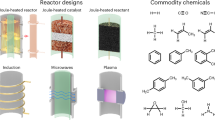
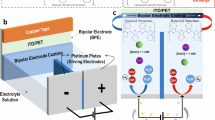
Abstract
High-throughput experimentation (HTE) has accelerated academic and industrial chemical research in reaction development and drug discovery and has been broadly applied in many domains of organic chemistry1,2. However, application of HTE in electrosynthesis—an enabling tool for chemical synthesis—has been limited by a dearth of suitable standardized reactors3,4,5,6,7. Here we report the development of microelectronic devices, which are produced using standard nanofabrication techniques, to enable wireless electrosynthesis on the microlitre scale. These robust and inexpensive devices are powered by visible light and convert any traditional 96-well or 384-well plate into an electrochemical reactor. We validate the devices in oxidative, reductive and paired electrolysis and further apply them to achieve the library synthesis of biologically active compounds and accelerate the development of two electrosynthetic methodologies. We anticipate that, by simplifying the way electrochemical reactions are set up, this user-friendly solution will not only enhance the experience and efficiency of current practitioners but also substantially reduce the barrier for nonspecialists to enter the field of electrosynthesis, thus allowing the broader community of synthetic chemists to explore and benefit from new reactivities and synthetic strategies enabled by electrochemistry8,9,10,11,12.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
199,00 € per year
only 3,90 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data supporting the findings of this work are available in the paper and its Supplementary Information.
References
Mennen, S. M. et al. The evolution of high-throughput experimentation in pharmaceutical development and perspectives on the future. Org. Process Res. Dev. 23, 1213–1242 (2019).
Buitrago Santanilla, A. et al. Nanomole-scale high-throughput chemistry for the synthesis of complex molecules. Science 347, 49–53 (2015).
Wills, A. G. et al. High-throughput electrochemistry: state of the art, challenges, and perspective. Org. Process Res. Dev. 25, 2587–2600 (2021).
Gütz, C., Klöckner, B. & Waldvogel, S. R. Electrochemical screening for electroorganic synthesis. Org. Process Res. Dev. 20, 26–32 (2016).
Rein, J., Lin, S., Kalyani, D. & Lehnherr, D. in The Power of High-Throughput Experimentation: General Topics and Enabling Technologies for Synthesis and Catalysis (Volume 1) Vol. 1419 (eds Emmert, M. H., Jouffroy, M. & Leitch, D. C.) 167–187 (American Chemical Society, 2022).
Chen, H. & Mo, Y. Accelerated electrosynthesis development enabled by high-throughput experimentation. Synthesis 55, 2817–2832 (2023).
Graaf, M. D. & Moeller, K. D. Introduction to microelectrode arrays, the site-selective functionalization of electrode surfaces, and the real-time detection of binding events. Langmuir 31, 7697–7706 (2015).
Yan, M., Kawamata, Y. & Baran, P. S. Synthetic organic electrochemical methods since 2000: on the verge of a renaissance. Chem. Rev. 117, 13230–13319 (2017).
Novaes, L. F. T. et al. Electrocatalysis as an enabling technology for organic synthesis. Chem. Soc. Rev. 50, 7941–8002 (2021).
Zhu, C., Ang, N. W. J., Meyer, T. H., Qiu, Y. & Ackermann, L. Organic electrochemistry: molecular syntheses with potential. ACS Cent. Sci. 7, 415–431 (2021).
Wiebe, A. et al. Electrifying organic synthesis. Angew. Chem. Int. Ed. 57, 5594–5619 (2018).
Little, R. D. & Moeller, K. D. Introduction: electrochemistry: technology, synthesis, energy, and materials. Chem. Rev. 118, 4483–4484 (2018).
Kolbe, H. Zersetzung der Valeriansäure durch den elektrischen Strom. Ann. Chem. Pharm. 64, 339–341 (1848).
Yan, M., Kawamata, Y. & Baran, P. S. Synthetic organic electrochemistry: calling all engineers. Angew. Chem. Int. Ed. 57, 4149–4155 (2018).
Gesmundo, N. J. et al. Nanoscale synthesis and affinity ranking. Nature 557, 228–232 (2018).
Hendrick, C. E. et al. Direct-to-biology accelerates PROTAC synthesis and the evaluation of linker effects on permeability and degradation. ACS Med. Chem. Lett. 13, 1182–1190 (2022).
Stevens, R. et al. Integrated direct-to-biology platform for the nanoscale synthesis and biological evaluation of PROTACs. J. Med. Chem. 66, 15437–15452 (2023).
Siu, T., Li, W. & Yudin, A. K. Parallel electrosynthesis of α-alkoxycarbamates, α-alkoxyamides, and α-alkoxysulfonamides using the spatially addressable electrolysis platform (SAEP). J. Comb. Chem. 2, 545–549 (2000).
Palkowitz, M. D. et al. Overcoming limitations in decarboxylative arylation via Ag–Ni electrocatalysis. J. Am. Chem. Soc. 144, 17709–17720 (2022).
Rein, J. et al. Unlocking the potential of high-throughput experimentation for electrochemistry with a standardized microscale reactor. ACS Cent. Sci. 7, 1347–1355 (2021).
Gerroll, B. H. R., Kulesa, K. M., Ault, C. A. & Baker, L. A. Legion: an instrument for high-throughput electrochemistry. ACS Meas. Sci. Au 3, 371–379 (2023).
Miskin, M. Z. et al. Electronically integrated, mass-manufactured, microscopic robots. Nature 584, 557–561 (2020).
Chen, J. & Mo, Y. Wireless electrochemical reactor for accelerated exploratory study of electroorganic synthesis. ACS Cent. Sci. 9, 1820–1826 (2023).
Molnar, A. C. et al. in Proc. 2021 IEEE Custom Integrated Circuits Conference (CICC) 1–6 (IEEE, 2021).
Ravetz, B. D. et al. Development of a platform for near-infrared photoredox catalysis. ACS Cent. Sci. 6, 2053–2059 (2020).
Pijper, B. et al. Addressing reproducibility challenges in high-throughput photochemistry. JACS A 4, 2585–2595 (2024).
Qi, N. et al. Development of a high intensity parallel photoreactor for high throughput screening. React. Chem. Eng. 7, 354–360 (2022).
Flamm, K. Measuring Moore’s Law: evidence from price, cost, and quality indexes. National Bureau of Economic Research Working Paper Series No. 24553. https://www.nber.org/papers/w24553 (2018).
Kirste, A., Elsler, B., Schnakenburg, G. & Waldvogel, S. R. Efficient anodic and direct phenol-arene C,C cross-coupling: the benign role of water or methanol. J. Am. Chem. Soc. 134, 3571–3576 (2012).
Hoque, M. A. et al. Electrochemical PINOylation of methylarenes: improving the scope and utility of benzylic oxidation through mediated electrolysis. J. Am. Chem. Soc. 144, 15295–15302 (2022).
Siu, J. C., Parry, J. B. & Lin, S. Aminoxyl-catalyzed electrochemical diazidation of alkenes mediated by a metastable charge-transfer complex. J. Am. Chem. Soc. 141, 2825–2831 (2019).
Lehnherr, D. et al. Electrochemical synthesis of hindered primary and secondary amines via proton-coupled electron transfer. J. Am. Chem. Soc. 142, 468–478 (2020).
Zhang, B. et al. Ni-electrocatalytic Csp3–Csp3 doubly decarboxylative coupling. Nature 606, 313–318 (2022).
Costa e Silva, R. et al. Electrosynthesis of aryliminophosphoranes in continuous flow. Adv. Synth. Catal. 366, 955–960 (2024).
Imada, Y. et al. Metal- and reagent-free dehydrogenative formal benzyl–aryl cross-coupling by anodic activation in 1,1,1,3,3,3-hexafluoropropan-2-ol. Angew. Chem. Int. Ed. 57, 12136–12140 (2018).
Huang, H. et al. Electrophotocatalysis with a trisaminocyclopropenium radical dication. Angew. Chem. Int. Ed. 58, 13318–13322 (2019).
Qiu, Y., Struwe, J., Meyer, T. H., Oliveira, J. C. A. & Ackermann, L. Catalyst- and reagent-free electrochemical azole C–H amination. Chem. Eur. J. 24, 12784–12789 (2018).
Anthony Romero, F. et al. The discovery of potent antagonists of NPBWR1 (GPR7). Bioorg. Med. Chem. Lett. 22, 1014–1018 (2012).
Thomas, R. P. et al. A direct-to-biology high-throughput chemistry approach to reactive fragment screening. Chem. Sci. 12, 12098–12106 (2021).
Wan, Z. et al. Electrochemical oxidative C(sp3)–H azolation of lactams under mild conditions. Green Chem. 22, 3742–3747 (2020).
Libendi, S. S., Demizu, Y. & Onomura, O. Direct electrochemical α-cyanation of N-protected cyclic amines. Org. Biomol. Chem. 7, 351–356 (2009).
Tajima, T. & Nakajima, A. Direct oxidative cyanation based on the concept of site isolation. J. Am. Chem. Soc. 130, 10496–10497 (2008).
Mäder, P. & Kattner, L. Sulfoximines as rising stars in modern drug discovery? Current status and perspective on an emerging functional group in medicinal chemistry. J. Med. Chem. 63, 14243–14275 (2020).
Sun, Q. et al. Cascade reactions of aryl-substituted terminal alkynes involving in situ-generated α-imino gold carbenes. Angew. Chem. Int. Ed. 63, e202313738 (2024).
Xie, X. & Sun, J. [4+3]-cycloaddition reaction of sulfilimines with cyclobutenones: access to benzazepinones. Org. Lett. 23, 8921–8925 (2021).
Tian, X. et al. Sulfilimines as versatile nitrene transfer reagents: facile access to diverse aza-heterocycles. Angew. Chem. Int. Ed. 58, 3589–3593 (2019).
Okamura, H. & Bolm, C. Sulfoximines: synthesis and catalytic applications. Chem. Lett. 33, 482–487 (2004).
Klein, M., Troglauer, D. L. & Waldvogel, S. R. Dehydrogenative imination of low-valent sulfur compounds─fast and scalable synthesis of sulfilimines, sulfinamidines, and sulfinimidate esters. JACS Au 3, 575–583 (2023).
Bandlish, B. K., Padilla, A. G. & Shine, H. J. Ion radicals. XXXIII. Reactions of 10-methyl- and 10-phenylphenothiazine cation radicals with ammonia and amines. Preparation and reactions of 5-(N-alkyl)sulfilimines and 5-(N,N-dialkylamino)sulfonium salts. J. Org. Chem. 40, 2590–2595 (1975).
Shine, H. J. & Kim, K. Cation radicals. XXVII. Sulfilimine derivatives from reaction of thianthrene and N-phenylphenothiazine cation radicals with t-butylamine and dimethylamine. Tetrahedron Lett. 15, 99–101 (1974).
Marzag, H., Schuler, M., Tatibouët, A. & Reboul, V. Synthesis of methionine-derived endocyclic sulfilimines and sulfoximines. Eur. J. Org. Chem. 2017, 896–900 (2017).
Luan, D. et al. Cyclic regulation of the sulfilimine bond in peptides and NC1 hexamers via the HOBr/H2Se conjugated system. Anal. Chem. 90, 9523–9528 (2018).
Dannenberg, C. A., Bizet, V. & Bolm, C. Direct access to N-alkylsulfoximines from sulfides by a sequential imidation/oxidation procedure. Synthesis 47, 1951–1959 (2015).
Acknowledgements
Financial support was provided by the National Institutes of Health (R01GM130928), the Camille and Henry Dreyfus Foundation, the College of Arts and Sciences at Cornell University (New Frontier Grant), the Cornell Center for Materials Research (DMR1719875) and the Cornell Ignite Innovation Acceleration programme. This work was performed in part at the Cornell NanoScale Facility, a member of the National Nanotechnology Coordinated Infrastructure (NNCI), which was supported by the National Science Foundation grant NNCI-2025233. We thank S. B. Zacate for experimental assistance; Cornell NanoScale Facility staff for guidance and support with the fabrication process; D. Kalyani, D. Lehnherr and M. K. Wismer for helpful discussion; I. Keresztes for nuclear magnetic resonance data analysis; J. Liu and W. Yue for data reproduction; L. Baker for providing a photograph of the Legion electrochemistry system; and K. Meinhaus for copyediting the manuscript.
Author information
Authors and Affiliations
Contributions
S.L., P.L.M. and J.R. conceived the project. S.L. and P.L.M. supervised the project. B.G. and J.R. conducted all synthetic experiments. B.G., S.N. and Y.J. designed and manufactured the devices. B.G., J.R. and S.L. wrote the initial draft of the manuscript. S.N., Y.J. and P.L.M. assisted in writing and editing the manuscript.
Corresponding authors
Ethics declarations
Competing interests
A US provisional patent (no. 63/592,209) was filed on the SPECS technology. S.L. and P.L.M. are commercializing the SPECS technology.
Peer review
Peer review information
Nature thanks Alastair Lennox and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Sections 1–11, including Supplementary Figs., Text and Data – see Contents for details.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Górski, B., Rein, J., Norris, S. et al. Light-harvesting microelectronic devices for wireless electrosynthesis. Nature 637, 354–361 (2025). https://doi.org/10.1038/s41586-024-08373-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-024-08373-1
This article is cited by
-
Electrochemical synthesis goes wireless
Nature (2025)