-tagged and lactylated h- and m- cGAS (middle) and ELISA of cGAMP (right) were shown. All data are representative of at least three independent experiments. Mean ± s.d., statistical analysis was performed using two-tailed Student’s t-test (a, d-g, j-m), or log-rank test (i). Supplementary Fig. 1 shows full gels.
Extended Data Fig. 3 Genome-wide CRISPR screen identifies AARS1/2 as intracellular L-lactate sensor.
a, Schematic overview of the GFP reporter construct. GFP expression is driven by interferon-stimulatory response elements (ISRE) (left). HT-1080 cells stably expressing the GFP reporter were incubated with L-lactate (25 mM) for 24 h, followed by HSV-1 infection (MOI, 10) for 12 h. GFP reporter response was analyzed by flow cytometry after the infection (right). b, Distribution of the fold change in the positively selected genes enriched in the GFP-high HT-1080 cells. c, Bacterial purified AARS1-wt, AARS2-wt and AlaRS-wt proteins were analyzed by SDS-PAGE and detected by Coomasssie blue staining. d, Human AARS1 (left), AARS2 (middle) and E. coli AlaRS (right) directly bound alanine and L-lactate in vitro with indicated Kd in Microscale thermophoresis (MST) binding affinity analysis. Data represent mean ± s.d. n = 3 per group. e, Boxplot of global lysine lactylation intensity of samples as in control, AARS1 and/or AARS2 depleted HeLa cells treated without or with L-lactate (Lac, 25 mM) for 24 h. In the box plots, the middle bar represents the median, and the box represents the interquartile range; bars extend to 1.5× the interquartile range. The P values were calculated by the two-sided Wilcoxon rank-sum test. n = 220 proteins. f-g, Scatter plots of global lysine lactylation intensity between indicated samples as in e. h, Sequence motif analysis showed the amino acid residue preference of AARS1 (upper), AARS2 (middle) and AARS1 + AARS2 (lower) around the identified lysine lactylation sites. The red horizontal lines denote thresholds of p < 0.05. These significance values are calculated using the binomial probability of residue frequencies, with respect to a genomic or proteomic background based on pLogo (https://plogo.uconn.edu/). Data are representative of at least three independent experiments (a, c, d). Supplementary Fig. 1 shows full gels.
Extended Data Fig. 4 E. coli AlaRS is functional conserved in catalyzing ATP-dependent conjugation of L-lactate on human (h-) and mouse (m-) cGAS.
a-c, Prokaryotic or eukaryotic Strep-purified indicated proteins were analyzed by SDS–PAGE and detected by coomassie blue staining. d, Left: lysate derived from HEK293T cells were in vitro incubated with prokaryotic Strep-purified AARS1-wt or AARS2-wt, L-lactate (2 mM) and ATP (2 mM) as indicated for 1 h at 37 °C, followed by immunoblot (IB) analysis. Right: lysate derived from HEK293T cells were in vitro incubated with L-lactate (2 mM), ATP (2 mM) and prokaryotic Strep-purified AARS1/2-wt or mutants as indicated for 1 h at 37 °C, followed by immunoblot (IB) analysis. e, Prokaryotic Strep-purified E. coli AlaRS wt or mutant proteins as indicated were analyzed by SDS–PAGE and detected by coomassie blue staining. f, Immunoblot (IB) of TCLs and proteins immunoprecipitated with antibody to (anti-) lactylated-Lys131 of h-cGAS (left) or to (anti-) lactylated-Lys156 of m-cGAS (right) from HEK293T cells transfected with indicated plasmids. g, Prokaryotic purified h-cGAS (left) and m-cGAS (right) His-tagged proteins were in vitro incubated with prokaryotic Strep-purified AARS1/2-wt or mutants, L-lactate (2 mM) and ATP (2 mM) as indicated for 1 h at 37 °C, followed by immunoblot (IB) analysis. h, Prokaryotic purified h-cGAS-His (left) and m-cGAS-His (right) proteins were in vitro incubated with prokaryotic Strep-purified E. coli AlaRS-wt or mutants, L-lactate (2 mM) and ATP (2 mM) as indicated for 1 h at 37 °C, followed by immunoblot (IB) analysis. i, Lysate derived from HEK293T cells were in vitro incubated with eukaryotic (left) and prokaryotic (right) Strep-purified AARS1/AARS2-wt protein, L-lactate (2 mM), ATP (2 mM) and L-alanine (1 mM) as indicated for 1 h at 37 °C, followed by immunoblot (IB) analysis. j, Eukaryotic purified Flag-tagged h-cGAS (left) or m-cGAS (right) proteins were in vitro incubated with eukaryotic Strep-purified AARS1/AARS2-wt, L-lactate (2 mM), ATP (2 mM) and L-alanine (1 mM) as indicated for 1 h at 37 °C, followed by immunoblot (IB) analysis. k, Prokaryotic purified h-cGAS (left) and m-cGAS (right) His-tagged proteins were in vitro incubated with prokaryotic Strep-purified AARS1/AARS2-wt, L-lactate (2 mM), ATP (2 mM) and L-alanine (1 mM) as indicated for 1 h at 37 °C, followed by immunoblot (IB) analysis. l, upper panel: L-Lactate-Adenosine monophosphate (Lac-AMP) production assayed by incubating human AARS1 (panel -left), AARS2 (panel -middle) or E. coli AlaRS (panel -right) wt and mutant as indicated with ATP (2 mM) and various concentrations of NaLac, without (w/o) protein substrate (left panels) or with h-cGAS protein as substrate (right panels) by mass-spectrometry analysis. lower panel: Pyrophosphate (PPi) production assayed by incubating prokaryotic Strep-purified human AARS1 (panel-left), AARS2 (panel-middle) or E. coli AlaRS (panel-right) wt and mutants with ATP (2 mM) and various concentrations of NaLac, without (w/o) protein substrate (left panels) or with prokaryotic purified h-cGAS His-tagged proteins as substrate (right panels), using fluorogenic PPi sensor. m, Kinetic parameters for L-alanine and NaLac activation of wild-type AARS by incubating prokaryotic Strep-purified AARS1, AARS2 or E. coli AlaRS with ATP (2 mM) and various concentrations of L-alanine (left panels) and NaLac (right panels) as indicated. Data were measured by the ATP-PPi exchange assay, using fluorogenic PPi sensor. All values were determined from the best fit of the data to the Michaelis-Menten equation. All data are representative of at least three independent experiments. Mean ± s.d., statistical analysis was performed using two-way ANOVA (l). Supplementary Fig. 1 shows full gels.
Extended Data Fig. 5 AARS2 promotes cGAS lactylation and is required for the L-lactate-induced innate immune suppression.
a, si-ARS library screen identified AARS2 as the endogenous lactyltransferase for human. ELISA of in vitro cGAMP synthesis (left, showing as relative cGAS activity) and immunoblot (IB) analysis (right) of Rec. h-cGAS after incubating at 37 °C for 1 h with ATP (2 mM), GTP (2 mM), HT-DNA (0.01 mg/ml) and lysate derived from HEK293T cells transfected with control siRNA or the si-ARS library. b, si-ARS library screen also identified AARS2 as the endogenous lactyltransferase for mouse (m-) cGAS. ELISA of in vitro cGAMP synthesis (left, showing as relative cGAS activity) and immunoblot (IB) analysis (right) of Rec. m-cGAS after incubating at 37 °C for 1 h with ATP (2 mM), GTP (2 mM), HT-DNA (0.01 mg/ml) and lysate derived from HEK293T cells transfected with control siRNA or the si-ARS library. c, Immunoblot (IB) of TCLs and proteins immunoaffinity purified with antibody to (anti-) lactyl-Lys131 of h-cGAS from HEK293T cells transfected with indicated expression plasmids. d, Immunoblot (IB) of TCLs and proteins immunoprecipitated with antibody to (anti-) lactyl-Lys156 of m-cGAS from HEK293T cells transfected with indicated expression plasmids. e, Immunoblot (IB) of TCLs and proteins immunoaffinity purified with antibody to control IgG, (anti-) AARS2 (upper) or (anti-) Myc-tag (lower), from PBMCs (upper) or HeLa cells transfected with AlaRS-Myc (lower) and stimulated with (+) or without (−) NaLac (25 mM) for 24 h. f, Immunoblot (IB) of TCLs and immunoprecipitates with antibody to control IgG, (anti-) AARS2 (upper) or (anti-) Myc tag (lower) from primary peritoneal macrophages (upper) or HeLa cells transfected with AlaRS-Myc (lower) and stimulated with (+) or without (−) NaLac (25 mM) for 24 h. g, Upper: in situ PLA for endogenous cGAS and AARS2 in HeLa cells treated with control NaCl (25 mM) or NaLac (25 mM) for 24 h. Lower: in situ PLA for endogenous cGAS with transfected AlaRS-Myc in HeLa cells. h, Immunoblot (IB) of TCLs and proteins immunoaffinity purified with antibody to (anti-) lactyl-Lys131 of h-cGAS (left) or to (anti-) lactyl-Lys156 of m-cGAS (right) from human THP1 cells (left) or mouse RAW264.7 cells (right) transfected with control and indicated siRNAs and stimulated for 24 h with NaLac (25 mM). i, Schematic diagram of Aars2 conditional knockout strategy (left). Immunoblot (IB) of the total cell lysate (TCL) from Lyz2-Cre−Aars2f/f and Lyz2-Cre+Aars2f/f peritoneal macrophages (right) were shown. j, ELISA of cGAMP (left), Ifnb1 mRNA (middle) and HSV-1 titer (right) in Lyz2-Cre−Aars2f/f and Lyz2-Cre+Aars2f/f peritoneal macrophages pre-treated for 12 h with NaCl (25 mM) or NaLac (25 mM), followed by mock infection or infection for 12 h with HSV-1 (MOI, 10).k, Immunoblot (IB) analysis of monomeric (Mono) and dimeric (Dimer) IRF3 (top blot; native PAGE) total lysates and immunocomplexes (bottom blot; denaturing gel) of Lyz2-Cre−Aars2f/f and Lyz2-Cre+Aars2f/f peritoneal macrophages pre-treated for 12 h with (+) or without (−) L-lactate (25 mM), followed by mock infection (PBS) or infection for 12 h with HSV-1 (MOI, 10).l, ELISA of cGAMP (left) and qPCR analysis of Ifnb1 mRNA (right) in Lyz2-Cre-Aars2f/f and Lyz2-Cre+Aars2f/f peritoneal macrophages pre-treated for 24 h with NaCl (25 mM) or NaLac (25 mM), followed by transfected with mock (PBS) or HT-DNA (2.5 μg/ml) for 8 h. m, ELISA of cGAMP (left), qPCR analysis of Ifnb1 mRNA (middle) and HSV-1-specific genomic DNA (gDNA) (right) in the lung of Lyz2-Cre−Aars2f/f and Lyz2-Cre+Aars2f/f mice (n = 5 animals per group) pre-educated with NaCl (1 g/kg; i.p.) or NaLac (1 g/kg; i.p.) every day for 10 days, followed by tail intravenous injection of HSV-1 for 24 h (5×108 PFU per mouse). n, Immunoblot (IB) of HSV-1 (UL46) in the lung, liver and spleen of infected mice as in m. All data are representative of at least three independent experiments. Mean ± s.d., statistical analysis was performed using two-tailed Student’s t-test (j, l, m). Supplementary Fig. 1 shows full gels.
Extended Data Fig. 6 Site-specific lactylation of cGAS abrogates their Liquid-Liquid Phase Separation (LLPS) and sensing of DNA.
a, A schematic diagram describing the generation of site-specifically-lactylated recombinant h- or m- cGAS proteins with an lactyl-lysine Methanosarcina barkeri (Mb) pyrrolysyl-tRNA synthetase/tRNACUA pair in pSupAR backbone vector (see Materials and Methods). b, Immunoblot (IB) of the purified non-lactylated and site-specifically-lactylated h-cGAS (left) or m-cGAS (right) proteins with specific antibodies. The anti-Strep blots indicate loading of lanes. c, MST binding affinity between Cy3-45-bp ISD and h-cGASNon-lac, h-cGASK131-lac (left), m-cGASNon-lac or m-cGASK156-lac (right) proteins purified in a. d, Representative images (left) and quantification (right) of droplet formation by mixing 45-bp ISD (1 μM, 5% labeled with Cy3) with cGAS proteins (2 μM) purified in a. e, Representative image (left) and quantification (right) of FRAP over a 120 s time course of 45-bp ISD (1 μM, 5% labeled with Cy3) with h-cGAS (upper, 2 μM) or m-cGAS (lower, 2 μM) proteins purified in a. f, Fluorescence of GFP-fused m-cGAS (10 μM) proteins mixed with 100-bp DNA (1 μM, 5% labeled with AF647) at room temperature with 150 mM NaCl at pH 7.0. Quantitative line profile of co-localization along the indicated arrow of the left image (right). n = 3. g, Fluorescence of m-cGAS (20 μM) proteins purified in a after incubated with HT-DNA (0.05 μg/μl) and fluorescent tetramethulrhodamine dextran (10 kDa, 40 kDa or 155 kDa as indicated). h, FRAP over a 150-s time course of droplet formation by mixing 45-bp ISD (1 μM), 100-bp DNA (1 μM), HT-DNA (0.05 μg/μl) with AF488-labeled h-cGASNon-Lac (upper-left), h-cGASK131-Lac (upper-right), m-cGASNon-Lac (lower-left) or m-cGAS-K156-Lac (lower-right) proteins purified in a. i, Working model on low-diffusive nature of the DNA-repellent cGASLac condensate. cGASNon-Lac condensates with DNA and forms liquid-like droplets that are highly diffusive and permeable by 10 kDa, 40 kDa and 155 kDa tetramethylrhodamine dextran molecules. After N-terminal lactylation, cGASLac tend to repel DNA and self-condensate into DNA-free, small gel-like droplets with high-viscosity, low-fluorescence recovery and low-permeability, resulting in the exclusion of 155 kDa tetramethylrhodamine dextran molecules (Rh ≈ 9 nm). cGAS and dextran molecules were depicted in blue and red respectively. cGAS lactylation (Lac) was highlighted with the orange circle. j, MST binding affinity between 100-bp DNA and m-cGAS proteins purified in a. Data are representative of at least three independent experiments (b-h, j). Scale bar, 5 μm (d-g). Mean ± s.d., statistical analysis was performed using two-tailed Student’s t-test (d) or two-way ANOVA (e, h). Supplementary Fig. 1 shows full gels.
Extended Data Fig. 7 Functional characterization of the site-specific cGAS lactylation in cells and lactyl-mimicking or lactyl-destructing knock-in mutations in mice.
a, A schematic depicting the engineering strategy for site-specific lactylation of h- and m-cGAS in cells using aminoacyl-tRNA synthetases and aminoacyl tRNA. b, Immunoblot (IB) of the TCLs of engineered cells as described in a. c, Immunoblot (IB) of the TCLs and streptavidin Biotin-DNA (HSV60) pull-down derived from HEK293T cells transfected to express indicated proteins. d-e, In situ PLA of HeLa cells engineered to express indicated proteins and stimulated with HT-DNA for 8 h. f-g, Immunofluorescence and DAPI staining of HeLa cells engineered to express indicated proteins, followed by HT-DNA stimulation for 8 h. h, ELISA of cGAMP in HEK293T cells engineered to express h-cGASNon-lac or h-cGASK131-lac (left), m-cGASNon-lac or m-cGASK156-lac (right) proteins as in a, followed by non-stimulation or HT-DNA stimulation for 8 h. i, Schematic diagram of CRIPSR/Cas9-mediated genome editing of the mouse cGAS gene locus. j, ELISA of IFN-β (left) and plaque assay of HSV-1 titers (right) in the serum of cGASwt/wt and cGASKQ/KQ mice (n = 5) treated with HSV-1 infection. k, Hematoxylin-and-eosin (H&E)-stained lung sections from mice (n = 3) as in i. l, ELISA of IFN-β (left) and plaque assay of HSV-1 titers (right) in the serum of cGASwt/wt and cGASKR/KR mice (n = 5) pre-educated with NaCl (1 g/kg; i.p.) or NaLac (1 g/kg; i.p.) every day for 10 days, followed by HSV-1 infection. m, Hematoxylin-and-eosin (H&E)-stained lung sections from mice (n = 3) as in l. n, ELISA of cGAMP (left), Ifnb1 mRNA (middle) and HSV-1 titer (right) in cGASwt/wt and cGASKQ/KQ peritoneal macrophages by mock infection or infection for 12 h with HSV-1 (MOI, 10). o, ELISA of cGAMP (left), Ifnb1 mRNA (middle) and HSV-1 titer (right) in cGASwt/wt and cGASKR/KR peritoneal macrophages pre-treated for 12 h with NaCl (25 mM) or NaLac (25 mM), followed by mock infection or infection for 12 h with HSV-1 (MOI, 10). The ratio of transfected cGASNon-Lac: cGASLac is 1:4 to ensure equal amount of protein expression. Data are representative of at least three independent experiments (b-h, j-o). Mean ± s.d., statistical analysis was performed using two-tailed Student’s t-test (h, j, l, n, o). Scale bar, 5 μm (d-g). 100 μm (k, m). Supplementary Fig. 1 shows full gels.
Extended Data Fig. 8 Lactylated cGAS failed to recognize cytosolic mtDNA and self-DNA in Trex1 knock-out mice.
a, Partial Least-squares discrimination analysis (PLS-DA) of the serum metabolome of normal (n = 6, 0–2 mM), high-lac (n = 4, 2–6 mM) and lac-acidosis (n = 2, above 6 mM) patients with HCMV infection. Each symbol represents the data of an individual patients. b, Gene signatures indicating ATP metabolic process (left) or mitochondrial-ATP coupled electron transport (right) are significantly enriched in normal people versus lac-acidosis patients (left) or high-lac patients (right); shown by preranked gene set enrichment analysis (GSEA) of RNA-seq analysis of PBMCs from patients. c, Left: measurement of ATP levels in PBMC of normal (n = 7, 0–2 mM), high-lac (n = 16, 2–6 mM) and lac-acidosis (n = 5, above 6 mM) patients with HCMV infection. Right: mitochondrial DNA (mtDNA) levels in cytosolic fractions from above samples assessed by qPCR amplification of mitochondrial CYTB. d, Fluorescence of GFP fused h-cGASNon-lac or h-cGASK131-lac and mtDNA (5% labeled with AF647) mixed at room temperature with 150 mM NaCl at pH 7.0. e, Fluorescence of non-lactylated and Lys156-lactylated m-cGAS-GFP mixing with mtDNA (5% labeled with AF647) at room temperature with 150 mM NaCl at pH 7.0. f, Cytosolic mtDNA assessment by qPCR amplification of mitochondrial CYTB from U2OS cells stimulated with either bis-2-(5-phenylacetamido-1,3,4-thiadiazol-2-yl) ethyl sulfide (BPTES, 30 μM) for 6 h, Doxorubicin (Dox, 500 nM) for 24 h or 5-Fluorouracil (5-FU, 5 μM) for 16 h. g, Mitotracker and Picogreen staining of U2OS cells with PBS or BPTES (30 μM) stimulation for 6 h. h, Immunofluorescence, Picogreen and DAPI staining of U2OS cells engineered to express wt and Lys156-specifically-lactylated m-cGAS, followed by stimulation with BPTES (30 μM) for 6 h. i, Data represent the fold change in enrichment of mtDNA fragments using anti-cGAS to co-precipitate DNA from THP1 cells transfected with control siRNA (Co.si) or si-AARS2, followed by treatment with NaLac (25 mM) for 12 h and BPTES (30 μM) for 6 h as indicated. DNA fragments were amplified by real-time qPCR using three primer pairs (ND1, DLOOP and CYTB) for mtDNA and two primer pairs (L1ORF1, POLG1) for gDNA. j, Data represent the fold change in enrichment of mtDNA fragments using anti-Flag to coprecipitate DNA in HEK293T cells engineered to express wt and site-specifically-lactylated h-cGAS, followed by stimulation with BPTES (30 μM) for 6 h. The quantification of DNA fragments amplified by real-time qPCR using primer pairs (ND1) for mtDNA and primer pairs (L1ORF1) for gDNA were also shown in Fig. 5f. k, Data represent the fold change in enrichment of mtDNA fragments using anti-Flag to coprecipitate DNA in HEK293T cells engineered to express wt and site-specifically-lactylated m-cGAS (K156-Lac), followed by stimulation with BPTES (30 μM) for 6 h. DNA fragments were amplified by real-time qPCR using three primer pairs (ND1, DLOOP and CYTB) for mtDNA and two primer pairs (L1ORF1, POLG1) for gDNA. l, Immunofluorescence and DAPI staining of U2OS cells engineered to express wt and Lys131-specifically-lactylated h-cGAS, followed by stimulation with H2O2 (10 mM) for 30 min (left). Quantitative line profile of co-localization along the indicated white arrow of the left image (right). n = 3. m, Representative image (left) and cell number (right) of spleens from Trex1−/−cGASwt/wt and Trex1−/−cGASKQ/KQ mice (n = 10). n, Representative image (left) and histology score (right) of hematoxylin-and-eosin (H&E)-stained heart sections from mice as in m. Scale bar, 100 µm. o, Representative image (left) and histology score (right) of hematoxylin-and-eosin (H&E)-stained skin sections from mice as in m. Scale bar, 100 µm. p, Quantification by RT-PCR of a panel of IFN-stimulated gene transcripts in PBMC (left) or heart (right) from Trex1−/−cGASwt/wt and Trex1−/−cGASKQ/KQ mice (n = 4). q, ELISA analysis of TNF-α, CCL5, CXCL10 and IFN-β in the serum from mice (n = 10) as in m. r, High intracellular L-lactate or AARS2 activity promotes cGAS lactylation thereby severely reducing self-DNA detection and down-regulating the autoimmune surveillance. Data are representative of at least three independent experiments (c-q). Scale bar, 5 μm (d, e, g, h, l). Mean ± s.d., statistical analysis was performed using two-tailed Student’s t-test (c, f, i, j-q). Supplementary Fig. 1 shows full gels.
Extended Data Fig. 9 Anxiety-induced cGAS lactylation impairs innate immune surveillance.
a, Measurement of L-lactate in serum of patients with HSV-1 but no anxiety (n = 30) or patients with HSV-1 and anxiety disorders (n = 20). b, cGAMP peak area intensity by mass-spectrometry analysis (left), ELISA of cGAMP (middle) and IFN-β (right) in serum of patients. c, Correlation between cGAMP (left), IFN-β (right) and L-lactate in serum of patients with HSV-1 infection and anxiety disorders. d, Schematic of RS model in vivo: Lyz2-Cre−Aars2f/f and Lyz2-Cre+Aars2f/f mice were non-treated (NT) or given RS, followed by behavioral test and HSV-1 infection. e, Representative tracks (left) of Lyz2-Cre−Aars2f/f and Lyz2-Cre+Aars2f/f mice in open-field test (OFT) (n = 12) on day 8 after inducing RS model, as well as travel distance (middle-left), percentage of distance in center area (middle-right) and the percentage of time spent in center (right). Lyz2-Cre−Aars2f/f and Lyz2-Cre+Aars2f/f mice were non-treated (NT) or given acute restraint stress (RS) for seven consecutive days, followed by behavioral test and HSV-1 infection (5×108 PFU per mouse, tail intravenous injection). Two-way ANOVA with Dunnett’s test. f, Representative tracks (left), the number of entries into the open arms (middle-left), time on open arms (middle-right) and the number of total entries into two arms (right) in elevated plus-maze (EPM) of NT and RS mice described as in d on day 8 (n = 12). Two-way ANOVA with Dunnett’s test. g, Measurement of L-lactate in the serum of mice as in d (n = 12). Two-way ANOVA with Dunnett’s test. h, Immunoblot analysis of K156-lactylated m-cGAS in PBMCs (upper) or BMDMs (lower) isolated from mice as in d. i, ELISA of cGAMP (left), IFN-β (middle) and plaque assay of the HSV-1 titers (right) in the serum of mice as in d (n = 6 animals per group) treated with tail intravenous injection of HSV-1 for 24 h (5×108 PFU per mouse). j, Survival of mice (n = 10 animals per group) as in d at various days after infection with HSV-1 (5×108 PFU per mouse, tail intravenous injection). k, ELISA of cGAMP (left), qPCR analysis of Ifnb1 mRNA (middle), and HSV-1-specific genomic DNA (gDNA) (right) in the lung of Lyz2-Cre−Aars2f/f and Lyz2-Cre+Aars2f/f mice (n = 6 animals per group). Lyz2-Cre-Aars2f/f and Lyz2-Cre+Aars2f/f mice were non-treated (NT) or given acute restraint stress (RS) for seven consecutive days, followed by behavioral test and HSV-1 infection (5×108 PFU per mouse, tail intravenous injection). l, Schematic of electric foot shock (ES) model and experimental analysis in vivo: Lyz2-Cre−Aars2f/f and Lyz2-Cre+Aars2f/f mice were non-treated (NT) or given ES for seven consecutive days, followed by behavioral test and HSV-1 infection (5×108 PFU per mouse, tail intravenous injection). m, Representative tracks (left) of Lyz2-Cre−Aars2f/f and Lyz2-Cre+Aars2f/f mice in open-field test (OFT) (n = 12) on day 8 after inducing ES model, as well as travel distance (middle-left), percentage of distance in center area (middle-right) and percentage of time spent in center (right). Two-way ANOVA with Dunnett’s test. n, Representative tracks (left), the number of entries into the open arms (middle-left), time on open arms (middle-right) and the number of total entries into two arms (right) in elevated plus-maze (EPM) of NT and RS mice described as in l on day 8 (n = 12). Two-way ANOVA with Dunnett’s test. o, Measurement of L-lactate in serum of Lyz2-Cre−Aars2f/f and Lyz2-Cre+Aars2f/f mice given NT or ES (n = 12). Two-way ANOVA with Dunnett’s test. p, Survival of mice (n = 10 animals per group) as in l at various days after infection with HSV-1 (5×108 PFU per mouse, tail intravenous injection). q, ELISA of cGAMP (left) and IFN-β (middle) in the serum and plaque assay of the HSV-1 titer (right) in the lung of mice as in l (n = 6 animals per group). Data are representative of at least three independent experiments (a-q). Mean ± s.d., statistical analysis was performed using two-tailed Student’s t-test (a, b, i, k, q), Dunnett’s test (e-g, m-o) or Log-rank test (j, p). Supplementary Fig. 1 shows full gels.
Extended Data Fig. 10 Efficient rescue of anxiety-induced immune dysfunction by MCT1 blockade.
a, Schematic of RS model in vivo: cGASwt/wt and cGASKR/KR mice were non-treated (NT) or given RS, followed by behavioral test and HSV-1 infection. b, Measurement of L-lactate in serum of cGASwt/wt and cGASKR/KR mice given NT or RS (n = 12). c, Representative tracks (left) of cGASwt/wt and cGASKR/KR mice in open-field test (OFT) (n = 12) on day 8 after inducing RS model, as well as travel distance (middle-left), percentage of distance in center area (middle-right) and percentage of time spent in center (right). d, Representative tracks (left), the number of entries into the open arms (middle-left), time on open arms (middle-right) and the number of total entries into two arms (right) in elevated plus-maze (EPM) of NT and RS mice described as in a on day 8 (n = 12). e, ELISA of cGAMP (left) and IFN-β (middle) in the serum of mice as in a (n = 6). Plaque assay of HSV-1 titers (right) in the lung of cGASwt/wt and cGASKR/KR mice as in a (n = 6). f, Survival of mice (n = 10) as in a after HSV-1 infection. g, Wild-type mice were given NT or RS for seven consecutive days and treated with control vehicle or 50 mg/kg AZD3965 orally twice daily, followed by behavioral test and HSV-1 infection (5×108 PFU per mouse, tail intravenous injection). Representative tracks (left) of the above mice in open-field test (OFT) (n = 12) on day 8 after inducing RS model, as well as travel distance (middle-left), percentage of distance in center area (middle-right) and percentage of time spent in center (right). h, Related to Fig. 5i, body weight of mice during AZD3965 treatment (n = 12). i, Related to Fig. 5k, immunoblot (IB) of the total cell lysate (TCL) and immunoaffinity purified proteins in PBMCs (left) or BMDMs (right) isolated from mice. Wild-type mice were given NT or RS for seven consecutive days and treated with control vehicle or 50 mg/kg AZD3965 orally twice daily, followed by behavioral test and HSV-1 infection (5×108 PFU per mouse, tail intravenous injection). j, ELISA of cGAMP (left) and qPCR analysis of Ifnb1 mRNA (right) in the lung, spleen and liver of mice as in g (n = 6 animals per group). Data are representative of at least three independent experiments (b-j). Mean ± s.d., statistical analysis was performed using two-tailed Student’s t-test (e, j), Dunnett’s test (b-d, g), Log-rank test (f) or two-way ANOVA analysis (h). Supplementary Fig. 1 shows full gels.
Supplementary information
Supplementary Information
Supplementary Figs. 1–5.
Supplementary Table 1
Lactylated peptides enriched from AARS1/2-depleted HeLa cells. Protein UniProt identifiers, lactylated peptides sequences and peptide relative intensities for peptides enriched by Klac beads in control, AARS1- or AARS2-depleted HeLa cells treated with PBS or lactate.
Supplementary Table 2
Lactylated peptides enriched from AARS1/2 overexpression HeLa cells. Protein UniProt identifiers, lactylated peptides sequences and peptide relative intensities for peptides enriched by Klac beads in HeLa cells transfected with control, AARS1 or AARS2.
Supplementary Table 3
AARS1, AARS2 and AlaRS mutation information. List of human AARS1, human AARS2 and E. coli AlaRS mutants included in the study.
Supplementary Table 4
Sequences for siRNA constructs. List of siRNA target sequence included in the study.
Supplementary Table 5
Features of patients with HCMV infection tested for cGAMP and IFNβ levels. List of features for patients with HCMV infection tested for cGAMP and IFNβ levels.
Supplementary Table 6
Features of patients with anxiety disorder tested for cGAMP and IFNβ levels. List of features for patients with anxiety disorder tested for cGAMP and IFNβ levels.
Supplementary Data 1
Statistical source data for Supplementary Fig. 2.
Supplementary Data 2
Statistical source data for Supplementary Fig. 5.
Source data
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, H., Liu, C., Li, R. et al. AARS1 and AARS2 sense l-lactate to regulate cGAS as global lysine lactyltransferases. Nature 634, 1229–1237 (2024). https://doi.org/10.1038/s41586-024-07992-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-024-07992-y
This article is cited by
-
Lactate and lactylation: novel perspectives on fibrosis pathogenesis and therapeutic directions
Journal of Translational Medicine (2025)
-
Lactylation-regulated biomolecular condensates: metabolic control of phase separation in physiology and disease
Cell Communication and Signaling (2025)
-
Mechanism and application of lactylation in cancers
Cell & Bioscience (2025)
-
L- and D-Lactate: unveiling their hidden functions in disease and health
Cell Communication and Signaling (2025)
-
Regulation of cGAS–STING signalling and its diversity of cellular outcomes
Nature Reviews Immunology (2025)