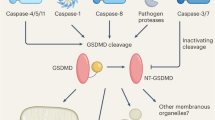
Abstract
The establishment of an early pro-regenerative niche is crucial for tissue regeneration1,2. Gasdermin D (GSDMD)-dependent pyroptosis accounts for the release of inflammatory cytokines upon various insults3,4,5. However, little is known about its role in tissue regeneration followed by homeostatic maintenance. Here we show that macrophage GSDMD deficiency delays tissue recovery but has little effect on the local inflammatory milieu or the lytic pyroptosis process. Profiling of the metabolite secretome of hyperactivated macrophages revealed a non-canonical metabolite-secreting function of GSDMD. We further identified 11,12-epoxyeicosatrienoic acid (11,12-EET) as a bioactive, pro-healing oxylipin that is secreted from hyperactive macrophages in a GSDMD-dependent manner. Accumulation of 11,12-EET by direct supplementation or deletion of Ephx2, which encodes a 11,12-EET-hydrolytic enzyme, accelerated muscle regeneration. We further demonstrated that EPHX2 accumulated within aged muscle, and that consecutive 11,12-EET treatment rejuvenated aged muscle. Mechanistically, 11,12-EET amplifies fibroblast growth factor signalling by modulating liquid–liquid phase separation of fibroblast growth factors, thereby boosting the activation and proliferation of muscle stem cells. These data depict a GSDMD-guided metabolite crosstalk between macrophages and muscle stem cells that governs the repair process, which offers insights with therapeutic implications for the regeneration of injured or aged tissues.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
199,00 € per year
only 3,90 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All raw and processed sequencing data generated in this study have been deposited in the NCBI Gene Expression Omnibus (GEO) under accession numbers GSE246007 (bulk RNA sequencing) and GSE250049 (scRNA-seq). The publicly available dataset used in this study is available at GEO under the accession numbers GSE113631 and GSE164471. The gating strategy for flow cytometry and raw, uncropped images of western blots are provided in the Supplementary Information. Source data are provided with this paper.
References
Gurtner, G. C., Werner, S., Barrandon, Y. & Longaker, M. T. Wound repair and regeneration. Nature 453, 314–321 (2008).
Eming, S. A., Murray, P. J. & Pearce, E. J. Metabolic orchestration of the wound healing response. Cell Metab. 33, 1726–1743 (2021).
Xia, S. et al. Gasdermin D pore structure reveals preferential release of mature interleukin-1. Nature 593, 607–611 (2021).
Devant, P. & Kagan, J. C. Molecular mechanisms of gasdermin D pore-forming activity. Nat. Immunol. 24, 1064–1075 (2023).
Evavold, C. L. et al. The pore-forming protein gasdermin D regulates interleukin-1 secretion from living macrophages. Immunity 48, 35–44.e36 (2018).
Martin, P. Wound healing-aiming for perfect skin regeneration. Science 276, 75–81 (1997).
Shang, M. et al. Macrophage-derived glutamine boosts satellite cells and muscle regeneration. Nature 587, 626–631 (2020).
Medzhitov, R. The spectrum of inflammatory responses. Science 374, 1070–1075 (2021).
Broz, P., Pelegrin, P. & Shao, F. The gasdermins, a protein family executing cell death and inflammation. Nat. Rev. Immunol. 20, 143–157 (2020).
Liu, X. & Lieberman, J. Knocking ‘em dead: pore-forming proteins in immune defense. Annu. Rev. Immunol. 38, 455–485 (2020).
Weindel, C. G., Ellzey, L. M., Martinez, E. L., Watson, R. O. & Patrick, K. L. Gasdermins gone wild: new roles for GSDMs in regulating cellular homeostasis. Trends Cell Biol. 33, 773–787 (2023).
Li, M. et al. Gasdermin D maintains bone mass by rewiring the endo-lysosomal pathway of osteoclastic bone resorption. Dev. Cell 57, 2365–2380.e2368 (2022).
Zhang, J. et al. Epithelial gasdermin D shapes the host-microbial interface by driving mucus layer formation. Sci. Immunol. 7, eabk2092 (2022).
Karmakar, M. et al. N-GSDMD trafficking to neutrophil organelles facilitates IL-1β release independently of plasma membrane pores and pyroptosis. Nat. Commun. 11, 2212 (2020).
Chen, Y. et al. Gasdermin D drives the nonexosomal secretion of galectin-3, an insulin signal antagonist. J. Immunol. 203, 2712–2723 (2019).
Tidball, J. G. Regulation of muscle growth and regeneration by the immune system. Nat. Rev. Immunol. 17, 165–178 (2017).
Sousa-Victor, P., Garcia-Prat, L. & Munoz-Canoves, P. Control of satellite cell function in muscle regeneration and its disruption in ageing. Nat. Rev. Mol. Cell Biol. 23, 204–226 (2022).
Relaix, F. et al. Perspectives on skeletal muscle stem cells. Nat. Commun. 12, 692 (2021).
Tierney, M. T. & Sacco, A. Satellite cell heterogeneity in skeletal muscle homeostasis. Trends Cell Biol. 26, 434–444 (2016).
Zhang, J. et al. Endothelial lactate controls muscle regeneration from ischemia by inducing M2-like macrophage polarization. Cell Metab. 31, 1136–1153.e1137 (2020).
Nakka, K. et al. JMJD3 activated hyaluronan synthesis drives muscle regeneration in an inflammatory environment. Science 377, 666–669 (2022).
Qiu, X. et al. Reversed graph embedding resolves complex single-cell trajectories. Nat. Methods 14, 979–982 (2017).
Massenet, J., Gardner, E., Chazaud, B. & Dilworth, F. J. Epigenetic regulation of satellite cell fate during skeletal muscle regeneration. Skelet. Muscle 11, 4 (2021).
De Micheli, A. J. et al. Single-cell analysis of the muscle stem cell hierarchy identifies heterotypic communication signals involved in skeletal muscle regeneration. Cell Rep. 30, 3583–3595.e3585 (2020).
Kayagaki, N. et al. NINJ1 mediates plasma membrane rupture during lytic cell death. Nature 591, 131–136 (2021).
Degen, M. et al. Structural basis of NINJ1-mediated plasma membrane rupture in cell death. Nature 618, 1065–1071 (2023).
Cui, M., Cheng, C. & Zhang, L. High-throughput proteomics: a methodological mini-review. Lab. Invest. 102, 1170–1181 (2022).
Zanoni, I., Tan, Y., Di Gioia, M., Springstead, J. R. & Kagan, J. C. By capturing inflammatory lipids released from dying cells, the receptor CD14 induces inflammasome-dependent phagocyte hyperactivation. Immunity 47, 697–709.e693 (2017).
Borges, J. P. et al. Glycine inhibits NINJ1 membrane clustering to suppress plasma membrane rupture in cell death. eLife 11, e78609 (2022).
Liu, X. et al. Context-dependent activation of STING-interferon signaling by CD11b agonists enhances anti-tumor immunity. Cancer Cell 41, 1073–1090.e1012 (2023).
Dennis, E. A. & Norris, P. C. Eicosanoid storm in infection and inflammation. Nat. Rev. Immunol. 15, 511–523 (2015).
Edin, M. L. et al. Epoxide hydrolase 1 (EPHX1) hydrolyzes epoxyeicosanoids and impairs cardiac recovery after ischemia. J. Biol. Chem. 293, 3281–3292 (2018).
Hu, J. J. et al. FDA-approved disulfiram inhibits pyroptosis by blocking gasdermin D pore formation. Nat. Immunol. 21, 736–745 (2020).
Palacios, D. et al. TNF/p38α/Polycomb signaling to Pax7 locus in satellite cells links inflammation to the epigenetic control of muscle regeneration. Cell Stem Cell 7, 455–469 (2010).
Xie, Y. et al. FGF/FGFR signaling in health and disease. Signal Transduct. Target. Ther. 5, 181 (2020).
Do, M. K. et al. Time-coordinated prevalence of extracellular HGF, FGF2 and TGF-β3 in crush-injured skeletal muscle. Anim. Sci. J. 83, 712–717 (2012).
Rodgers, J. T. et al. mTORC1 controls the adaptive transition of quiescent stem cells from G0 to GAlert. Nature 510, 393–396 (2014).
Maddaluno, L., Urwyler, C. & Werner, S. Fibroblast growth factors: key players in regeneration and tissue repair. Development 144, 4047–4060 (2017).
Richardson, T. P., Trinkaus-Randall, V. & Nugent, M. A. Regulation of basic fibroblast growth factor binding and activity by cell density and heparan sulfate. J. Biol. Chem. 274, 13534–13540 (1999).
Xue, S. et al. Phase separation on cell surface facilitates bFGF signal transduction with heparan sulphate. Nat. Commun. 13, 1112 (2022).
Venkataraman, G. et al. Preferential self-association of basic fibroblast growth factor is stabilized by heparin during receptor dimerization and activation. Proc. Natl Acad. Sci. USA 93, 845–850 (1996).
Beenken, A. & Mohammadi, M. The FGF family: biology, pathophysiology and therapy. Nat. Rev. Drug Discov. 8, 235–253 (2009).
Goldstein, M. H., Silva, F. Q., Blender, N., Tran, T. & Vantipalli, S. Ocular benzalkonium chloride exposure: problems and solutions. Eye 36, 361–368 (2022).
Park, M. et al. Visualizing the contribution of keratin-14+ limbal epithelial precursors in corneal wound healing. Stem Cell Rep. 12, 14–28 (2019).
Neves, J., Sousa-Victor, P. & Jasper, H. Rejuvenating strategies for stem cell-based therapies in aging. Cell Stem Cell 20, 161–175 (2017).
Benjamin, D. I. et al. Fasting induces a highly resilient deep quiescent state in muscle stem cells via ketone body signaling. Cell Metab. 34, 902–918.e906 (2022).
Baker, S. A. & Rutter, J. Metabolites as signalling molecules. Nat. Rev. Mol. Cell Biol. 24, 355–374 (2023).
Liu, L., Cheung, T. H., Charville, G. W. & Rando, T. A. Isolation of skeletal muscle stem cells by fluorescence-activated cell sorting. Nat. Protoc. 10, 1612–1624 (2015).
Hoeffel, G. et al. Sensory neuron-derived TAFA4 promotes macrophage tissue repair functions. Nature 594, 94–99 (2021).
Rowland, M. B., Moore, P. E., Bui, C. & Correll, R. N. Assessing wound closure in mice using skin-punch biopsy. STAR Protoc. 4, 101989 (2023).
Chi, Z. et al. Histone deacetylase 3 couples mitochondria to drive IL-1β-dependent inflammation by configuring fatty acid oxidation. Mol. Cell 80, 43–58.e47 (2020).
Wu, T. et al. clusterProfiler 4.0: a universal enrichment tool for interpreting omics data. Innovation 2, 100141 (2021).
Hao, Y. et al. Integrated analysis of multimodal single-cell data. Cell 184, 3573–3587.e3529 (2021).
DeTomaso, D. et al. Functional interpretation of single cell similarity maps. Nat. Commun. 10, 4376 (2019).
Castanza, A. S. et al. Extending support for mouse data in the Molecular Signatures Database (MSigDB). Nat. Methods 20, 1619–1620 (2023).
Gulati, G. S. et al. Single-cell transcriptional diversity is a hallmark of developmental potential. Science 367, 405–411 (2020).
Browaeys, R., Saelens, W. & Saeys, Y. NicheNet: modeling intercellular communication by linking ligands to target genes. Nat. Methods 17, 159–162 (2020).
Assarsson, E. et al. Homogenous 96-plex PEA immunoassay exhibiting high sensitivity, specificity, and excellent scalability. PLoS ONE 9, e95192 (2014).
Acknowledgements
The authors thank I. C. Bruce for reading the manuscript; Z. Zhao for support with flow cytometric experiments; S.-Z. Luo for providing eGFP–bFGF plasmids; X. Gao and S. Xu for support with phase separation experiments; and Q. Han for technical support. This work was supported by the National Natural Science Foundation of China (82025017 and 81930042 to D.W., 32370919 and 32100692 to Z.C. and 32370832 to W.Y.), the China Postdoctoral Science Foundation (2023M733063 to Z.W.), the Huadong Medicine Joint Funds of the Zhejiang Provincial Natural Science Foundation of China (LHDMD23H160002 to D.W.), the Key R&D Program of Zhejiang (2024SSYS0024 to D.W.), and the Dr Li Dak Sum & Yip Yio Chin Development Fund for Regenerative Medicine. Graphics in Figs. 3c, 4i, 5n and 6e,i,n,s and Extended Data Figs. 1a,d,j, 5a,e,f,q and 10a,j were created using BioRender.com.
Author information
Authors and Affiliations
Contributions
Z.C., S.C., D.Y., W.C., Y. Lu, Z.W., M.L., Y.J., W.Y., J.Z., Q.Y., T.H., X.L., Q.D., Y.Y., T.Z. and M.C. performed animal and cell experiments. S.C. and R.S. performed scRNA-seq downstream analysis. Q.X. and K.D. provided human muscle samples. Z.C., S.C., D.Y., M.L., W.C., Y. Li, M.S., X.Z. and D.W. conceived and designed the study. Z.C., S.C., D.Y. and D.W. wrote the manuscript, and Z.C. and D.W. supervised the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Myeloid GSDMD deficiency compromises tissue repair.
a, Schematic of the tissue repair process coupled with an inflammatory response. b,c, Representative flow cytometry analysis contour plots (b, left), quantification (b, right) of baseline MuSC number and mean CSA (c), related to Fig. 1d (n = 4), from tibialis anterior muscles of Gsdmdf/f and GsdmdCKO mice (n = 6). d-i, Schematic of GsdmdCKO and littermate controls subjected to CTX-induced injury upon macrophage depletion by anti-CSF1R antibody intraperitoneally (d). Representative images and quantification of macrophages at 3 dpi (e). Muscle strength (n = 3) (f), myofiber CSA (g), frequency distribution (h) and mean CSA (i) of TA muscles at 14 dpi with isotype or anti-CSF1R injection (n = 5, isotype; n = 6, anti-CSF1R). j, Schematic of MuSC myogenic gene programs during regeneration. k,l, Immunoblots of GSDMD in muscle extracts post CTX injury at indicated time. m, Representative images and quantitation (n = 3, 0 dpi; n = 6, 1–10 dpi) of F4/80+ staining in injured TA muscles. n, Immunoblots of muscle extracts for GSDMD from mice with isotype or anti-CSF1R injection at 3dpi. o, Representative images and quantitation (n = 3, 1dpi; n = 4, 3dpi; n = 3, 5dpi; n = 3 Gsdmdf/f, n = 4 GsdmdCKO, 7 dpi) of CD31+ staining in injured TA muscles. A representative (b,c-i,k-o) of at least two independent experiments is shown. Unpaired two-tailed t test applied for (b,c,e,m,o). Two-way ANOVA applied for (f,h,i) with Šidák correction. Mean ± SEM.
Extended Data Fig. 2 Single-cell data analysis of muscles from Gsdmdf/f and GsdmdCKO mice.
a,b, Heatmap (a) and featureplot (b) showing the expression of signature markers of each cell type. c,d, UMAP plot of dynamics of cell populations during regeneration. e-g, CytoTRACE score of each state of MuSCs (e,f). Dotplot of the enrichment score of indicated gene sets calculated by AddModuleScore, related to Fig. 2d (g). h, Immunoblots and grayscale statistics of the PI3K-AKT-mTOR and MAPK signaling pathways downstream of FGF-FGFR in TA muscles from Gsdmdf/f and GsdmdCKO mice subjected to CTX-induced injury. A representative (h) of at least two independent experiments is shown.
Extended Data Fig. 3 Single-cell data analysis of intramuscular immune components of Gsdmdf/f and GsdmdCKO mice.
a-c, Analysis of intramuscular immune components of Gsdmdf/f and GsdmdCKO mice. Dynamics of percentages of each cell type in Gsdmdf/f and GsdmdCKO muscles (a). Stacked violin plot of signature marker genes of each immune cell type (b) and dynamics of immune cell percentages in Gsdmdf/f and GsdmdCKO muscles (c). d, Flow cytometry analysis of myeloid cell composition at 2 dpi (n = 8). e,f, Violin plot (e) and dotplot of VISION enrichment analysis of indicated signature genesets (f). g, Dotplot of the enrichment score of indicated gene sets in highly dynamic immune cells determined in Ccr2high monocytes, Spp1high and Mrc1high macrophages.h, Trajectory analysis of the monocyte-macrophage lineage. i, Expression of the top ranked ligands in immune cells upon injury. j,k, Cell-cell interaction analysis using NicheNet. Top ranked intramuscular ligands at 2 dpi (j) and expression of top ranked receptors in each immune cell type (k). A representative of at least two independent experiments is shown. Unpaired two-tailed t test applied for (d). Mean ± SEM.
Extended Data Fig. 4 Gsdmd deficiency has little impact on the muscle microenvironment upon injury.
a, Immunoblots of GSDMD and NINJ1 oligomerization (a) in injured TA muscle lysates (2dpi) after treatment with/without the membrane-impermeable BS3 crosslinker, which stabilizes protein-protein interactions for further analysis by immunoprecipitation. The intensity of Vinculin was used as control. b, Immunoblots of intracellular proteins using total muscle lysates and TIF to confirm the purity of the interstitial fluid. Ponceau S is used as loading control. c, OLINK analysis of muscle TIF. PCA plot of each sample during regeneration. d, Intramuscular levels of inflammatory and regenerating proteins determined by OLINK (n = 3 per group),and boxchart of intramuscular Il1β protein levels (n = 3). e, Immunoblots of HMGB1 secretion in muscle TIF at 2 dpi. f, Dotplot of pyroptosis index and indicated gene expression of three highly dynamic immune cell types. A representative (a,b,e) of at least two independent experiments is shown. Unpaired two-tailed t test applied for (d). Abbreviation: BS3, bis-(sulfosuccinimidyl)-suberate, used as a crosslinker.
Extended Data Fig. 5 Release of GSDMD-dependent metabolites promotes tissue regeneration.
a, Schematic of supernatant collection and ultracentrifugation. Macrophages were stimulated with 500 ng/mL LPS for 4 h. Thirty minutes before the second signal (3.5 h post LPS stimulation), 10 mM glycine was supplemented to maintain plasma membrane integrity. The second signals, 10 µM nigericin or 1 µg/mL poly(dA:dT), were added to induce GSDMD pore formation. b-d, Quantification of IL-1β (b), IL-6 (c) and TNFα (d) by ELISA (n = 3). e, f, LDH levels in supernatants determined by LDH releasing assay (n = 4) and PI+ cells detected by flow cytometry (n = 3). g-j, Volcano plot of metabolite levels with indicated comparisons, representing total lytic release (g), glycine-induced secretion (h), active secretion (i), and GSDMD pore-dependent active release (j), related to Fig. 3c,d. k, Schematic of 11,12-EET biogenesis and hydrolysis. l, 11,12-EET levels in supernatants determined by ELISA (n = 2, technical replicates). m, PCA plot of targeted metabolites of cell lysates and supernatants, related to Fig. 3e. n, The proportion of 11,12-EET secretion in the supernatant relative to the intracellular quantity, related to Fig. 3e. o,p, Representative images (o) and quantification (p) of C2C12 cell fusion index and myotube size with/without 11,12-EET treatment (n = 6). Scale bar, 200 μm. q, Schematic of primary MuSC isolation procedure. r,s, Representative scanning electron microscope image (r) and statistics (s) of 11,12-EET treated (n = 30) and control (n = 40) MuSCs. t,u, Representative flow cytometry contour plots, related to Fig l,m. A representative (l,o,p,r) or a pool (b-f, s-u) of at least two independent experiments is shown. Unpaired two-tailed t test applied for (b-d,n,p,s). Two-way ANOVA applied for (e,f) with Šidák correction. Mean ± SEM, except Mean ± SD for 5 l.
Extended Data Fig. 6 Accumulation of 11,12-EET boosts tissue regeneration in vivo.
a, Immunoblots and quantification (n = 3) of EPHX2 expression in peritoneal macrophages. b, Mean CSA (n = 5), related to Fig. 4c. c,d, Muscle strength (n = 3) (c) and weight (n = 6) (d) of TA muscles from Ephx2f/f and Ephx2CKO mice at 14 dpi. e, Expression of Myod and Myog mRNA levels in Ephx2f/f and Ephx2CKO muscle at indicated dpi (n = 4). f,g, The frequency distribution (n = 3, GsdmdCKOEphx2CKO and GsdmdCKOEphx2Het; n = 6, GsdmdHetEphx2CKO and GsdmdHetEphx2Het) of myofiber CSA at 14 dpi, related to Fig. 4j. h,i, Representative images of Ephx2f/f and Ephx2CKO TA muscle cross-sections (h) and the frequency distribution (n = 4, DMSO; n = 6, DSF) (i) of myofiber CSA at 14 dpi with or without DSF treatment. A representative of at least two independent experiments is shown. Unpaired two-tailed t test applied for (a-d); Two-way ANOVA applied for (e-g,i) with Šidák correction. Mean ± SEM. Abbreviation: DSF, disulfiram (MCE, HY-B0240).
Extended Data Fig. 7 11,12-EET propels MuSC proliferation via enhancing FGF-FGFR signaling.
a, Correlation between gene fold-changes of 11,12-EET versus control, and gene fold-changes of activated versus quiescent MuSCs (from GSE113631). b, Dotplot of expressions of growth factor receptor genes in different MuSC celltype from scRNAseq data of Fig. 2b. c,d Immunoblots of the PI3K-AKT-mTOR and MAPK signaling pathways downstream of FGF-FGFR in NIH-3T3 cells treated with bFGF with/without 11,12-EET (c), or with vehicle, 11,12-EET or 11,12-EET + FGFR inhibitor (d). e, Immunoblots of EGFR activation in NIH-3T3 with or without 11,12-EET. f,g, Analysis pipeline of the correlation level between each gene and FGF signaling score (f) and GO enrichment with the top 300 correlated genes (g), related to Fig. 5d. FGF signaling score was determined using AddModuleScore with gene set ‘RESPONSE_TO_FIBROBLAST_GROWTH_FACTOR’. h, Representative images of FGF condensates on cell surface. Scale bars, 10 μm. i, Immunoblots of eGFP-bFGF oligomerization in NIH-3T3 cells after FGF treatment with/without 11,12-EET.j, Schematic of 11,12-EET treatment and scRNAseq design. k,l, Immunoblots of the PI3K-AKT-mTOR and MAPK signaling pathways downstream of FGF-FGFR in TA muscles subjected to CTX-induced injury with vehicle, vehicle+FGFR inhibitor, 11,12-EET or 11,12-EET + FGFR inhibitor intramuscularly treatment (k), or from Ephx2f/f and Ephx2CKO mice at 3 dpi (l). A representative (c-e,h,i,k,l) of at least two independent experiments is shown. Pearson correlation analysis for (a). Abbreviation: FGFRi, FGFR inhibitor.
Extended Data Fig. 8 scRNAseq analysis of muscles with or without 11,12-EET treatment.
a-i, Heatmap of signature marker genes of each cell type (a, upper), and dynamics of cell type population after injury (a, lower). UMAP plot and percentage of each cell type with indicated treatment (b). Feature plot of signature marker genes of each cell type (c). UMAP plot of 6 consecutive MuSC states and changes of cell proportion upon 11,12-EET treatment (d). Dotplot of signature marker genes of each state of MuSCs (e). Trajectory analysis and the density plots of the relative number of MuSCs separately for 11,12-EET treatment or vehicle (f,g). Red arrow indicates the enriched proliferation lineage and more terminally-differentiated Myoghi MuSCs upon 11,12-EET treatment. Dotplot of the enrichment scores of the indicated gene sets (h). Correlation between FGF binding capacity and P38MAPK cascade level of MuSCs with the indicated treatment (i). Pearson correlation analysis for (i).
Extended Data Fig. 9 11,12-EET has multi-organ pro-regenerating capacity.
a, Mean CSA (n = 4), related to Fig. 6b. b, Representative H&E staining of TA muscle cross-sections with/without 11,12-EET supplement and the percentage of necrotic area at 14 dpi (n = 8). c, F4/80 immunofluorescence and statistics at 10 dpi (n = 7). d, Representative images of eMyHC+ myofibers and quantification (n = 4). e, The strength (n = 4) of TA muscles with indicated treatment post CTX-induced injury. f,g, Representative images (f left panel), the frequency distribution (f right panel) and mean of myofiber CSA (g) at 14 dpi with vehicle, vehicle+FGFR inhibitor, 11,12-EET or 11,12-EET + FGFR inhibitor treatment (n = 6). h-l, Representative H&E staining of corneal (h), statistics of epithelium and anterior stroma layer thickness (i) and inflammation infiltration (j) with 11,12-EET (50 ng 11,12-EET, twice daily) or vehicle treatment (n = 6). F4/80 staining (k) and quantification (n = 8) (l). m, Images of mice subjected to skin punch biopsy with 11,12-EET (300 ng daily) or vehicle treatment at 7 dpi. A representative of at least two independent experiments is shown. Unpaired two-tailed t test applied for (a-d,i,j,l); One-way ANOVA for (e,g) with tukey’s multiple comparisons test. Two-way ANOVA applied for (f) with Šidák correction. Mean ± SEM. Abbreviation: FGFRi, FGFR inhibitor; ROI, region of interest.
Extended Data Fig. 10 11,12-EET rejuvenates aged muscle.
a-d, Schematic of UV-induced skin injury model (a). Statistics of wound closure over time (n = 10) (b), and images of mouse ear post UV irradiation with/without 11,12-EET (300 ng daily) treatment (n = 4) (c). Representative H&E staining of ears 14 days after UV irradiation (d). e, Quantification of EPHX2 expression in TA muscles from young and aged mice (n = 3)., related to Fig. 6l. f, Micrographs and quantification of collagen deposition in TA muscles of aged mice by Masson’s trichrome staining (n = 8). g, Quantification of muscle weight (n = 8) and strength (n = 4) in vehicle- and 11,12-EET-treated aged mice. h,i, Representative cross-sections of TA muscles stained with H&E (h) and myofiber CSA quantification (n = 8) i, Working model. A representative (c-i) or a pool (b) of at least two independent experiments is shown. Unpaired two-tailed t test applied for (e-h). Two-way ANOVA applied for (b) with Šidák correction; Mean ± SEM.
Supplementary information
Supplementary Information
Supplementary Figs 1–4.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chi, Z., Chen, S., Yang, D. et al. Gasdermin D-mediated metabolic crosstalk promotes tissue repair. Nature 634, 1168–1177 (2024). https://doi.org/10.1038/s41586-024-08022-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-024-08022-7
This article is cited by
-
Unveiling the dynamic drivers: phase separation’s pivotal role in stem cell biology and therapeutic potential
Stem Cell Research & Therapy (2025)
-
Targeting NINJ1-mediated cell rupture to treat inflammatory diseases
Molecular Medicine (2025)
-
LC-MS/MS based metabolomics reveals the mechanism of skeletal muscle regeneration
BMC Musculoskeletal Disorders (2025)
-
GSDMD-mediated pyroptosis: molecular mechanisms, diseases and therapeutic targets
Molecular Biomedicine (2025)
-
Complosome as a new intracellular regulatory network in both normal and malignant hematopoiesis
Leukemia (2025)