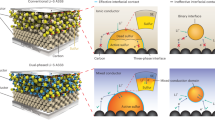
Abstract
With promises for high specific energy, high safety and low cost, the all-solid-state lithium–sulfur battery (ASSLSB) is ideal for next-generation energy storage1,2,3,4,5. However, the poor rate performance and short cycle life caused by the sluggish solid–solid sulfur redox reaction (SSSRR) at the three-phase boundaries remain to be solved. Here we demonstrate a fast SSSRR enabled by lithium thioborophosphate iodide (LBPSI) glass-phase solid electrolytes (GSEs). On the basis of the reversible redox between I− and I2/I3−, the solid electrolyte (SE)—as well as serving as a superionic conductor—functions as a surficial redox mediator that facilitates the sluggish reactions at the solid–solid two-phase boundaries, thereby substantially increasing the density of active sites. Through this mechanism, the ASSLSB exhibits ultrafast charging capability, showing a high specific capacity of 1,497 mAh g−1sulfur on charging at 2C (30 °C), while still maintaining 784 mAh g−1sulfur at 20C. Notably, a specific capacity of 432 mAh g−1sulfur is achieved on charging at an extreme rate of 150C at 60 °C. Furthermore, the cell demonstrates superior cycling stability over 25,000 cycles with 80.2% capacity retention at 5C (25 °C). We expect that our work on redox-mediated SSSRR will pave the way for developing advanced ASSLSBs that are high energy and safe.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
199,00 € per year
only 3,90 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The datasets analysed and generated during the course of this study are included in the paper and the Supplementary information.
References
Han, F. et al. High-performance all-solid-state lithium–sulfur battery enabled by a mixed-conductive Li2S nanocomposite. Nano Lett. 16, 4521–4527 (2016).
Gao, X. et al. All-solid-state lithium–sulfur batteries enhanced by redox mediators. J. Am. Chem. Soc. 143, 18188–18195 (2021).
Yan, H. et al. In situ generated Li2S–C nanocomposite for high-capacity and long-life all-solid-state lithium sulfur batteries with ultrahigh areal mass loading. Nano Lett. 19, 3280–3287 (2019).
Wang, D. et al. Realizing high-capacity all-solid-state lithium-sulfur batteries using a low-density inorganic solid-state electrolyte. Nat. Commun. 14, 1895 (2023).
Kim, J. T. et al. Manipulating Li2S2/Li2S mixed discharge products of all-solid-state lithium sulfur batteries for improved cycle life. Nat. Commun. 14, 6404 (2023).
Kato, Y. et al. High-power all-solid-state batteries using sulfide superionic conductors. Nat. Energy 1, 16030 (2016).
Janek, J. & Zeier, W. G. A solid future for battery development. Nat. Energy 1, 16141 (2016).
Ning, Z. et al. Dendrite initiation and propagation in lithium metal solid-state batteries. Nature 618, 287–293 (2023).
Wan, H., Wang, Z., Zhang, W., He, X. & Wang, C. Interface design for all-solid-state lithium batteries. Nature 623, 739–744 (2023).
Li, Y. et al. A lithium superionic conductor for millimeter-thick battery electrode. Science 381, 50–53 (2023).
Han, F. et al. High electronic conductivity as the origin of lithium dendrite formation within solid electrolytes. Nat. Energy 4, 187–196 (2019).
Yin, Y.-C. et al. A LaCl3-based lithium superionic conductor compatible with lithium metal. Nature 616, 77–83 (2023).
Tan, D. H. S. et al. Carbon-free high-loading silicon anodes enabled by sulfide solid electrolytes. Science 373, 1494–1499 (2021).
Kochetkov, I. et al. Different interfacial reactivity of lithium metal chloride electrolytes with high voltage cathodes determines solid-state battery performance. Energy Environ. Sci. 15, 3933–3944 (2022).
Zuo, T. et al. A mechanistic investigation of the Li10GeP2S12|LiNi1-x-yCoxMnyO2 interface stability in all-solid-state lithium batteries. Nat. Commun. 12, 6669 (2021).
Wang, L. et al. High-energy all-solid-state lithium batteries enabled by Co-free LiNiO2 cathodes with robust outside-in structures. Nat. Nanotechnol. 19, 208–218 (2024).
Wang, S. et al. Thermal stability between sulfide solid electrolytes and oxide cathode. ACS Nano 16, 16158–16176 (2022).
Rui, X. et al. Distinct thermal runaway mechanisms of sulfide-based all-solid-state batteries. Energy Environ. Sci. 16, 3552–3563 (2023).
Pang, Q., Liang, X., Kwok, C. Y. & Nazar, L. F. Advances in lithium–sulfur batteries based on multifunctional cathodes and electrolytes. Nat. Energy 1, 16132 (2016).
Hayashi, A., Ohtsubo, R., Ohtomo, T., Mizuno, F. & Tatsumisago, M. All-solid-state rechargeable lithium batteries with Li2S as a positive electrode material. J. Power Sources 183, 422–426 (2008).
Kwok, C. Y., Xu, S., Kochetkov, I., Zhou, L. & Nazar, L. F. High-performance all-solid-state Li2S batteries using an interfacial redox mediator. Energy Environ. Sci. 16, 610–618 (2023).
Pan, H. et al. Carbon-free and binder-free Li-Al alloy anode enabling an all-solid-state Li-S battery with high energy and stability. Sci. Adv. 8, eabn4372 (2022).
Hakari, T., Hayashi, A. & Tatsumisago, M. Li2S-based solid solutions as positive electrodes with full utilization and superlong cycle life in all-solid-state Li/S batteries. Adv. Sustain. Syst. 1, 1700017 (2017).
Pang, Q. et al. Fast-charging aluminium–chalcogen batteries resistant to dendritic shorting. Nature 608, 704–711 (2022).
Meini, S., Elazari, R., Rosenman, A., Garsuch, A. & Aurbach, D. The use of redox mediators for enhancing utilization of Li2S cathodes for advanced Li–S battery systems. J. Phys. Chem. Lett. 5, 915–918 (2014).
Liu, T. et al. Cycling Li-O2 batteries via LiOH formation and decomposition. Science 350, 530–533 (2015).
Ren, Y. X., Zhao, T. S., Liu, M., Zeng, Y. K. & Jiang, H. R. A self-cleaning Li-S battery enabled by a bifunctional redox mediator. J. Power Sources 361, 203–210 (2017).
Park, J. B., Lee, S. H., Jung, H. G., Aurbach, D. & Sun, Y. K. Redox mediators for Li–O2 batteries: status and perspectives. Adv. Mater. 30, 1704162 (2018).
Lim, H. D. et al. Rational design of redox mediators for advanced Li–O2 batteries. Nat. Energy 1, 16066 (2016).
Menetrier, M., Hojjaji, A., Estournes, C. & Levasseur, A. Ionic conduction in the B2S3-Li2S glass system. Solid State Ion. 48, 325–330 (1991).
Chi, X. et al. An electrochemically stable homogeneous glassy electrolyte formed at room temperature for all-solid-state sodium batteries. Nat. Commun. 13, 2854 (2022).
Wada, H., Menetrier, M., Levasseur, A. & Hagenmuller, P. Preparation and ionic conductivity of new B2S3–Li2S–LiI glasses. Mater. Res. Bull. 18, 189–193 (1983).
Liu, X. et al. Local electronic structure variation resulting in Li ‘filament’ formation within solid electrolytes. Nat. Mater. 20, 1485–1490 (2021).
Larink, D., Eckert, H. & Martin, S. W. Structure and ionic conductivity in the mixed-network former chalcogenide glass system [Na2S]2/3[(B2S3)x(P2S5)1−x]1/3. J. Phys. Chem. C 116, 22698–22710 (2012).
Ohara, K. et al. Structural and electronic features of binary Li2S-P2S5 glasses. Sci. Rep. 6, 21302 (2016).
Kaup, K. et al. A lithium oxythioborosilicate solid electrolyte glass with superionic conductivity. Adv. Energy Mater. 10, 1902783 (2020).
Feng, X. et al. Synthesis and characterizations of highly conductive and stable electrolyte Li10P3S12I. Energy Storage Mater. 22, 397–401 (2019).
Imanaka, N., Misran, M. R. I. B. & Nunotani, N. Evidence for enormous iodide anion migration in lanthanum oxyiodide-based solid. Sci. Adv. 7, eabh0812 (2021).
Vinatier, P., Ménétrier, M. & Levasseur, A. Aggregation of the doping salt in B2S3-Li2S-LiI glasses, effect on the dynamical properties. Solid State Ion. 116, 35–45 (1999).
See, K. A. et al. Effect of hydrofluoroether cosolvent addition on Li solvation in acetonitrile-based solvate electrolytes and its influence on S reduction in a Li–S Battery. ACS Appl. Mater. Interfaces 8, 34360–34371 (2016).
Morgan, W. E., Wazer, J. R. & Stec, W. J. Inner-orbital photoelectron spectroscopy of the alkali metal halides, perchlorates, phosphates, and pyrophosphates. J. Am. Chem. Soc. 95, 751–755 (1973).
Dillard, J. G. et al. An X-ray photoelectron and Auger electron spectroscopic study of the adsorption of molecular iodine on uranium metal and uranium dioxide. J. Phys. Chem. 88, 4104–4111 (1984).
Wang, S. et al. Lithium argyrodite as solid electrolyte and cathode precursor for solid-state batteries with long cycle life. Adv. Energy Mater. 11, 2101370 (2021).
Schwietert, T. K. et al. Clarifying the relationship between redox activity and electrochemical stability in solid electrolytes. Nat. Mater. 19, 428–435 (2020).
Kerner, R. A. et al. Organic hole transport material ionization potential dictates diffusion kinetics of iodine species in halide perovskite devices. ACS Energy Lett. 6, 501–508 (2021).
Ahn, S. et al. Why charging Li–air batteries with current low-voltage mediators is slow and singlet oxygen does not explain degradation. Nat. Chem. 15, 1022–1029 (2023).
Gerber, L. C. H. et al. Three-dimensional growth of Li2S in lithium–sulfur batteries promoted by a redox mediator. Nano Lett. 16, 549–554 (2016).
Ravel, B. & Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 12, 537–541 (2005).
Strauss, F. et al. Gas evolution in lithium-ion batteries: solid versus liquid electrolyte. ACS Appl. Mater. Interfaces 12, 20462–20468 (2020).
Teo, J. H. et al. Design-of-experiments-guided optimization of slurry-cast cathodes for solid-state batteries. Cell Rep. Phys. Sci. 2, 100465 (2021).
Zhou, L. et al. Solvent-engineered design of argyrodite Li6PS5X (X = Cl, Br, I) solid electrolytes with high ionic conductivity. ACS Energy Lett. 4, 265–270 (2019).
Adeli, P. et al. Boosting solid-state diffusivity and conductivity in lithium superionic argyrodites by halide substitution. Angew. Chem. Int. Ed. 58, 8681–8686 (2019).
Royle, M., Cho, J. & Martin, S. W. Raman spectroscopy studies of xNa2S+(1−x)B2S3 glasses and polycrystals. J. Non-Cryst. Solids 279, 97–109 (2001).
Bischoff, C., Schuller, K., Haynes, M. & Martin, S. W. Structural investigations of yNa2S+(1−y)PS5/2 glasses using Raman and infrared spectroscopies. J. Non Cryst. Solids 358, 3216–3222 (2012).
Kudu, O. U. et al. A review of structural properties and synthesis methods of solid electrolyte materials in the Li2S–P2S5 binary system. J. Power Sources 407, 31–43 (2018).
Hertle, J. et al. Miniaturization of reference electrodes for solid-state lithium-ion batteries. J. Electrochem. Soc. 170, 040519 (2023).
Lu, Y., Zhao, C.-Z., Huang, J.-Q. & Zhang, Q. The timescale identification decoupling complicated kinetic processes in lithium batteries. Joule 6, 1172–1198 (2022).
Cao, D., Sun, X., Li, F., Bak, S.-M. & Ji, T. Understanding electrochemical reaction mechanisms of sulphur in all-solid-state batteries through operando and theoretical studies. Angew. Chem. Int. Ed. 62, e202302363 (2023).
Alvarez, L. et al. High-pressure behavior of polyiodides confined into single-walled carbon nanotubes: a Raman study. Phys. Rev. B 82, 205403 (2010).
Tan, D. H. S. et al. Elucidating reversible electrochemical redox of Li6PS5Cl solid electrolyte. ACS Energy Lett. 4, 2418–2427 (2019).
Koerver, R. et al. Capacity fade in solid-state batteries: interphase formation and chemomechanical processes in nickel-rich layered oxide cathodes and lithium thiophosphate solid electrolytes. Chem. Mater. 29, 5574–5582 (2017).
Kimura, T. et al. Stabilizing high-temperature α-Li3PS4 by rapidly heating the glass. J. Am. Chem. Soc. 145, 14466–14474 (2023).
Sedlmaier, S. J. et al. Li4PS4I: a Li+ superionic conductor synthesized by a solvent-based soft chemistry approach. Chem. Mater. 29, 1830–1835 (2017).
Acknowledgements
This work was supported by the National Key R&D Program of China (grant no. 2021YFB2500200), the National Natural Science Foundation of China (NSFC) (grant nos. 92372115, 22075002). We are also grateful for the support from the Beijing Natural Science Foundation (no. Z220020) and NSFC (52103329, 22409006 and 52203347). We acknowledge the financing from BMBF (Bundesministerium für Bildung und Forschung, Germany) within the project SOLIS (03XP0395D). We thank the beamline station 4B7A at the Beijing Synchrotron Radiation Facility (BSRF) for the XANES measurements. We appreciate T. L. Song (Experimental Center of Advanced Materials, School of Materials Science & Engineering, Beijing Institute of Technology) for the contribution to the discussion of the TOF-SIMS study.
Author information
Authors and Affiliations
Contributions
H.S. and Q.P. conceived the concept and designed the experiments. H.S. conducted the materials synthesis. H.S. carried out the characterizations and electrochemical measurements, with the help of X.L., K.S., X.H., J.M., Y.Liu, M.H. and Y.Li. D.J. assisted in performing the Raman measurements. K.M., T.W. and Y.Y. performed the TOF-SIMS analyses and discussed other spectroscopy data under the supervision of J.J. R.Z. and P.H. conducted the DEMS measurements and data analyses under the supervision of T.B. All authors participated in the discussion of the results and contributed to the data analysis. H.S., Q.P., K.M., T.B. and J.J. wrote the manuscript, with contributions from all authors. Q.P. supervised the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Rezan Demir Çakan, Kentaro Yamamoto and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Structural characterizations and physicochemical properties of the LBPSI GSEs (the ratio x is defined as n(P2S5)/n(P2S5 + B2S3)).
a, XRD patterns for the LBPSI electrolytes with x of 0, 0.11, 0.17, 0.29, 0.50, 0.67, 0.75 and 0.80. b, Raman spectra of LBPSI electrolytes with x from 0 to 0.80. c, Enlarged view focused on the [PmSn] clusters. d, Schematic drawing of the clusters with their characteristic Raman shift. e, 31P MAS NMR spectra of LBPSI electrolytes with x of 0.17 and 0.29. f–h, DC polarization curves with an ion-blocking cell configuration of Ti|electrolyte|Ti for the three LBPSI electrolytes with x of 0 (f), 0.17 (g) and 0.29 (h). The thicknesses of the pellets during measurement for the three electrolytes are 0.028 cm, 0.022 cm and 0.023 cm, respectively. The measurements were conducted at 25 °C. i, The trend of electron conductivities obtained from DC polarization curves and the corresponding Ea values. The electronic conductivities for the three LBPSI electrolytes with x of 0, 0.17 and 0.29 are 6.4 × 10−10 S cm−1, 2.8 × 10−11 S cm−1 and 2.8 × 10−10 S cm−1, respectively. It is clear that, by fine-tuning the P/B ratio in the sulfide glass, the electronic conductivity of the electrolytes can be reduced. The values are much lower than the typical electron conductivity of argyrodite Li6PS5Cl and Li5.5PS4.5Cl1.5 (typically measured to be 10−8–10−9 S cm−1)51,52, which may lead to low self-discharge in a cell. Discussions on the evolution of local clustering structures: on the basis of the XRD patterns (a), for the P2S5-free electrolyte (x = 0), a crystalline LiI phase is present, indicating that the LBSI glass framework cannot fully include the amount of LiI added, which leads to the precipitation of crystalline-phase LiI on quenching. With the addition of P2S5, the fraction of the crystalline phase of LiI markedly decreases and is barely observed at x = 0.17, indicating that, with a small amount of P2S5, the glass structure can better dissolve and integrate the LiI. The generated I− in the glass structure can therefore contribute to a weaker coulombic attraction for Li+. However, at high P2S5/(P2S5 + B2S3) ratios of x = 0.75 and 0.80, the peak of crystalline LiI gradually appears again, suggesting that excessive P2S5 counteracts on the integration of LiI and would impede the Li+ diffusion. The Raman spectrum of P2S5-free electrolyte (x = 0) (b,c) shows the presence of only the [BmSn] group: the bands around 394 cm−1 and 433 cm−1 correspond to the B–S breathing mode of [BS3] groups34; the bands around 312 cm−1, 497 cm−1 and 763 cm−1 correspond to the vibrations of metathioborate [B3S6], thiopyroborate [B2S5] and [BS4] groups, respectively34,53. With x increased to 0.11 and 0.17, a strong Raman peak corresponding to the [PS4] group appears at 420 cm−1 (ref. 35), along with decrease of the [BmSn] peaks. Notably, at x = 0.29, two more peaks appear at 386 cm−1 and 407 cm−1, corresponding to the polyanionic groups of [P2S6]hypo and [P2S7], respectively34,35. Further, when x = 0.50, the Raman peak corresponding to [PS4] greatly fades, whereas those of [P2S6]hypo and [P2S7] groups are more pronounced, along with the appearance of a new peak at 582 cm−1 ascribed to [P2S6]hypo (refs. 54,55). Here the two peaks at 386 cm−1 and 582 cm−1 represent the symmetric and asymmetric stretching modes of the [P2S6]hypo group, respectively (d)54. As x further increases to 0.67, the [PS4] peak is no longer observed and a new peak appears at 425 cm−1, along with two subtle peaks at 315 cm−1 and 368 cm−1, corresponding to the [P2S6]meta group54,55. Further increasing the fraction of P2S5 to x = 0.75 and 0.80 leads to a much higher intensity of the [P2S7] peak, along with the peaks of [P2S6]meta and [P2S6]hypo. On the basis of these results, we can clearly observe two transitions of the local structures in the LBPSI electrolytes as x increases: (1) the appearance of the [PS4] group at x = 0.11; (2) the gradual disappearance of the [PS4] group, along with the dominance of the [P2S6] and [P2S7] groups at x = 0.50. By associating the local structural changes with the evolution of ionic conductivity (Fig. 1b), we can draw the following conclusions: at x = 0.11, the appearance of the [PS4] group substantially enhances the ionic conductivity, accounting for the high conductivity of more than 1 mS cm−1 at x = 0.11, 0.17 and 0.29. We believe that the [PS4] group promotes disruption of the large B–S network and formation of island-like structures, thereby enhancing the Li+ ion transport. The fragmentation is probably because of the different coordination preference of P (fourfold coordinated) and B (threefold coordinated), that is, the incorporation of P thermodynamically breaks the threefold coordinated B–S network. As x increases to 0.50, the appearance and dominance of [P2S6] and [P2S7] polyanions decreases the ionic conductivity, which ultimately reduces to 10−3 mS cm−1 at x = 0.80, which is probably because of the highly charged polyanions exhibiting higher coulombic attraction for Li+ ions than the [PS4] group35. Further, as shown in 31P MAS NMR spectra (e), the resonances around 83 ppm, 99 ppm and 108 ppm can be ascribed to the [PS4], [P2S7] and [P2S6] groups, respectively34. It is clear that the resonance of [PS4] appears in the electrolytes with x = 0.17 and 0.29. Further, the resonance of polyanionic [P2S7] and [P2S6] groups appear in the electrolyte with x = 0.29 (and not in the one with 0.17), which is in accordance with the Raman results. Altogether, in LBPSI, we find evidence for a more fragmented network (which should provide more free volume) and effective inclusion of I−, which results in higher ionic conductivity and easier breaking of the bond between Li+ and the anionic framework ligands at the electrode–electrolyte interface.
Extended Data Fig. 2 Detailed electrochemical performance data for the evaluation of fast-charging ASSLSBs.
a, Differential capacity (dQ/dV) plots of the ASSLSB fitted with LBPSI with varied charging rates from 2C to 35C and a fixed discharging rate of 1C at 30 °C (voltage profiles shown in Fig. 2b; sulfur loading: 1.0 mg cm−2). b,c, The discharge–charge voltage profiles at varied rates for the first several activation cycles (shown in Fig. 2a) of the cells with LBPSI (b) and Li5.5PS4.5Cl1.5 (c). d,e, The discharge–charge voltage profiles of the ASSLSB fitted with the Li5.5PS4.5Cl1.5 electrolyte with charging rates from 2C to 35C and a fixed discharging rate of 1C at 30 °C (d) and the corresponding differential capacity (dQ/dV) plots (e) (sulfur loading: 1.0 mg cm−2). On the basis of the dQ/dV plots, we can observe that charging and discharging of the cell fitted with LBPSI exhibits distinct redox peaks when charged at 2C to 35C rates, indicating faradaic processes; in contrast, the cell fitted with Li5.5PS4.5Cl1.5 exhibits very small oxidation peaks when charged at rates exceeding 15C. f–h, The specific capacity (f), discharge–charge voltage profiles (g) and dQ/dV plots (h) of the cell using LBPSI as the active material (containing no sulfur in cathode; mass based on LBPSI), measured at the same areal current density as that for the ASSLSB in Fig. 2a (discharge current density is 1.7 mA cm−2). The loading of LBPSI is 1.9 mg cm−2, close to the amount of LBPSI used in the cathode of an ASSLSB. In the dQ/dV plots, we can observe distinct oxidation and reduction peaks owing to the redox behaviour of the LBPSI electrolyte itself. It should be noted that a small but constant fraction (7.8–8.9%) of the capacity comes from the fully reversible redox behaviour of the LBPSI electrolyte at all investigated rates, as quantified by the cell using LBPSI as the only active material. i, The dQ/dV plots of the ASSLSB fitted with LBPSI at 60 °C, with varied charging rates from 5C to 150C and a fixed discharging rate of 2C (sulfur loading: 1.05 mg cm−2). j, The dQ/dV plots of the ASSLSB fitted with Li5.5PS4.5Cl1.5 at charging rates from 5C to 150C and a fixed discharging rate of 2C at 60 °C. k,l, The discharge–charge voltage profiles (k) and dQ/dV plots (l) of the cell with LBPSI as the active material (containing no sulfur in cathode) at 60 °C (discharging current density is 3.5 mA cm−2; the loading of LBPSI is 1.9 mg cm−2).
Extended Data Fig. 3 Galvanostatic cycling of the In/InLi|LBPSI|In/InLi symmetric cell, the three-electrode sulfur-containing cell and long-term cycling of the ASSLSB at extreme charging rates.
a,b, The galvanostatic cycling of the In/InLi|LBPSI|In/InLi symmetric cell (In/InLi foil as both electrodes) with current increasing from 0.26 mA cm−2 to 31.8 mA cm−2 at 25 °C (the Li plating/stripping time is 30 min for measurements with current density ≤ 3.8 mA cm−2 and 5 min for those with current density ≥ 6.4 mA cm−2) and enlarged section at high currents (reaching the voltage limit) (b). The symmetric cell can indeed operate at higher currents without soft or hard short-circuit at the areal capacity used, but in fact suffers from much greater overpotential than at low currents; this indicates that the In/InLi electrode shows limited reaction kinetics. This result offers some qualitative hints on the overall poor reaction kinetics for In/InLi electrodes. Such a high overpotential at the anode, along with the ohmic resistance from the SE, contributes to the high overpotential of full cells at high currents. c–f, Three-electrode cell measurement to examine the origin of the high overpotential of full cells at high currents. c, The scheme of the three-electrode cell, with a thin Li wire placed in the electrolyte layer close to the In/InLi counter electrode as a reference electrode. d–f, Discharge–charge voltage profiles at the current density of 0.08 mA cm−2 (d), 8.3 mA cm−2 (e) and 15.0 mA cm−2 (f) (sulfur loading of 1.0 mg cm−2; 25 °C). At a very low current density of 0.08 mA cm−2, the potential of the In/InLi electrode stabilizes at around 0.61 V. As the current density increases to 8.3 mA cm−2, the potential of the In/InLi electrode increases to about 0.75 V (on discharge) and reduces to about 0.46 V (on charge), resulting in a discharging overpotential of 0.14 V and charging overpotential of 0.15 V. On further increasing of the current to 15.0 mA cm−2, the potential of the In/InLi electrode increases to about 0.90 V (on discharge) and the potential decreases to about 0.31 V (on charge), resulting in a discharging overpotential of 0.29 V and charging overpotential of 0.30 V. Therefore, the reaction overpotential of the In/InLi electrode is high and increases greatly with the current density. Furthermore, there is a large increase of the ohmic resistance when the current is increased from 0.08 mA cm−2 to 8.3 mA cm−2. The ohmic resistance is qualitatively recognized by the abrupt voltage change (IR drop) on supplying of the charging/discharging currents. The increased ohmic resistance is the result of the thick layer of the electrolyte separator between the sulfur working electrode and the Li reference electrode, and the moderate ionic conductivity of the SE (2.4 mS cm−1). The high overpotential of the cell at high charging rates is largely because of the In/InLi anode and IR ohmic polarization56. g–j, The actual discharge–charge voltage profiles by using the potential versus In/InLi counter electrode of the ASSLSBs fitted with the LBPSI and Li5.5PS4.5Cl1.5 electrolyte at different current densities at 30 °C (g,h) and 60 °C (i,j). k,l, Cycling performance (k) and discharge–charge voltage profiles (l) of ASSLSB fitted with LBPSI electrolyte at a charging rate of 100C and a discharging rate of 2C at 60 °C. The cell was subjected to an activation process by cycling at 1–80C (charging rates) for several cycles. The cell sustains 5,000 cycles with a capacity retention greater than 80.4% (sulfur loading: 1.0 mg cm−2). It is clear that the cell experienced marginal voltage degradation over the cycling. The fluctuation in Coulomb efficiency is because of the limited resolution for data acquisition from the equipment with such a short duration of charging.
Extended Data Fig. 4 The electronic and ionic conductivity of sulfur electrodes prepared with LBPSI-0.17, LBPSI-0.29 and Li5.5PS4.5Cl1.5 and the morphological characterization of the electrolytes.
a, DC polarization curves of sulfur electrodes with an ion-blocking configuration of Ti|sulfur electrode|Ti. b–d, DC polarization curves of sulfur electrodes with an electron-blocking configuration of Ti|Cu|In/InLi|SE|sulfur electrode|SE|In/InLi|Cu|Ti. e, Nyquist plot of the symmetric cell of In/InLi|SE|In/InLi. f–k, Scanning electron microscopy (SEM) images (f–h) and corresponding particle size distributions (derived from the SEM imaging by statistical analysis; scale bars, 10 μm) (i–k) of LBPSI-0.17 (f,i), LBPSI-0.29 (g,j) and Li5.5PS4.5Cl1.5 (h,k). The electronic conductivity of the sulfur cathodes was obtained by measuring the electronic resistance with an ion-blocking cell configuration of Ti|sulfur electrode|Ti (a). The room-temperature electronic conductivities for the three sulfur cathodes prepared with LBPSI-0.17, LBPSI-0.29 and Li5.5PS4.5Cl1.5 is 0.031 S cm−1, 0.022 S cm−1 and 0.042 S cm−1, respectively. The ionic conductivities of the sulfur cathodes was obtained by measuring the total ionic resistance with an electron-blocking configuration of Ti|Cu| In/InLi|SE|sulfur electrode|SE|In/InLi|Cu|Ti (ref. 21) (b–d). The contributions of the SE and In/InLi electrode are measured to be 23 Ω and 0.9 Ω with the equivalent circuit fitting of the symmetric cell Ti|Cu|In/InLi|SE|In/InLi|Cu|Ti (e). The room-temperature ionic conductivity for the three sulfur cathodes prepared with LBPSI-0.17, LBPSI-0.29 and Li5.5PS4.5Cl1.5 after subtracting the contributions of SE and In/InLi electrode is 0.56 × 10−4 S cm−1, 0.39 × 10−4 S cm−1 and 0.41 × 10−4 S cm−1, respectively. The three composite sulfur cathodes exhibit similar ionic and electronic conductivities. The SEM images and particle-size distributions (derived from the SEM imaging by statistical analysis) (f–k) show that the mean particle sizes of LBPSI-0.17, LBPSI-0.29 and Li5.5PS4.5Cl1.5 are somewhat close at 2.13, 2.34 and 2.18 μm, respectively. Likewise, the three electrolytes all exhibit wide particle-size distribution over 0–10 μm, with a concentration in the range 0.5–5.0 μm.
Extended Data Fig. 5 Further electrochemical data of the cells prepared with electrolyte–carbon composites containing no sulfur in the cathode and the cells with sulfur cathodes.
a,b, The cycling performance with capacity and Coulomb efficiency of the cell prepared with a LBPSI–C composite cathode (wt% LBPSI:wt% C = 8:2) containing no sulfur (0.25 mA cm−2; LBPSI loading of 6.5 mg cm−2) (a) and the corresponding differential capacity plots (dQ/dV) (b). c, The CV curves of the cell for the first five cycles. The cell in a–c is configured as In/InLi|LBPSI|LBPSI–C. The long-term cycling performance, dQ/dV plots and CV curves altogether show the high stability and reversibility of the redox reaction of LBPSI within the potential window of interest. d, The dQ/dV plots of the cell prepared with Li5.5PS4.5Cl1.5 as the active material in the cathode (wt% Li5.5PS4.5Cl1.5:wt% C = 8:2), configured as In/InLi|Li5.5PS4.5Cl1.5|Li5.5PS4.5Cl1.5-C for the first, second and fiftieth cycles; the dQ/dV plots of first and fiftieth cycles show notable differences in terms of the peak positions and intensity, indicating irreversible decomposition of Li5.5PS4.5Cl1.5 after the first charging process, which may cause some permanent damage to the interface in a sulfur cell. e,f, First-cycle discharge–charge voltage profiles of the cells with sulfur cathodes at a discharging/charging rate of 0.1C (e) and the corresponding differential capacity (dQ/dV) (f) (25 °C), which shows the presence of LBPSI redox that occurs in the cell at a slightly higher voltage than that of Li2S/S, whereas the Li5.5PS4.5Cl1.5 redox, if any, occurs with a very much overlapped voltage range with that of Li2S/S. g,h, In situ EIS studies on a sulfur cell fitted with LBPSI for the first charge (after the first discharge process) and following discharge at 0.1C: Nyquist plots at representative states of charge/discharge (g) and the corresponding distribution of relaxation time analysis (h). The in situ EIS study shows that, when charged to 2.8 V, a new semicircle at the medium frequencies appears, indicating that a new interface forms, which is herein tentatively ascribed to the surficial oxidation of LBPSI forming a surface layer. The corresponding distribution of relaxation time analysis that mathematically converts the frequency-dependent EIS spectra to relaxation-based functions γ(τ) (ref. 57), shows the appearance of relaxation distribution (γ) at the characteristic time constant (τ) of 10−3–100 s and further confirms the formation of a new interface. The reversibility of the redox behaviour of LBPSI is indicated by the observation of the additional interface disappearing during discharge.
Extended Data Fig. 6 Ex situ synchrotron sulfur K-edge XANES profiles of the sulfur cathode fitted with LBPSI electrolyte and ex situ Raman and XPS studies of the cells prepared with LBPSI as the active material in the cathode (configured as In/InLi|LBPSI|LBPSI–C).
a, Sulfur K-edge XANES spectra of the sulfur cathode fitted with LBPSI electrolyte performed ex situ at different discharge/charge states, along with the sulfur standards for references. b, Ex situ Raman spectra of the pristine cathode and the cathode charged to 3.2 V of the cell using LBPSI as the active material (containing no sulfur) in the cathode. The Raman spectra of LiI, I2 and milled I2/LiI ((I2+LiI)-BM) are measured as references. c,d, The I 3d (c) and S 2p (d) XPS data of the pristine LBPSI material, the pristine cathode, the electrode charged to 3.2 V and the electrode subsequently discharged to 1.4 V in the cell configured as In/InLi|LBPSI|LBPSI–C (dotted lines, raw data; black lines, fitted total; coloured lines, fitted components). e, The I 3d spectra of the pristine electrode and the electrode charged to 3.2 V in the cell for better visibility of the signal shift. We performed ex situ sulfur K-edge XANES measurements and observe that the sulfur cathode using LBPSI electrolyte discharges with the formation of Li2Sx intermediates and that Li2S/Li2Sx is completely converted to sulfur on charge. The S/KB and Li2S/KB samples prepared by mixing the respective sulfur species with KB were used as references. The S/KB shows two features at 2,472.6 eV (the white line) and 2,480.0 eV; the Li2S/KB shows three features at 2,473.6, 2,476.2 and 2,484.0 eV. The spectrum of the pristine cathode shows the features of S0 at 2,472.6 and 2,480.0 eV. When discharged to 1.4 V, the features of Li2S at 2,473.6, 2,476.2 and 2,484.0 eV as well as that for Li2Sx at 2,471.8 eV appear5,58. After the subsequent charging, the features corresponding to Li2S or Li2Sx disappear and S0 features at 2,472.6 and 2,480.0 eV emerge again. This indicates that the charging has fully converted Li2S (and Li2Sx) back to sulfur. When discharged to 1.4 V again, the features of S0 disappear and the features of Li2S and Li2Sx appear. This demonstrates the reversibility of the SSSRR during charging and discharging. In the Raman spectra of the LBPSI–C electrodes (b), compared with the pristine electrode, for the electrode charged to 3.2 V, a broad band appears at 100–200 cm−1. The peak at around 181 cm−1 is attributed to I2 and the peaks around 110 cm−1 and 167 cm−1 are attributed to I3− (ref. 59). For a clear reference of the assignment of these peaks, we measured the Raman spectra of crystalline LiI, crystalline I2 and a milled mixture of I2/LiI (which forms LiI3). We can see that the peak around 181 cm−1 is clearly attributed to the vibrations of I2 and the peaks around 110 cm−1 and 167 cm−1 are attributed to the vibrations of I3−. From the I 3d XPS spectra (c,e), we can clearly observe the signal shift of the I 3d spectra to higher binding energy on charging the cell to 3.2 V (e), confirming the I on the surface of the SE being present with a higher valence state than that in LBPSI (I−). By a closer observation of the fitted I 3d spectra, the pristine LBPSI electrolyte and LBPSI–C electrode show anionic I−, as evidenced by the I 3d doublet signals at 618.8 eV (3d5/2 energy)41 (c). On charging to 3.2 V, the oxidized iodine species are clearly observed (tentatively assigned to I2/I3−)42, which locates at around 619.9 eV (3d5/2 energy). Furthermore, after subsequent discharging to 1.4 V, the signals of oxidized iodine species disappear, demonstrating the reversible oxidation/reduction of iodine species. For the S 2p spectra (d), the pristine LBPSI electrolyte and electrode contain [PS4] units (and [BS4], that is, non-bridging sulfur) and P–S–P (and B–S–B, that is, bridging sulfur) signals, with characteristic S 2p signals located at 161.5 eV and 162.5 eV (2p3/2 energy)43,60. The LBPSI electrode charged to 3.2 V contains oxidized sulfur (S0), which is evident by the characteristic S 2p doublet signals at around 163.6 eV (2p3/2 energy)61. After discharge to 1.4 V, the signal of oxidized sulfur (S0) disappears and signals of bridging sulfur and non-bridging sulfur appear, which demonstrate the reversible oxidation/reduction of sulfur species.
Extended Data Fig. 7 The experimental evidence of diffusion of I2 along the SE particle surface and the occurrence of the reaction between I2 as redox mediator and Li2S.
a–c, Evidence of diffusion of I2 along the SE particle surface. a, The LBPSI electrolyte pellet on direct contact with I2 for 5 min. b, The LBPSI electrolyte pellet placed at the I2 bottle cap for indirect contact with I2 for 3 days. c, EDX mapping of the vertical cross-section of Li5.5PS4.5Cl1.5 electrolyte pellet after indirect contact with I2 for 3 days. d, Pictures showing the process of mixing Li2S and I2 in anhydrous ethanol. e,f, XRD pattern (e) and Raman spectrum (f) of the precipitated product. g,h, Characterization of the reaction of Li2S and I2 by a gentle ball-milling at 100 rpm for 2 h, with the XRD pattern (g) and Raman spectrum (h) of the product. i,j, Characterization of the reaction of Li2S and I2 by simple hand grinding in the mortar (5 min), with the XRD pattern (i) and Raman spectrum (j) of the product. The insets of j are visual pictures of the mixture of Li2S and I2 before and after hand grinding in the mortar. The diffusion and permeation of I2 into the electrolyte pellet is shown in a,b. On direct contact of the LBPSI SE pellet with the iodine (brown), I2 sublimes and diffuses very quickly over the LBPSI pellet surface within 5 min; the surface of the electrolyte is covered with brown iodine (a). The LBPSI pellet was placed at the cap of a bottle that contains I2 and the bottle was sealed with grease to prevent escape of iodine vapour through the gap (that is, indirect contact; b). After 3 days without direct contact with I2, the outer/top surface of the electrolyte pellet turns brown, indicating penetration of the iodine vapour across the SE pellet (b), which further proves the diffusion of I2 along the grain boundaries and throughout the SE pellet. Note that these experiments were conducted in an Ar-filled glovebox and not in vacuum. Further, we performed the same indirect contact experiment with the iodine-free Li5.5PS4.5Cl1.5 electrolyte. The cross-section of the pellet was subjected to scanning electron microscopy imaging and EDX analysis (c). We can see that the element I is uniformly distributed, along with the P, S and Cl, over the imaged area (several hundred µm; c). To mimic the reactions between Li2S and I2, equal molar amounts of Li2S and I2 were first added separately into anhydrous ethanol, the solutions of which are, respectively, colourless and purple (step 1; d) and, subsequently, the two solutions of Li2S and I2 were mixed. Along with the change of colour (pale yellow, step 2), we observed precipitation, which is confirmed to consist of crystallized LiI along with S (as shown by the XRD pattern in e). The Raman spectrum further shows a strong signal corresponding to S (f). Therefore, the products of Li2S reacting with I2 are confirmed to be S and LiI. With an effort to further mimic the condition of the reaction occurring within the all-solid-state Li–S cells, the reaction was performed in solid state. Specifically, equimolar amounts of Li2S and I2 were mixed by a gentle ball-milling at 100 rpm for 2 h (to prevent overheating of the jar) and LiI and S are formed on solid–solid mixing of the two materials (based on XRD pattern; g). As shown in h, the Raman spectrum exhibits strong S signals (154 cm−1, 219 cm−1 and 473 cm−1), I2 (signal around 181 cm−1) and I3− (110 cm−1 and 167 cm−1)59. In fact, even by simple hand grinding in the mortar, the two materials react very quickly (j); during 5 min of grinding, the two materials react to form LiI (based on XRD pattern; i), S and I3− (Raman spectrum; j). The feasibility of the chemical reaction between I2 and Li2S that we experimentally confirmed above is strong evidence for us to conclude that the occurrence of redox mediation in the cell at high charging rates are not cascade reactions (that is, occurring sequentially without interplay). Further, the 0.3–0.4 V higher potential for iodine redox over that of sulfur redox (Fig. 3a) serves as the theoretical basis for a chemical reaction between the two couples. This is distinct from the behaviour of sulfur cells using Li5.5PS4.5Cl1.5, in which the SE redox is expected to occur simultaneously with sulfur redox owing to the lack of potential difference (Fig. 3b).
Extended Data Fig. 8 Characterizations and electrochemical performance of ASSLSBs using Li3PS4 and 70Li3PS4·30LiI.
Li5.5PS4.5Cl1.5 was used as the electrolyte separator for the ASSLSBs for its high ionic conductivity. a,b, Powder XRD patterns of the Li3PS4 (a) and 70Li3PS4·30LiI (b). c, Nyquist plots of impedance spectra of Li3PS4 and 70Li3PS4·30LiI for the measurement of the respective ionic conductivity. d, The first-cycle discharge–charge voltage profile of ASSLSBs at 0.05C. e, Charging-rate performance with varied charging rates (labelled) and a constant discharging rate of 1C. Cells were subjected to an activation process by charging at progressively increasing rates of 0.05–0.8C for several cycles before being charged at 2C (sulfur loading: 1.0 mg cm−2). f,g, Discharge–charge voltage profiles of ASSLSBs with charging rates from 2C to 30C using Li3PS4 (f) and 70Li3PS4·30LiI (g) as the catholyte, respectively. h, Nyquist plot for the measurement of the ionic conductivity of LiI (the room-temperature ionic conductivity is measured to be 0.7 × 10−6 S cm−1). On discharge, the iodine species (more precisely, the LiI) can serve as ionically conducting facilitator in the cathode (h) because the as-formed LiI would remain in close contact to the sulfur that it has redox-mediated, and as a moderate ionic conductor (0.7 × 10−6 S cm−1; h) can thus assist in bridging the ionic transport path and enhance the discharge kinetics. We have further demonstrated the much-improved SSSRR kinetics enabled by another iodine-containing sulfide electrolyte (70Li3PS4·30LiI), which highlights the universality of the iodine-based surficial redox-mediating strategy. Li3PS4 and 70Li3PS4·30LiI electrolytes were synthesized to investigate whether the iodine-based redox mediating takes effect in these electrolytes. The two electrolytes are glass ceramics, of which the crystalline phase herein is confirmed to be Li3PS4 (Pnma phase)62 and Li4PS4I (P4/nmm phase)63 by XRD, respectively (a,b). The Li3PS4 and 70Li3PS4·30LiI electrolytes show similar room-temperature ionic conductivity of 0.17 mS cm−1 and 0.13 mS cm−1, respectively (c). We prepared sulfur cathodes using Li3PS4 and 70Li3PS4·30LiI electrolytes in the same way as for those using LBPSI. The Li5.5PS4.5Cl1.5 electrolyte was used as the separator for the cells. The cell with 70Li3PS4·30LiI, when charged at 0.05C, shows a further voltage plateau at 2.7 V, which is consistent with the LBPSI cell and is the result of the redox of iodine (d). The rate performance of the sulfur cathode with 70Li3PS4·30LiI is superior to that using Li3PS4. The cell with 70Li3PS4·30LiI exhibits a high specific capacity of 1,300 mAh g−1 at a charging rate of 2C and maintains 660 mAh g−1 at 10C (discharging rate constant at 1C). At low rates of 0.05C to 0.5C for the first several cycles, the cell fitted with Li3PS4 shows a higher capacity than the cell using 70Li3PS4·30LiI. However, at charging rates of 2C and 10C, it shows lower capacities of 1,113 mAh g−1 and 422 mAh g−1, respectively, than that using 70Li3PS4·30LiI in the cathode. Therefore, we consider that the iodine-based redox mediation effect should apply to a variety of SEs containing iodine on the surface.
Extended Data Fig. 9 Long-term cycling performance of ASSLSBs.
a,b, Discharge–charge voltage profiles of the cell fitted with Li5.5PS4.5Cl1.5 electrolyte (a) and LBPSI electrolyte (b) at a discharging/charging rate of 0.2C over different numbers of cycles (sulfur loading: 1.5 mg cm−2). c, Discharge–charge voltage profiles of the cell fitted with LBPSI electrolyte at a discharging/charging rate of 2C over 5,000 cycles (sulfur loading: 1.1 mg cm−2). d, Discharge–charge voltage profiles of the cell fitted with LBPSI electrolyte at a discharging/charging rate of 5C over 25,000 cycles (sulfur loading: 1.1 mg cm−2). e, Discharge–charge voltage profiles of the cell fitted with Li5.5PS4.5Cl1.5 electrolyte at a discharging/charging rate of 5C over 10,000 cycles (sulfur loading: 1.1 mg cm−2) as a comparison. f, The evolution of stack pressure of ASSLSBs during discharge–charge cycles at 30 °C with LBPSI and Li5.5PS4.5Cl1.5 electrolyte, respectively. Δp represents the change of stack pressure within one full discharge–charge cycle and \(\overline{\Delta p}\) is the average over five cycles. Both cells show apparent stack-pressure variations during cycling, indicating volume expansion and contraction of the electrodes; however, the average amplitude of pressure variation within one cycle for the cell using LBPSI is much lower than that with Li5.5PS4.5Cl1.5 (\({\overline{\Delta p}}_{{\rm{LBPSI}}}=0.45\,{\rm{MPa}}\); \({\overline{\Delta p}}_{{\rm{LiPSCI}}}=1.08\,{\rm{MPa}}\)), which is indicative of a more profound buffering of the microscopic volume change in the case of LBPSI. g,h, Long-term cycling of the cell fitted with LBPSI at a discharging/charging rate of 8C (sulfur loading: 1.0 mg cm−2) (g) and corresponding discharge–charge voltage profiles at 25 °C (h). The cell was subjected to an activation process by cycling at 0.05–7C for several cycles. The cell exhibits a high capacity of 744 mAh g−1 at 8C, with 78% capacity retention over 10,000 cycles.
Extended Data Fig. 10 Long-term cycling performance of ASSLSBs.
a, Discharge–charge voltage profiles of the cell fitted with LBPSI at a discharging/charging rate of 1C over 1,000 cycles (sulfur loading: 3.0 mg cm−2). b, Discharge–charge voltage profiles of the cell with a high sulfur fraction of 45 wt% in the cathode, fitted with LBPSI at a 0.2C rate (sulfur loading: 1.9 mg cm−2) and 25 °C. c,d, Long-term cycling of the cell fitted with LBPSI with a high sulfur loading of 6.3 mg cm−2 (c) and corresponding discharge–charge voltage profiles at 25 °C at a discharging/charging rate of 0.1C (d). e,f, Discharge–charge voltage profiles of the cell fitted with LBPSI for the first two activation cycles (e) and discharge–charge voltage profiles at a discharging/charging rate of 15C over 10,000 cycles (f) at 60 °C (sulfur loading: 1.0 mg cm−2). g, Discharge–charge voltage profiles of the cell fitted with Li5.5PS4.5Cl1.5 electrolyte at a discharging/charging rate of 15C over 10,000 cycles (sulfur loading: 1.0 mg cm−2) as a comparison. h,i, Low-temperature performance of ASSLSBs at −20 °C (sulfur loading: 1.1 mg cm−2). h, Charging-rate performance of cells using LBPSI and Li5.5PS4.5Cl1.5 electrolytes with varied charging rates (labelled) and a constant discharging rate of 0.2C. The first cycle was operated at room temperature at 0.05C for activation. At the low rates of 0.05–0.20C, the cell with Li5.5PS4.5Cl1.5 shows a slightly higher capacity than one with LBPSI at −20 °C, which is probably because of the lower ionic conductivity of LBPSI. At a charging rate of 0.3C, the two cells show comparable specific capacity of about 820 mAh g−1. Beyond that rate, the cell with LBPSI exhibits higher capacities, that is, a specific capacity of 652 mAh g−1 at a charging rate of 0.5C, maintaining 466 mAh g−1 at 1C, whereas the cell with Li5.5PS4.5Cl1.5 exhibits a specific capacity of 514 mAh g−1 at 0.5C and maintains only 295 mAh g−1 at 1C. i, Long-term cycling of the cells at a 1C charge/discharge rate at −20 °C. The cell with LBPSI electrolyte shows stable cycling with 406 mAh g−1 at 200 cycles and almost no capacity fading over 1,000 cycles. By contrast, the cell with Li5.5PS4.5Cl1.5 shows only 128 mAh g−1 at 200 cycles, which is maintained over 1,000 cycles.
Supplementary information
Supplementary Information
Supplementary Figs. 1–3, Supplementary Table 1 and Supplementary References.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Song, H., Münch, K., Liu, X. et al. All-solid-state Li–S batteries with fast solid–solid sulfur reaction. Nature 637, 846–853 (2025). https://doi.org/10.1038/s41586-024-08298-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-024-08298-9