Abstract
Cancer cells have been shown to exploit neurons to modulate their survival and growth, including through the establishment of neural circuits within the central nervous system1,2,3. Here we report a distinct pattern of cancer–nerve interactions between the peripheral nervous system and gastric cancer. In multiple mouse models of gastric cancer, nociceptive nerves demonstrated the greatest degree of nerve expansion in an NGF-dependent manner. Neural tracing identified CGRP+ peptidergic neurons as the primary gastric sensory neurons. Three-dimensional co-culture models showed that sensory neurons directly connect with gastric cancer spheroids. Chemogenetic activation of sensory neurons induced the release of calcium into the cytoplasm of cancer cells, promoting tumour growth and metastasis. Pharmacological ablation of sensory neurons or treatment with CGRP inhibitors suppressed tumour growth and extended survival. Depolarization of gastric tumour membranes through in vivo optogenetic activation led to enhanced calcium flux in jugular nucleus complex and CGRP release, defining a cancer cell–peptidergic neuronal circuit. Together, these findings establish the functional connectivity between cancer and sensory neurons, identifying this pathway as a potential therapeutic target.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
199,00 € per year
only 3,90 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The sequencing data reported in this paper are available at the GEO under accession number GSE243578. The single-cell publicly available data used in this study are available in the GEO (GSE157694 and GSE116514) and OMIX (OMIX001073) databases. Data from the TCGA study are publicly available online (https://portal.gdc.cancer.gov). Source data are provided with this paper.
Code availability
No newly generated codes were used in this paper.
References
Zeng, Q. et al. Synaptic proximity enables NMDAR signalling to promote brain metastasis. Nature 573, 526–531 (2019).
Venkatesh, H. S. et al. Electrical and synaptic integration of glioma into neural circuits. Nature 573, 539–545 (2019).
Venkataramani, V. et al. Glutamatergic synaptic input to glioma cells drives brain tumour progression. Nature 573, 532–538 (2019).
Hanahan, D. & Monje, M. Cancer hallmarks intersect with neuroscience in the tumor microenvironment. Cancer Cell 41, 573–580 (2023).
Venkataramani, V. et al. Glioblastoma hijacks neuronal mechanisms for brain invasion. Cell 185, 2899–2917 (2022).
Krishna, S. et al. Glioblastoma remodelling of human neural circuits decreases survival. Nature 617, 599–607 (2023).
Hyman, S. E. Neurotransmitters. Curr. Biol. 15, R154–R158 (2005).
Balood, M. et al. Nociceptor neurons affect cancer immunosurveillance. Nature 611, 405–412 (2022).
Zhang, Y. et al. Cancer cells co-opt nociceptive nerves to thrive in nutrient-poor environments and upon nutrient-starvation therapies. Cell Metab. 34, 1999–2017.e10 (2022).
Reavis, H. D., Chen, H. I. & Drapkin, R. Tumor innervation: cancer has some nerve. Trends Cancer 6, 1059–1067 (2020).
Zhao, C. M. et al. Denervation suppresses gastric tumorigenesis. Sci. Transl. Med. 6, 250ra115 (2014).
Berthoud, H. R. & Neuhuber, W. L. Functional and chemical anatomy of the afferent vagal system. Auton. Neurosci. 85, 1–17 (2000).
Hayakawa, Y. et al. Nerve growth factor promotes gastric tumorigenesis through aberrant cholinergic signaling. Cancer Cell 31, 21–34 (2017).
Chang, W. et al. Hormonal suppression of stem cells inhibits symmetric cell division and gastric tumorigenesis. Cell Stem Cell 26, 739–754 (2020).
Till, J. E. et al. Oncogenic KRAS and p53 loss drive gastric tumorigenesis in mice that can be attenuated by E-cadherin expression. Cancer Res. 77, 5349–5359 (2017).
Ma, L., Lei, L., Eng, S. R., Turner, E. & Parada, L. F. Brn3a regulation of TrkA/NGF receptor expression in developing sensory neurons. Development 130, 3525–3534 (2003).
Lee, S. et al. NGF-TrkA signaling dictates neural ingrowth and aberrant osteochondral differentiation after soft tissue trauma. Nat. Commun. 12, 4939 (2021).
Ji, R. R., Samad, T. A., Jin, S. X., Schmoll, R. & Woolf, C. J. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron 36, 57–68 (2002).
Hayakawa, Y. et al. Mist1 expressing gastric stem cells maintain the normal and neoplastic gastric epithelium and are supported by a perivascular stem cell niche. Cancer Cell 28, 800–814 (2015).
Ahluwalia, A. et al. Reduced NGF in gastric endothelial cells is one of the main causes of impaired angiogenesis in aging gastric mucosa. Cell. Mol. Gastroenterol. Hepatol. 6, 199–213 (2018).
Callaway, E. M. & Luo, L. Monosynaptic circuit tracing with glycoprotein-deleted rabies viruses. J. Neurosci. 35, 8979–8985 (2015).
Russell, F. A., King, R., Smillie, S. J., Kodji, X. & Brain, S. D. Calcitonin gene-related peptide: physiology and pathophysiology. Physiol. Rev. 94, 1099–1142 (2014).
Kim, Y. J. & Granstein, R. D. Roles of calcitonin gene-related peptide in the skin, and other physiological and pathophysiological functions. Brain Behav. Immun. Health 18, 100361 (2021).
Yang, D. et al. Nociceptor neurons direct goblet cells via a CGRP-RAMP1 axis to drive mucus production and gut barrier protection. Cell 185, 4190–4205 (2022).
Horie, S., Michael, G. J. & Priestley, J. V. Co-localization of TRPV1-expressing nerve fibers with calcitonin-gene-related peptide and substance P in fundus of rat stomach. Inflammopharmacology 13, 127–137 (2005).
Okabe, S. & Amagase, K. An overview of acetic acid ulcer models-the history and state of the art of peptic ulcer research. Biol. Pharm. Bull. 28, 1321–1341 (2005).
Lai, N. Y. et al. Gut-innervating nociceptor neurons regulate peyer’s patch microfold cells and SFB levels to mediate Salmonella host defense. Cell 180, 33–49 (2020).
Le, T. T. et al. Sensory nerves enhance triple-negative breast cancer invasion and metastasis via the axon guidance molecule PlexinB3. npj Breast Cancer 8, 116 (2022).
Filliol, A. et al. Opposing roles of hepatic stellate cell subpopulations in hepatocarcinogenesis. Nature 610, 356–365 (2022).
Carr, R. & Frings, S. Neuropeptides in sensory signal processing. Cell Tissue Res. 375, 217–225 (2019).
Bocchi, R., Masserdotti, G. & Gotz, M. Direct neuronal reprogramming: fast forward from new concepts toward therapeutic approaches. Neuron 110, 366–393 (2022).
Vermeiren, S., Bellefroid, E. J. & Desiderio, S. Vertebrate sensory ganglia: common and divergent features of the transcriptional programs generating their functional specialization. Front. Cell Dev. Biol. 8, 587699 (2020).
Tomlinson, R. E. et al. NGF-TrkA signaling in sensory nerves is required for skeletal adaptation to mechanical loads in mice. Proc. Natl Acad. Sci. USA 114, E3632–E3641 (2017).
Umoh, N. A. et al. Calcitonin gene-related peptide regulates cardiomyocyte survival through regulation of oxidative stress by PI3K/Akt and MAPK signaling pathways. Ann. Clin. Exp. Hypertens. 2, 1007 (2014).
Venkatesh, H. S. et al. Neuronal activity promotes glioma growth through neuroligin-3 secretion. Cell 161, 803–816 (2015).
Peterson, S. C. et al. Basal cell carcinoma preferentially arises from stem cells within hair follicle and mechanosensory niches. Cell Stem Cell 16, 400–412 (2015).
Saloman, J. L. et al. Ablation of sensory neurons in a genetic model of pancreatic ductal adenocarcinoma slows initiation and progression of cancer. Proc. Natl Acad. Sci. USA 113, 3078–3083 (2016).
Restaino, A. C. et al. Functional neuronal circuits promote disease progression in cancer. Sci. Adv. 9, eade4443 (2023).
Zhong, F., Christianson, J. A., Davis, B. M. & Bielefeldt, K. Dichotomizing axons in spinal and vagal afferents of the mouse stomach. Dig. Dis. Sci. 53, 194–203 (2008).
Lobikin, M., Chernet, B., Lobo, D. & Levin, M. Resting potential, oncogene-induced tumorigenesis, and metastasis: the bioelectric basis of cancer in vivo. Phys. Biol. 9, 065002 (2012).
Yang, M. & Brackenbury, W. J. Membrane potential and cancer progression. Front. Physiol. 4, 185 (2013).
Lukyanetz, E. A. Different secretory vesicles can be involved in depolarization-evoked exocytosis. Biochem. Biophys. Res. Commun. 288, 844–848 (2001).
Bohorquez, D. V. et al. Neuroepithelial circuit formed by innervation of sensory enteroendocrine cells. J. Clin. Invest. 125, 782–786 (2015).
Zhang, W. et al. Gut-innervating nociceptors regulate the intestinal microbiota to promote tissue protection. Cell 185, 4170–4189 (2022).
Gao, X. et al. Nociceptive nerves regulate haematopoietic stem cell mobilization. Nature 589, 591–596 (2021).
McIlvried, L. A., Atherton, M. A., Horan, N. L., Goch, T. N. & Scheff, N. N. Sensory neurotransmitter calcitonin gene-related peptide modulates tumor growth and lymphocyte infiltration in oral squamous cell carcinoma. Adv. Biol. 6, e2200019 (2022).
Edvinsson, L., Haanes, K. A., Warfvinge, K. & Krause, D. N. CGRP as the target of new migraine therapies—successful translation from bench to clinic. Nat. Rev. Neurol. 14, 338–350 (2018).
Elser, H. et al. Cancer risk in patients with migraine: a population-based cohort study in Denmark. Headache 62, 57–64 (2022).
Hayakawa, Y. et al. BHLHA15-positive secretory precursor cells can give rise to tumors in intestine and colon in mice. Gastroenterology 156, 1066–1081 (2019).
Zhan, J., Komal, R., Keenan, W. T., Hattar, S. & Fernandez, D. C. Non-invasive strategies for chronic manipulation of DREADD-controlled neuronal activity. J. Vis. Exp. https://doi.org/10.3791/59439 (2019).
de Sousae Melo, F. et al. A distinct role for Lgr5+ stem cells in primary and metastatic colon cancer. Nature 543, 676–680 (2017).
Ayer, A. et al. Techniques of sleeve gastrectomy and modified Roux-en-Y gastric bypass in mice. J. Vis. Exp. https://doi.org/10.3791/54905 (2017).
Kaelberer, M. M. et al. A gut-brain neural circuit for nutrient sensory transduction. Science 361, eaat5236 (2018).
Wolf, F. A. et al. SCANPY: large-scale single-cell gene expression data analysis. Genome Biol. 19, 15 (2018).
Acknowledgements
This work was supported by grants UO1DK103155, R01DK128195, R01CA272901, R01CA224428 and W81XWH-21-10901 as well as an NCI Outstanding Investigator Award (R35CA210088) to T.C.W. S.W.R. is supported by grants R01CA272891 and P50CA127003. This research was supported in part through the NIH/NCI Cancer Center Support Grant P30CA013696 and used the resources of the Herbert Irving Comprehensive Cancer Center Flow Cytometry Shared Resources, Molecular Pathology (MPSR), Genomics and High Throughput Screening, the Oncology Precision Therapeutics and Imaging Core (OPTIC) and the Genetically Modified Mouse Model Shared Resource (GMMMSR). This research was also supported by the Columbia University Digestive and Liver Disease Research Center (CU-DLDRC) P30DK132710 grant and its Bio-Imaging, Organoid and Bioinformatics/Single-Cell Analysis Cores. We thank the core directors, managers and staff for their expert assistance with our studies; the staff at Tonix Pharmaceuticals and the Torrey Coast Foundation for support; the members of the Core facilities, especially the Animal Facility at the Institute of Comparative Medicine at Columbia University for support; and the members of the Wang and Ryeom laboratories for input throughout the course of this study.
Author information
Authors and Affiliations
Contributions
X.Z. and T.C.W. conceived, designed the study and wrote the manuscript. X.Z., F.W., J.Q., Y.O., G.L., B.Z., R.T., Y.Z., H.K. and Q.S. performed the in vivo experiments. E.M. performed the computational analyses of single-cell RNA-seq data. R.W. and Y.P. performed electrophysiology experiments. Z.X. performed western blotting experiments. S.W.R. generated and characterized Tcon mice and ACKP cells. E.M., Y.P., D.C. and S.W.R. participated in the discussion. T.C.W. generated the concepts, analysed and interpretated the data, developed the idea and hypothesis, and secured the funding.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Brian Schmidt, Sébastien Talbot and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Neural expansion in GCs.
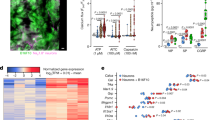
Representative images of (a) sensory (CGRP), (b) sympathetic (TH) and parasympathetic (VAChT) nerves in mouse gastric cancers (n = 5 mice/group). Scale bar, 100 μm. (c) Representative images and quantification of CGRP+, NF200+, Piezo2+, TH+, and VAChT+ nerves in mouse orthotopic and subcutaneous tumours (n = 6 mice/group). Scale bar, 100 μm. (d) Distribution of CGRP+ nerves in GCs (n = 5 mice/group). Data represent mean ± SEM, and P values were calculated by ANOVA in c. The statistical tests were two-sided.
Extended Data Fig. 2 Gastric epithelial injure upregulated Ngf expression.
(a) Representative images of HE staining in mouse stomach treated with vehicle or DMP-777, and Ngf expression in epithelial cells (n = 5 mice/group). Scale bar, 100 μm. (b) Representative images of HE staining in mouse stomach treated with vehicle or high dose of MNU, and Ngf expression in epithelial cells (n = 5 mice/group). Scale bar, 100 μm. (c) Relative expression of Ngf in non-mutant (GFP−) and Kras-mutant (GFP+) gastric epithelial cells (n = 3 mice/group). Mist1+ Kras-mutant gastric epithelial cells were isolated from Mist1-CreERT; KrasG12D; tGFP mice after tamoxifen induction 1 month. (d) Relative expression of Ngf in total epithelial cells, YFP- epithelial cells and YFP+ malignant cells from Atp4b-Cre; Cdh1fl/fl; KrasG12D; Trp53 fl/fl; YFP mice (n = 5 mice/group). (e) Representative images of Ngf in situ hybridization in mouse GCs (n = 5 mice/group). Scale bar, 100 μm. (f) Representative images and (g) quantification of sensory nerves and micro vessels in NGF-overexpression mice and control mice (n = 5 mice/group). Scale bar, 100 μm. (h) Representative images of sensory nerves in mouse gastric cancers treated by Entrectinib or Vehicle (n = 5 mice/group). Scale bar, 100 μm. Data represent mean ± SEM, and P values were calculated by ANOVA in a, b and d, by t test in c and g. The statistical tests were two-sided.
Extended Data Fig. 3 Nociceptive neurons in ganglia.
(a) Size distribution of tracer-positive neurons. (b) Representative images and quantification of CGRP+ neurons and IB4+ neurons in ganglia (n = 3 mice). Scale bar, 100 μm. (c) Representative images and quantification of CGRP+, SP+, SST+ neurons in ganglia (n = 3 mice). Scale bar, 100 μm. SP, Substance P. SST, Somatostatin. (d) Representative images and (e) quantification of neural tracing in NGF-overexpression mouse (n = 4 mice/group). Scale bar, 100 μm. (f) Quantification of molecular subtypes of the stomach-innervating sensory neurons in NGF-overexpression mouse (n = 4 mice/group). (g) Representative images of neural tracing in RTX-treated mice (n = 3 mice). Scale bar, 100 μm. (h) Relative expression of TrkA in sympathetic, parasympathetic and GC-traced sensory neurons (n = 5 mice). Data represent mean ± SEM, and P values were calculated by t test in e, by ANOVA in h. The statistical tests were two-sided.
Extended Data Fig. 4 CGRP/Ramp1 expression in GCs.
(a) Expression levels of Calca in the stomach-innervating sensory neurons from the NGF-overexpression mice and the control mice (n = 10 mice/group). (b) Concentrations of CGRP peptide in the stomach from the NGF-overexpression mice and the control mice (n = 10 mice/group). (c) Dot plot of single-cell transcriptome data of Substance P receptors and Somatostatin receptors from mouse stomach (GSE157694 and GSE116514) and human stomach (OMIX001073). (d) UMAP of single-cell transcriptome data from mouse stomach (GSE157694). (e) Representative image of in situ hybridization of Ramp1 RNA in mouse gastric antrum (n = 5 mice/group). Scale bar, 100 μm. (f) Representative images and (g) quantification of in situ hybridization (RNAscope) of Calcrl RNA in mouse stomach and gastric cancers (n = 5 mice/group). Scale bar, 100 μm. (h) RNA levels of Ramp1 and Calcrl in YFP+ cell from Atp4b-Cre; Cdh1fl/fl; KrasG12D; Trp53fl/fl; YFP mice (n = 3 mice/group). (i) protein levels of Ramp1 and Calcrl in mouse GC cell (ACKP) and human GC cells (KATO III and AGC). (j-k) Kaplan-Meier curves of TCGA survival data. Data represent mean ± SEM, and P values were calculated by t test in a, b and g, by Logrank in j and k. The statistical tests were two-sided.
Extended Data Fig. 5 Nociceptive neurons enhanced gastric ulcer regeneration and gastric cancer growth.
(a) Representative images of gastric ulcer samples and Ki-67 staining (n = 5 mice/group). Scale bar, 100 μm. Quantification of (b) ulcer area and (c) Ki-67 staining. (d) Representative MRI images from orthotopic GC model. (e) Representative images and (f) quantification of DT-induced nociceptive neuron ablation (n = 5 mice/group). Scale bar, 100 μm. (g) Representative images and (h) quantification of CGRP+ nerves in GCs from DT-treated mice (n = 10 mice/group). Scale bar, 100 μm. (i) Concentrations of CGRP peptide in GCs from DT-treated mice (n = 10 mice/group). (j) Representative images and (k) quantification of MNU-induced GCs from nociceptive neuron-ablated mice or control mice (n = 10 mice/group). (l) Representative images and (m) quantification of syngeneic orthotopic tumours from nociceptive neuron-ablated mice or control mice (n = 10 mice/group). (n) Representative images and (o) quantification of syngeneic orthotopic tumours from nociceptive neuron-ablated mice treated with vehicle or Rimegepant (n = 10 mice/group). (p) Representative images and (q) quantification of co-staining of mCherry and CGRP (n = 10 mice/group). Scale bar, 100 μm. (r) Representative images and (s) quantification of syngeneic orthotopic tumours from Calca-Cre mice with injection of AAV-hSyn-DIO-hM3Dq-mCherry (n = 10 mice/group). (t) Representative images and (u) quantification of syngeneic orthotopic tumours from C21-activated mice (n = 10 mice/group). Data represent mean ± SEM, and P values were calculated by t test in b, c, f, h, i, k, m, o, q, s and u. The statistical tests were two-sided.
Extended Data Fig. 6 CGRP promoted the proliferation of gastric cancer spheroids.
(a) Representative images and (b) quantification of gastric cancer spheroids treated by CGRP or Rimegepant (n = 60 spheroids/group). Scale bar, 100 μm. (c) Representative images and (d) quantification of Edu staining in (a) (n = 20 spheroids/group). Scale bar, 100 μm. (e) Proliferation curve of ACKP cells (n = 3 experiments/group). Data represent mean ± SEM, and P values were calculated by ANOVA in b and d. The statistical tests were two-sided.
Extended Data Fig. 7 Nociceptive neurons regulated the number and function of gastric CAFs.
(a) Representative images and (b) quantification of Pdgfra+ CAFs in orthotopic tumours (n = 5 mice/group). Scale bar, 100 μm. (c) Flow chart of gastric CAFs isolation. (d) Proliferation curve of gastric CAFs (n = 3 mice/group). (e) Expression levels of CAF-associated cytokines in gastric CAFs treated with vehicle of CGRP (n = 3 mice/group). Data represent mean ± SEM, and P values were calculated by t test in b and e. The statistical tests were two-sided.
Extended Data Fig. 8 Nociceptive neurons promote GC metastasis in a spontaneous metastasis model.
(a) The spontaneous metastatic model was done by resecting the primary tumour followed by an esophagojejunostomy with Roux-en-Y anastomosis. (b) Mice were monitored weekly with bioluminescence (IVIS) following resection of the primary tumour. Liver metastases were detectable from the third week. (c) Representative images and (d) quantification of Ki-67 staining in liver metastasis (n = 6 lesions/group). Scale bar, 100 μm. (e) Representative images and (f) quantification of sensory nerves (red) in liver metastatic area (yellow) and adjacent area (n = 5 lesions/group). Scale bar, 100 μm. (g) Representative images of sensory nerves in normal and metastatic lymph nodes. Scale bar, 100 μm. (h) Representative images and (i) quantification of α-SMA staining in liver metastasis (n = 5 lesions/group). Scale bar, 100 μm. (j) UMAP of single-cell transcriptome data from mouse liver (GSE174748). Data represent mean ± SEM, and P values were calculated by t test in d, f and i. The statistical tests were two-sided.
Extended Data Fig. 9 Interaction between nociceptive neurons and GC.
(a) Co-staining of tdTomato and TUBB3 in DRGs from Trpv1-Cre; tdTomato mice. Scale bar, 100 μm. (b) Representative current traces in ChR2-expressing ACKP cell (ChR2+) and non-ChR2 ACKP cell (ChR2−). The waveforms of currents were obtained by depolarizing the membrane with 4 s test pluses from −125 to +100 mV at 25 mV steps (9 sweeps, 5 s interval). The 473 nm laser was delivered to clamped cells for 2 s (blue). (c) Current-voltage relationships (I-V) in ChR2+ (n = 14) and ChR2− (n = 15) cells. The currents (pA) of each cell were normalized by dividing to its cell capacitance (pF). (d) Representative traces of neurons recorded in Fig. 5d responding to GC cells. (e) Representative images and (f) quantification of coculture of DRG (from Trpv1-Cre; tdTomato mice) and GC spheroids (with Ngf-knockout ACKP cells) (n = 5 experiments/group). Scale bar, 100 μm. Representative images of in situ hybridization (RNAscope) of Ngf in Cck2r+ organoids (g) and Ramp1-knockout GC spheroids (h). Scale bar, 100 μm. (i) Coculture of DRG (from Trpv1-Cre; hM3Dq; tdTomato mouse) and Ramp1-knockout GC spheroids (infected with rAAV-CMV-jRGECO1a), nociceptive neurons were treated with CNO and calcium indicator jRGECO1a (red) in GC spheroids was monitored (n = 3 experiments). Scale bar, 100 μm. (j) Coculture of Botox-treated DRG (from Trpv1-Cre; hM3Dq; tdTomato mouse) and GC spheroids (infected with rAAV-CMV-jRGECO1a), nociceptive neurons were treated with CNO and calcium indicator jRGECO1a (red) in GC spheroids was monitored (n = 3 experiments). Scale bar, 100 μm. (k-l) GC spheroids proliferation was detected with CellTiter-Glo 3D cell viability assay (n = 5 experiments/group). Data represent mean ± SEM, and P values were calculated by t test in f, and by ANOVA in k and l. The statistical tests were two-sided.
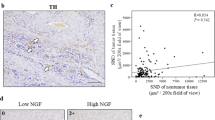
Extended Data Fig. 10 Nociceptive neurons activate Rb/E2F signalling depending on CaMK and PI3K.
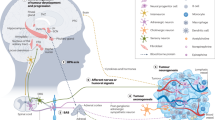
(a) Neuron-connected spheroids and unconnected spheroids were split. Expression levels of chemical synaptic genes, electrical synaptic genes and neurodevelopmental genes were analysed (n = 3 experiments). (b) Concentrations of CGRP peptide in orthotopic ACKP-ChR2 tumours from Trpv1-Cre; GCamp6s; tdTomato mice (n = 3 mice/group). (c) PCA plot of bulk RNA sequencing data from cancer spheroids alone, cancer spheroids cocultured with DRG, and cancer spheroids cocultured with CNO-activated DRG (n = 3 experiments/group). (d) GSEA plot of top 20 enhanced pathways in each comparison. (e) Heat map of proliferation genes, Rb-E2F signal enhancer genes and Rb-E2F signal repressor genes. (f) Relative E2F activity in ACKP cells treated with CGRP or kinase inhibitors. Wortmannin, PI3K inhibitor (n = 3 experiments/group). KN-93, CaMK inhibitor. ASN007, ERK inhibitor. ST034307, PKA inhibitor. (g) Representative images and (h) quantification of p-Rb staining in orthotopic tumours from CNO-treated mice (n = 5 mice/group). Scale bar, 100 μm. (i) Representative images and correlation test of sensory nerve and p-Rb staining in human GC tissue microarray (n = 50 cases). Scale bar, 100 μm. (j) Graphical abstract: Gastric cancer is innervated by nociceptive neurons from ipsilateral jugular nucleus complex and dorsal root ganglia (T7-T13). Gastric cancer cells increase the expression of NGF which attracts the expansion of nociceptive nerves. NGF binds to TrkA receptor causing CGRP synthesis and release from nociceptive nerves. In turn, CGRP activates Calcrl/Ramp1 receptor and promotes E2F activity through PI3K signalling and CaMK signalling in gastric cancer cells. Data represent mean ± SEM, and P values were calculated by ANOVA in f, by t test in b and h, and by spearman’s rank correlation test in i. The statistical tests were two-sided. The diagram in j was created using BioRender.
Supplementary information
Supplementary Figures
Supplementary Figs. 1–4.
Supplementary Video 1
In vivo calcium imaging in the jugular nucleus complex. In vivo calcium imaging was done with Trpv1-cre;GCaMP6s;tdTomato mice. GCaMP6s fluorescence (green) was continuously recorded at 488 nm excitation/510–550 nm emission.
Supplementary Video 2
The connections between nociceptive neurons and GC spheroids. DRGs (from Trpv1-cre;hM3Dq;tdTomato mice) were cocultured with GC spheroids (from Atp4b-cre;Cdh1fl/fl;KrasG12D;Trp53 fl/fl;YFP mice). The connections between nociceptive neurons (red) and GC spheroids (yellow) are shown as a 3D confocal video.
Supplementary Video 3
Coculture DRGs with normal gastric organoid. DRGs (from Trpv1-cre;tdTomato mice) were cocultured with normal gastric organoids (from Cck2r-creERT;ZsGreen mice). Nociceptive neurons (red) and normal gastric organoid (green) are shown as a 3D confocal video.
Source data
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhi, X., Wu, F., Qian, J. et al. Nociceptive neurons promote gastric tumour progression via a CGRP–RAMP1 axis. Nature 640, 802–810 (2025). https://doi.org/10.1038/s41586-025-08591-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-025-08591-1
This article is cited by
-
Cancer neuroscience in head and neck: interactions, modulation, and therapeutic strategies
Molecular Cancer (2025)
-
Cancer-nervous system crosstalk: from biological mechanism to therapeutic opportunities
Molecular Cancer (2025)
-
Neuro-immune cross-talk in cancer
Nature Reviews Cancer (2025)
-
A sensory neuron–gastric cancer circuit
Nature Reviews Gastroenterology & Hepatology (2025)
-
Functional cancer-cell-nociceptive neuronal circuits drive gastric tumor progression
Science China Life Sciences (2025)