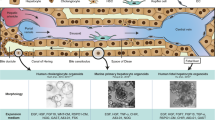
Abstract
Proliferating hepatocytes often undergo ductal metaplasia to balance the energy trade-off between cellular functions and replication, hindering the expansion of human adult hepatocytes with functional competency1. Here we demonstrate that the combined activation of Wnt and STAT3 signalling enables long-term self-renewal of human adult hepatocyte organoids. YAP activation facilitates hepatocyte proliferation but commits it towards the biliary duct lineage. By contrast, STAT3 activation by oncostatin M induces hepatocyte proliferation while counteracting ductal metaplasia and maintaining the hepatic identity. Xenotransplanted hepatocyte organoids repopulate the recipient mouse liver and reconstitute the metabolic zonation structure. Upon niche factor removal and hormone supplementation, hepatocyte organoids form cord-like structures with bile canalicular networks and exhibit major liver metabolic functions comparable to those of in vivo hepatocytes. Hepatocyte organoids are amenable to gene editing, prompting functional modelling of inherent metabolic liver diseases. The new culture system offers a promising avenue for developing therapeutic strategies against human liver diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
199,00 € per year
only 3,90 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Whole-genome sequencing data are deposited to SRA under the BioProject accession number PRJNA948890. RNA-seq and ChIP–seq data are available from GEO with accession numbers GSE228249 and GSE228248, respectively. The human reference genome version GRCh37 (hg19) and GRCh38 (hg38) were obtained from GENCODE. Source data are provided with this paper.
References
Pek, N. M. Q., Liu, K. J., Nichane, M. & Ang, L. T. Controversies surrounding the origin of hepatocytes in adult livers and the in vitro generation or propagation of hepatocytes. Cell. Mol. Gastroenterol. Hepatol. 11, 273–290 (2021).
Martini, T., Naef, F. & Tchorz, J. S. Spatiotemporal metabolic liver zonation and consequences on pathophysiology. Annu. Rev. Pathol.: Mech. Dis. 18, 439–466 (2023).
Rinella, M. E. et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J. Hepatol. 79, 1542–1556 (2023).
Dunn, J. C., Tompkins, R. G. & Yarmush, M. L. Hepatocytes in collagen sandwich: evidence for transcriptional and translational regulation. J. Cell Biol. 116, 1043–1053 (1992).
Xiang, C. et al. Long-term functional maintenance of primary human hepatocytes in vitro. Science 364, 399–402 (2019).
Walldorf, J. et al. Expanding hepatocytes in vitro before cell transplantation: donor age‐dependent proliferative capacity of cultured human hepatocytes. Scand. J. Gastroenterol. 39, 584–593 (2004).
Huch, M. et al. Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell 160, 299–312 (2015).
Cai, J. et al. Directed differentiation of human embryonic stem cells into functional hepatic cells. Hepatology 45, 1229–1239 (2007).
Sekiya, S. & Suzuki, A. Direct conversion of mouse fibroblasts to hepatocyte-like cells by defined factors. Nature 475, 390–393 (2011).
Huang, P. et al. Induction of functional hepatocyte-like cells from mouse fibroblasts by defined factors. Nature 475, 386–389 (2011).
Katsuda, T. et al. Generation of human hepatic progenitor cells with regenerative and metabolic capacities from primary hepatocytes. eLife 8, e47313 (2019).
Brown, J. W., Cho, C. J. & Mills, J. C. Paligenosis: cellular remodeling during tissue repair. Annu. Rev. Physiol. 84, 461–483 (2022).
Peng, W. C. et al. Inflammatory cytokine TNFα promotes the long-term expansion of primary hepatocytes in 3D culture. Cell 175, 1607–1619.e1615 (2018).
Hu, H. et al. Long-term expansion of functional mouse and human hepatocytes as 3D organoids. Cell 175, 1591–1606.e1519 (2018).
Marsee, A. et al. Building consensus on definition and nomenclature of hepatic, pancreatic, and biliary organoids. Cell Stem Cell 28, 816–832 (2021).
Peng, W. C., Kraaier, L. J. & Kluiver, T. A. Hepatocyte organoids and cell transplantation: what the future holds. Exp. Mol. Med. 53, 1512–1528 (2021).
Sato, T. & Clevers, H. Growing self-organizing mini-guts from a single intestinal stem cell: mechanism and applications. Science 340, 1190–1194 (2013).
Fujii, M. et al. A colorectal tumor organoid library demonstrates progressive loss of niche factor requirements during tumorigenesis. Cell Stem Cell 18, 827–838 (2016).
Ally, A. et al. Comprehensive and integrative genomic characterization of hepatocellular carcinoma. Cell 169, 1327–1341.e1323 (2017).
Jung, P. et al. Isolation of human colon stem cells using surface expression of PTK7. Stem Cell Rep. 5, 979–987 (2015).
Cressman, D. E. et al. Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science 274, 1379–1383 (1996).
Kiuchi, N. et al. STAT3 is required for the gp130-mediated full activation of the c-myc gene. J. Exp. Med. 189, 63–73 (1999).
Pepe-Mooney, B. J. et al. Single-cell analysis of the liver epithelium reveals dynamic heterogeneity and an essential role for YAP in homeostasis and regeneration. Cell Stem Cell 25, 23–38.e28 (2019).
Zhang, K. et al. In vitro expansion of primary human hepatocytes with efficient liver repopulation capacity. Cell Stem Cell 23, 806–819.e804 (2018).
Poncy, A. et al. Transcription factors SOX4 and SOX9 cooperatively control development of bile ducts. Dev. Biol. 404, 136–148 (2015).
Okada, H. et al. The transcription factor Klf5 is essential for intrahepatic biliary epithelial tissue remodeling after cholestatic liver injury. J. Biol. Chem. 293, 6214–6229 (2018).
Yimlamai, D. et al. Hippo pathway activity influences liver cell fate. Cell 157, 1324–1338 (2014).
Liu, Y. et al. Yap-Sox9 signaling determines hepatocyte plasticity and lineage-specific hepatocarcinogenesis. J. Hepatol. 76, 652–664 (2022).
Schaub, J. R. et al. De novo formation of the biliary system by TGFβ-mediated hepatocyte transdifferentiation. Nature 557, 247–251 (2018).
Wu, B. et al. A spatiotemporal atlas of cholestatic injury and repair in mice. Nat. Genet. 56, 938–952 (2024).
Bluhme, E. et al. Procurement and evaluation of hepatocytes for transplantation from neonatal donors after circulatory death. Cell Transplant. 31, 09636897211069900 (2022).
Vansaun, M. N., Mendonsa, A. M. & Lee Gorden, D. Hepatocellular proliferation correlates with inflammatory cell and cytokine changes in a murine model of nonalchoholic fatty liver disease. PLoS ONE 8, e73054 (2013).
Kaffe, E. et al. Humanized mouse liver reveals endothelial control of essential hepatic metabolic functions. Cell 186, 3793–3809.e3726 (2023).
Ardisasmita, A. I. et al. A comprehensive transcriptomic comparison of hepatocyte model systems improves selection of models for experimental use. Commun. Biol. 5, 1094 (2022).
Kim, D. S. et al. A liver‐specific gene expression panel predicts the differentiation status of in vitro hepatocyte models. Hepatology 66, 1662–1674 (2017).
García-Cañaveras, J. C., Donato, M. T., Castell, J. V. & Lahoz, A. Targeted profiling of circulating and hepatic bile acids in human, mouse, and rat using a UPLC-MRM-MS-validated method. J. Lipid Res. 53, 2231–2241 (2012).
Tilg, H., Adolph, T. E. & Trauner, M. Gut-liver axis: pathophysiological concepts and clinical implications. Cell Metab. 34, 1700–1718 (2022).
Rader, D. J. & Kastelein, J. J. P. Lomitapide and mipomersen. Circulation 129, 1022–1032 (2014).
Gerbal-Chaloin, S. et al. The WNT/beta-catenin pathway is a transcriptional regulator of CYP2E1, CYP1A2, and aryl hydrocarbon receptor gene expression in primary human hepatocytes. Mol. Pharmacol. 86, 624–634 (2014).
Zhong, Y., Yu, J. S., Wang, X., Binas, B. & Yoo, H. H. Chemical‐based primary human hepatocyte monolayer culture for the study of drug metabolism and hepatotoxicity: comparison with the spheroid model. FASEB J. 35, e21379 (2021).
Horcas-Nieto, J. M. et al. Organoids as a model to study intestinal and liver dysfunction in severe malnutrition. Biochim. Biophys. Acta, Mol. Basis Dis. 1869, 166635 (2023).
Merritt, M. E., Harrison, C., Sherry, A. D., Malloy, C. R. & Burgess, S. C. Flux through hepatic pyruvate carboxylase and phosphoenolpyruvate carboxykinase detected by hyperpolarized 13C magnetic resonance. Proc. Natl Acad. Sci. USA 108, 19084–19089 (2011).
Hendriks, D. et al. Mapping of mitogen and metabolic sensitivity in organoids defines requirements for human hepatocyte growth. Nat. Commun. 15, 4034 (2024).
Baudy, A. R. et al. Liver microphysiological systems development guidelines for safety risk assessment in the pharmaceutical industry. Lab Chip 20, 215–225 (2020).
Morgan, K. et al. Oncostatin M induced α1-antitrypsin (AAT) gene expression in Hep G2 cells is mediated by a 3′ enhancer. Biochem. J. 365, 555–560 (2002).
Baumann, H., Onorato, V., Gauldie, J. & Jahreis, G. P. Distinct sets of acute phase plasma proteins are stimulated by separate human hepatocyte-stimulating factors and monokines in rat hepatoma cells. J. Biol. Chem. 262, 9756–9768 (1987).
Maione, D. et al. Coexpression of IL-6 and soluble IL-6R causes nodular regenerative hyperplasia and adenomas of the liver. EMBO J. 17, 5588–5597 (1998).
Schirmacher, P. et al. Hepatocellular hyperplasia, plasmacytoma formation, and extramedullary hematopoiesis in interleukin (IL)-6/soluble IL-6 receptor double-transgenic mice. Am. J. Pathol. 153, 639–648 (1998).
Nakamura, K., Nonaka, H., Saito, H., Tanaka, M. & Miyajima, A. Hepatocyte proliferation and tissue remodeling is impaired after liver injury in oncostatin M receptor knockout mice. Hepatology 39, 635–644 (2004).
Kamiya, A. Fetal liver development requires a paracrine action of oncostatin M through the gp130 signal transducer. EMBO J. 18, 2127–2136 (1999).
Gramignoli, R. et al. Development and application of purified tissue dissociation enzyme mixtures for human hepatocyte isolation. Cell Transplant. 21, 1245–1260 (2012).
Kozakai, K. et al. Reliable high-throughput method for inhibition assay of 8 cytochrome P450 isoforms using cocktail of probe substrates and stable isotope-labeled internal standards. Drug Metab. Pharmacokinet. 27, 520–529 (2012).
Ni, X. et al. Functional human induced hepatocytes (hiHeps) with bile acid synthesis and transport capacities: a novel in vitro cholestatic model. Sci. Rep. 6, 38694 (2016).
Yamamoto, T. et al. PRMT1 sustains de novo fatty acid synthesis by methylating PHGDH to drive chemoresistance in triple-negative breast cancer. Cancer Res. 84, 1065–1083 (2024).
Tanosaki, S. et al. Fatty acid synthesis is indispensable for survival of human pluripotent stem cells. iScience 23, 101535 (2020).
Lengler, J. et al. Development of an in vitro biopotency assay for an AAV8 hemophilia B gene therapy vector suitable for clinical product release. Mol. Ther. Methods Clin. Dev. 17, 581–588 (2020).
De Castilho Fernandes, A. et al. Stable and high-level production of recombinant factor IX in human hepatic cell line. Biotechnol. Appl. Biochem. 58, 243–249 (2011).
Biron-Andréani, C., Raulet, E., Pichard-Garcia, L. & Maurel, P. in Hepatocytes. Methods in Molecular Biology, vol 640 (ed. Maurel, P.) 431–445 (Humana, 2010).
Fujii, M., Matano, M., Nanki, K. & Sato, T. Efficient genetic engineering of human intestinal organoids using electroporation. Nat. Protoc. 10, 1474–1485 (2015).
Nanki, K. et al. Somatic inflammatory gene mutations in human ulcerative colitis epithelium. Nature 577, 254–259 (2020).
Hasegawa, M. et al. The reconstituted ‘humanized liver’ in TK-NOG mice is mature and functional. Biochem. Biophys. Res. Commun. 405, 405–410 (2011).
Seino, T. et al. Human pancreatic tumor organoids reveal loss of stem cell niche factor dependence during disease progression. Cell Stem Cell 22, 454–467.e456 (2018).
Saitou, M. et al. Occludin-deficient embryonic stem cells can differentiate into polarized epithelial cells bearing tight junctions. J. Cell Biol. 141, 397–408 (1998).
Schmidl, C., Rendeiro, A. F., Sheffield, N. C. & Bock, C. ChIPmentation: fast, robust, low-input ChIP-seq for histones and transcription factors. Nat. Methods 12, 963–965 (2015).
Schmidt, U., Weigert, M., Broaddus, C. & Myers, G. in Medical Image Computing and Computer Assisted Intervention – MICCAI 2018. MICCAI 2018. Lecture Notes in Computer Science, vol 11071 (eds Frangi, A. et al.) 265–273 (Springer International, 2018).
Ollion, J., Cochennec, J., Loll, F., Escudé, C. & Boudier, T. TANGO: a generic tool for high-throughput 3D image analysis for studying nuclear organization. Bioinformatics 29, 1840–1841 (2013).
Poell, J. B. et al. ACE: absolute copy number estimation from low-coverage whole-genome sequencing data. Bioinformatics 35, 2847–2849 (2019).
Scheinin, I. et al. DNA copy number analysis of fresh and formalin-fixed specimens by shallow whole-genome sequencing with identification and exclusion of problematic regions in the genome assembly. Genome Res. 24, 2022–2032 (2014).
Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet.journal 17, 10 (2011).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Li, B. & Dewey, C. N. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinf. 12, 323 (2011).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012).
Zhang, Y. et al. Model-based analysis of ChIP-Seq (MACS). Genome Biol. 9, R137 (2008).
Zhu, L. J. et al. ChIPpeakAnno: a Bioconductor package to annotate ChIP-seq and ChIP-chip data. BMC Bioinf. 11, 237 (2010).
Ramírez, F. et al. deepTools2: a next generation web server for deep-sequencing data analysis. Nucleic Acids Res. 44, W160–W165 (2016).
Robinson, J. T. et al. Integrative genomics viewer. Nat. Biotechnol. 29, 24–26 (2011).
Mayakonda, A., Lin, D.-C., Assenov, Y., Plass, C. & Koeffler, H. P. Maftools: efficient and comprehensive analysis of somatic variants in cancer. Genome Res. 28, 1747–1756 (2018).
Acknowledgements
This study was supported by JSPS KAKENHI (grant nos. 22H04995, 20K17030 and 23K05742), AMED-CREST (grant no. JP20gm1210001), JST ERATO (grant no. JPMJER2303) and JSR Corporation as a JKiC Strategic Project. We thank the Collaborative Research Resources, School of Medicine and Keio University for their technical assistance. We also thank N. Hayakawa and T. Matsuura for their assistance with mass spectrometry analysis. The super-computing resource was provided by the Human Genome Center, Institute of Medical Science, University of Tokyo (http://sc.hgc.jp/shirokane.html).
Author information
Authors and Affiliations
Contributions
R.I., N.M., M.I. and T.S. conceptualized the study. R.I., M.O., R.O., T.Y., N.M. and T.S. developed the study methodology. R.I., R.O., M.M. and N.M. conducted in vitro experiments. R.I. and S.S. performed animal experiments. R.I., T.Y. and S.T. performed immunostaining. S.P., R.I. and Y.O. performed imaging analysis. R.I., R.O., S.S., T.H., M.S. and M.I. performed metabolite analysis. M.O. and K.T. conducted bioinformatics analysis. R.I., M.O., M.F. and T.S. managed data curation. R.I., M.O. and T.S. wrote the original draft. R.I., M.O., M.F. and T.S. reviewed and edited the paper. T.S. was responsible for acquiring funding and resources. T.S. supervised the study.
Corresponding author
Ethics declarations
Competing interests
R.I., M.O., R.O., N.M. and T.S. are inventors on filed patents related to hepatocyte organoids (PCT/JP2020/046781, PCT/JP2024/016542 and US63/745,798). R.O. and M.I. are employees of JSR Corporation. N.M. was an employee of JSR Corporation while conducting experiments in this study and is now employed by MBL. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature thanks Jan Tchorz and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Optimization of human hepatocyte organoid culture conditions.
a. The landscape of genetic alterations related to the WNT, RAS, TGF-β, IL-6, and PKA pathways in the TCGA liver hepatocellular carcinoma dataset. Long bars show genetic mutations, and short bars indicate copy number alterations and gene fusions. The genes in each pathway were manually curated. b. Expression of receptor genes related to Ras signaling in PHHs and organoids in RNA-seq dataset. c. Effect of growth factor combinations on cell growth. Growth factor component (EGF, FGF10, HGF) in EM were omitted as indicated in the graph. The Y-axis indicated the fold change of the estimated cell amount compared to the non-growth factor control. Data from three HHO lines derived from different donors biological replicates (donors), each dot shows one well and is colored according to the PHH donor. Error bars show mean ± SD from four technical replicates per donor. HHO growth in each condition was compared versus the EGF+FGF10+ HGF+ control (◊) using one-way ANOVA followed by Dunnett’s two-sided multiple comparison test. From the left, p = 0.63290, 0.00116, 0.99775, 0.87587, 0.99386, 0.99997, and 0.00092. *p < 0.05; **p < 0.01; ***p < 0.001. d. A representative image of primary organoids expanded from PHHs with EM(+IL6) condition. Black arrowheads indicate HHO with small organoids with a thick lining, and a green one indicates ICO with a sizeable hollow wall. Arrows indicate the individual organoids in magnified images. Scale bar: 50 μm. Similar results were obtained from 18 donors. e. Expression of hepatocyte (HNF-4α) and ductal (EpCAM) markers in an HHO and ICO cultured in the OSM-containing medium. Nuclear counterstaining, Hoechst 33342. Scale bar: 50 μm. Similar results were obtained from observation of at least 20 organoids from two donors. f. Proportion of EpCAM+ cells in viable cells in PHHs from different donors. g. Organoid thickness-to-diameter ratio in HHOs and ICOs derived from the PY9 donor. Data from n = 20 (HHO) and 11 (ICO) organoids. Each dot shows one organoid. Statistics, Welch’s two-sided t-test. h. The impact of growth factor withdrawal on organoid growth from PHHs. PHHs were cultured with EM including IL-6 (EM (+IL-6)), or with several conditions that lack the indicated growth factors. The organoids were cultured for 14 days, and their viability was measured using ATP luminescence assay. The organoid viability is shown as ATP luminescence relative to the value of EM (+IL-6)-cultured organoids. Data from three PHH donors. Error bars show mean ± SD of four technical replicates per donor, and each dot indicates one technical replicate. The dots are colored according to the PHH donors. Each condition was compared against the EM(+IL6) control (◊) condition using one-way ANOVA followed by Dunnett’s two-sided multiple comparison test. From the left, p = 7.7 × 10−5, 6.9 × 10−5, 0.06382, 1.00000, 0.00029, 0.59587, and 0.34387. *p < 0.05; **p < 0.01; ***p < 0.001. i. The impact of growth factor withdrawal on PHH-derived organoid passageability. PHHs were cultured with the indicated conditions. The circle indicates successful passage with subsequent HHO growth, and the cross (x) indicates passage failure.
Extended Data Fig. 2 OSM-STAT3 promotes stable expansion of HHOs.
a. The effect of STAT3-activating cytokines on HHO expansion. 5 × 103 HHO cells were seeded per well and cultured with the indicated cytokines. Concentration (ng/ml) are shown for each cytokine. Cell viability was measured on day 11 using ATP luminescence assay and used for cell count estimation. Each dot shows one well. b. For EdU assay for organoid proliferation, we cultured 7days with or without OSM. EdU+ cell number was measured 3 h after EdU administration. (left) Representative image of EdU-stained organoids. Nuclear counterstaining, Hoechst 33342. Scale bar: 50 μm. (right) The percentage of EdU+ cells. From the left, n = 207, 277, 251 and 781 cells were analyzed. c. Representative image of STAT3 staining in the untreated control (top left) and OSM-treated (bottom left) HHOs. The organoids were treated with 20 ng/ml OSM for 0.5 h. Nuclear counterstaining, Hoechst 33342. Scale bar: 20 μm. Measurement of nuclear STAT3 intensity (right). n = 5048 (OSM−) and 5713 (OSM+) cells were analyzed. Data are shown by boxplot with upper whisker, box top, center, box bottom, and lower whisker as –(1.5-fold inter-quartile range(IQR)), 25, 50, 75 percentiles, and 1.5-fold IQR of cells, respectively. p < 2.2 × 10−16, Welch’s two-sided t-test. d. The effect of OSM and a STAT3 inhibitor (C188-9, 10 μM) on organoid formation efficiency. 3 × 103 HHO cells were plated and cultured with the indicated conditions. Each dot shows one well. Statistics, Welch’s two-sided t-test. e. Sorting strategy for EpGAM−/ASGR1+ cells. PHHs were staind by human α-EpCAM-APC/α-ASGR-PE/7-AAD. The acquisition gate for sorting was set to include no more than 1% of events from the non-staining negative control. f. Organoid formation from flow-sorted single ASGR+/EpCAM− cells. Organoid formation efficiency from different donors is shown. Data from PHHs derived from four different donors. Each dot shows one well. Data are shown as mean ± SD. g. Sorting strategy for selection by BSC (cell complexity) levels. DAPI-viable cells were divided into three BSC levels. All gating strategy is shown in Supplementary Fig. 2. h. Differential interference contrast (DIC) images without or with nuclear staining overlay of PHHs with different BSC levels. Nuclei were stained with Hoechst33342. Scale bar: 10 μm. Representative data from three donors with similar results. i. Efficiency of organoid formation from PHHs with different BSC levels. Each dot shows one technical replicates. Equal number of sorted PHHs were plated per well, and the number of formed organoids was analyzed on day 14. Data from three donors are shown. Error bars show mean ± SD of three technical replicates. Statistics, two-way ANOVA followed by Tukey’s two-sided post-hoc test. j. BSC shift of PHHs after incubation at room temperature or 4 °C. PHHs (PY53) were resuspended in PBS and incubated at different temperatures. All gating strategy is shown in Supplementary Fig. 2. k. Copy number analysis of HHOs cultured over three months and their parental PHHs. No copy number alteration was observed.
Extended Data Fig. 3 Transcriptome and epigenetic analyses of OSM-treated HHOs.
a. Quantification of surface integrin-β4 expression intensity (related to Fig. 1d). Relative intensity (edge/organoid) were analyzed using ImageJ. For −OSM and +OSM conditions, 29 and 55 (PY30), 45 and 53 (PY31), 57 and 54 (PY39a), 74 and 121 (PY53) organoids were analyzed, respectively. Data are shown by boxplot with upper whisker, box top, center, box bottom, and lower whisker as –(1.5-fold IQR), 25, 50, 75 percentiles, and 1.5-fold IQR of organoids, respectively. Each dot shows one organoid. Statistics, Welch’s two-sided t-test. Four biologically independent measurements (donors) were shown. b. Comparative transcriptome analysis of eHHOs cultured in the EM condition with OSM (+OSM) or without OSM (−OSM). Statistical analyses were performed using DESeq2 with the Wald test (nbinomWaldTest). The test was two-sided, evaluating both up- and down-regulation of gene expression. Adjustments for multiple comparisons were performed using the Benjamin-Hochberg method to control the false discovery rate (FDR). Mean normalized counts and Wald statistics from the DESeq2 output are shown. Significantly upregulated genes (log2FoldChange > 1 and padj <0.05) are shown in red dots, and significantly downregulated genes (log2FoldChange <−1 and padj <0.05) are shown in light blue dots (Supplementary Table 3). Red diamonds indicate liver-specific gene expression panel (LiGEP) genes. c. Gene ontology analysis using genes differentially expressed between eHHOs with EM (+OSM) and EM (−OSM). The top 7 terms in GO_BP_DIRECT are shown. A modified Fisher’s two-sided exact test (EASE score) was used with the Benjamini-Hochberg method for FDR adjustment. d. STAT3 ChIP-seq peaks in eHHOs collected immediately (−OSM) or 0.5 hr (+OSM) after OSM treatment. Normalized read counts in common peaks and peaks gained in the +OSM group are shown. e. Motif enrichment in the 5813 peaks gained in eHHOs cultured with EM (+OSM). P-values were calculated based on a Poisson distribution. The test is one-sided. No adjustments for multiple comparisons. f. STAT3 ChIP-seq reads at MYC, FOSL2, JUNB, and ITGB4 gene loci. Results from the −OSM (collected immediately after OSM treatment) and +OSM (0.5 hr after OSM treatment) arms are shown. The list of STAT3 peak-related genes are shown in Supplementary Table 4.
Extended Data Fig. 4 OSM-STAT3 promotes stable expansion of HHOs OSM and YAP signaling synergistically promote expansion of HHOs.
a. Representative images of YAP staining (green) in single eHHO cells cultured with the indicated medium and duration. Nuclear counterstaining, Hoechst 33342. Scale bar: 10 μm. b. Percentage of YAP-positive cells at different time points in the OSM+ or OSM− condition after passaging. Each dot shows the proportion of nuclear YAP-positive cells of four biological replicates (donors), and error bars show mean ± SD of the replicates. The OSM− and OSM+ data was compared using two-way ANOVA followed by Tukey’s two-way post-hoc (p = 0.0025475). For the comparison at each time point, Welch’s two-sided t-test and Bonferroni’s correction were used. c. The effect of OSM and/or TEAD inhibitor (TEADi; MYF-01-37, 20 μM) on HHO expansion. 1,000 cells were plated per well, and the number of cells in each well was analyzed at day 14. Each dot shows one well (each three wells per sample). Statistics, Welch’s two-sided t-test. d. Nuclear YAP localization of HHOs after administration of LATS inhibitor (LATSi; TDI-011536, 3 μM). The organoids were fixed and stained 24 h after LATSi administration. Representative images of YAP localization of HHOs (left). Percentage of nuclear YAP+ cells in LATSi− and LATSi+ conditions (right). Each dot represents the mean of technical replicates of one donor. Data and error bars are shown as mean ± SD. N = 5 HHO lines (biological replicates) derived from different donors. For LATSi− and LATSi+ conditions, 86 and 84 (PY12), 44 and 65 (PY30), 74 and 72 (PY31), 82 and 126 (PY39a), 51 and 50 (PY53) organoids were analyzed, respectively. Statistics, Welch’s two-sided t-test. e. The effect of LATSi with or without a TEAD inhibitor (TEADi; MYF-01-37, 20 or 40 μM) on CYR61 mRNA expression. RNA was harvested 24 h after treatment. For each condition, n = 3 biological replicates. Data are shown as mean ± SD. Statistics, Welch’s two-sided t-test. f. The effect of LATSi with or without a TEADi on the growth of HHO lines. Data from two HHO lines derived from different donors. Each dot shows one well. Statistics, Welch’s two-sided t-test. g. Growth curves of HHOs cultured with or without OSM and LATSi. Each dot denotes passaging. Data from three HHO lines derived from different donors. h. Percentage of EdU + cells in HHOs cultured with or without LATSi and TEADi. Data from two HHO lines derived from different donors. n indicates the number of cells analyzed. i. Comparative transcriptome analysis of PHH-derived organoids, PHHs and ICOs generated based on RNA-seq data. Genes differentially expressed in PHHs versus ICOs (top 20 upregulated and downregulated genes) are used for visualization. j. Expression of EMT markers SNAI2 and ZEB1 in eHHOs with or without OSM and in 3D or 2D culture format. n = 3 biological replicates. Data are shown as mean ± SD. Statistics, Welch’s two-sided t-test.
Extended Data Fig. 5 HHO xenotransplantation.
a. Concentration of human albumin in mouse sera following xenotransplantation of PM11 HHOs. HHOs with different passage counts are shown in different colors. n shows the number of mice analyzed. b. STEM121 staining of PM11 HHO xenografts. Scale bar: 1 mm. Quantification shown in Extended Data Fig. 5c. c. Repopulation efficiency of PM11 PHHs and eHHO with different culture duration. Each dot denotes one mouse. Statistics, one-way ANOVA followed by Dunnett’s test with the control (PHHs). d. Concentration of human albumin in mouse sera following xenotransplantation of PM16 HHOs. HHOs with different passage counts are shown in different colors. e. Repopulation efficiency of PM16 PHHs and eHHO with different culture duration. Each dot denotes one mouse. Statistics, one-way ANOVA followed by Dunnett’s test with the control (PHHs). f. Repopulation efficiency of PM10, PY9, PY12 eHHO, dHHO and dHHO+WR. Each dot denotes one mouse. g. Concentration of human albumin in mouse sera following xenotransplantation of PY39a HHOs. h. STEM121 staining of PY39a HHO xenografts. Scale bar: 1 mm. Quantification shown in Extended Data Fig. 5i. i. Repopulation efficiency of PY39a PHHs and eHHO with different culture duration. Each dot denotes one mouse. Statistics, one-way ANOVA followed by Dunnett’s test with the control (PHHs). j. The length of telomere in HHOs and their parental PHHs after prolonged culture. Telomere length was measured with quantitative real-time PCR. Data from PHHs and HHOs derived from four different donors. Data are shown as mean ± SD. k. Representative Ki67 (red) staining in a STEM121-positive (light blue) HHO xenograft in the mouse liver. Nuclear counterstaining, Hoechst 33342 (green). Scale bar: 20 μm. Quantification shown in Extended Data Fig. 5l. l. The relationship between the repopulation efficiency and percentage of Ki67+ cells in xenograft. Each dot shows one mouse. One section slide was analyzed per mouse. r, Pearson correlation coefficient, p = 0.8801, t-statistics, two-sided. m. Area overlap ratio between hMRP2 and hALB. (related to Fig. 2i). For each PHH or HHO, two to three mice were analyzed, and the ratio between hALB+ area and hMRP2+ area was calculated. Each dot shows the mean ratio. One section slide was analyzed per mouse. n. Representative images of a xenograft in the PV-CV area stained for the PV zone marker ASS1 and STEM121. Nuclear counterstaining, Hoechst 33342. Scale bar: 100 μm. Hatched areas indicate the venous lumens. *Indicates PV lumen. Representative data from three donors with similar results. o. Representative immunostaining images of CV area with the CV zone marker GS (red), CD31 (blue) and STEM121 (green). Scale bar: 100 μm. Similar results were obtained from three donors.
Extended Data Fig. 6 Characterization of differentiated HHOs.
a. PCA plotting of HLCs and HPCs with their original cells (modified from Ardisasmita et al.34 Commun Biol). PCA was performed using 5,000 genes with the highest variance as per the original study. Circles represent training data from Ardisasmita et al., while triangles and squares represent the data introduced in this study. The upper right panel illustrates the individual culture conditions of dHHO and eHHOs. b. Expression levels of marker genes in the same PCA coordinates as in Extended Data Fig. 6a. Color code indicates normalized expression levels. c. Expression level of marker genes in the same PCA coordinates as in a. The color code indicates normalized expression levels. d. Expression of genes in the liver-specific gene expression panel (LiGEP). Hierarchical clustering was performed using the Euclidean distance, and the color code indicates a mean-centered log-fold change of log-normalized counts. e. Distance-based similarity scores of different hepatocyte model systems to PHH based on LiGEP expression. Each dot shows the median score of term-related genes by biological replication of cell type and hepatocyte models. From the left, n = 4 (PSC), 4 (Fibroblast) 3 (ICO), 3(CBD), 5(Chol_HLC), 10 (Hep_HPC), 20 (PSC_HLC), 3 (HepG2), 11 (Fib_HLC), 6 (Fetal_Hep), 2 (FHep_HLC), 15(eHHO), 5 (Hep_HLC),18 (dHHO), 5 (Liver), and 29 (PHH). The upper whisker, box top, center, box bottom, and lower whisker of the boxplot shows −(1.5-fold IQR), 25, 50, 75 percentiles, and 1.5-fold IQR, respectively. f. Distance-based similarity scores of different hepatocyte model systems to PHH based on the expression of the indicated gene ontology gene sets related to liver metabolic functions. Each dot shows the median score of term-related genes of one biological replicate. n and boxplot definitions are the same as those in e.
Extended Data Fig. 7 Fatty acid metabolism and bile acid synthesis in HHOs.
a. Representative ZO-1 (green) and MRP2 (red) immunostaining of dHHOs are shown by a 3D-reconstructed image. Scale bar; 50 μm. Similar results were obtained from three donors. b. 3D-reconstructed visualization of BSEP-mediated efflux of CLF-labeled bile acid in a dHHO. Scale bar: 50 μm. c. BSEP-mediated efflux of CLF-labeled bile acid (green) in an eHHO and dHHO. F-actin was visualized with a silicon rhodamine F-actin probe (red). Scale bar, 50 μm. Similar results were obtained from two donors. d. The bile acid metabolism pathway (top) and bile acid secretion from hepatocytes to the bile canaliculus (bottom). HOC; hydroxycholesterol, C4; hydroxy-4-cholesten-3-one, CA; cholic acid, CDCA; 7α-hydroxy-chenodeoxy cholic acid, GCA; glyco-cholic acid, TCA; tauro-cholic acid, GCDCA; glyco-chenodeoxy cholic acid, TCDCA; tauro-chenodeoxy cholic acid, CDFDA; 5(6)-Carboxy-2′,7′-dichloro-fluorescein diacetate, and CLF; cholyl-lysine fluorescein. e. The expression of genes related to bile acid metabolism in PHHs and HHOs generated based on RNA-seq data. f. FXR agonist (INT747, 10 μM) and FGF19 (100 ng/ml) suppress bile acid secretion from dHHOs. Data from two HHO lines derived from different donors (biological replicates). Data and error bars show mean ± SD of three technical replicates. Each dot shows one technical replicate. Statistics, Welch’s two-sided t-test. g. Suppression of CYP7A1 mRNA expression by INT747 and FGF19 in dHHOs. The gene expression was measured using quantitative real-time PCR. Data from two HHO lines derived from different donors. n = 3 technical replicates. Data are shown as mean ± SD. Statistics, Welch’s two-sided t-test. h. Lipid volume in HHOs cultured with the indicated conditions after short and long culture maintenance. Lipids were measured using 3D-reconstructed images of organoids and averaged by the cell (nuclei) count. Data from two HHO lines derived from different donors (biological replicates). For each line, data was collected at two time points as indicated. Each dot shows one organoid. Whiskers; 1.5 × interquartile ranges, box; 25th and 75th percentiles and the median. Statistics, Welch’s two-sided t-test. From the left, n = 7, 6, 6 and 6 replicates for PY31 (day 69), n = 8, 8, 8 and 7 replicates for PY31 (day 132), n = 6, 5, 6 and 6 replicates for PY39a (day 72), and n = 11, 11, 7 and 7 replicates for PY39a (day 275). The upper whisker, box top, center, box bottom, and lower whisker of the boxplot show –(1.5-fold IQR)), 25, 50, 75 percentiles, and 1.5-fold IQR of organoids, respectively. i. Representative bright field of an eHHO and dHHO+WR. Scale bar: 50 μm. Similar results were obtained from three donors. j. Representative H&E staining images of an eHHO and dHHO+WR. dHHO+WR undergoes cell ballooning by lipid accumulation. Scale bar: 50 μm. Similar results were obtained from three donors.
Extended Data Fig. 8 Drug metabolism in HHOs.
a. The expression of genes related to cytochrome P450-mediated drug metabolism in PHHs, eHHOs, and dHHOs based on RNA-seq data. b. A workflow for analyzing basal and drug-induced CYP activity (top). The percentage of 2D plating efficiency of PHH and their derivative dHHOs (bottom). Total culture days are shown in parentheses. c. Basal CYP activities in PHHs and dHHOs related to Fig. 3j. Workflow in b. Drugs used for CYP induction indicated in parenthesis. Three donors at different time points. Each dot shows an experimental replicate. dHHOs were cultured for the indicated durations before 2D plating. Error bars show mean ± SD for PY12 (PHH; n = 5, dHHO; n = 5 with 4 conditions), PY30 (PHH; n = 8, dHHO; n = 5 with 4 conditions, and PY39b (PHH; n = 7 with 2 replicates, dHHO condition; n = 3 with 2 conditions). d. Drug-induced activity of CYPs in PHHs and dHHOs. Data from HHOs derived from three different donors. Each dot shows one well. Data are shown as mean ± SD of triplicates. e. Coumarin metabolism by CYP2A6, UGT, and SULT. f. Measurement of coumarin metabolites in PHHs and dHHOs with LC-MS/MS. The proportion of unconjugated and conjugated derivatives is shown. g. Dose-response curves of acetaminophen treatment on dHHOs. Organoid viability and damage was measured using ATP activity (top) and lactate dehydrogenase (LDH) release (bottom), respectively. Error bars show mean ± SD of three technical replicates for each condition.
Extended Data Fig. 9 Characterization of periportal vein hepatocytes organoids.
a. Glycogen visualization in pre-fasted (top) and post-fasted (bottom) dHHOs by Periodic acid-Schiff (PAS) staining. Serial sections treated with α-amylase are shown as negative controls. Scale bar: 50 μm. b. Glycogen measurement in dHHOs using glycogen bioluminescent assay. Data from two organoid lines derived from different donors (biological replicates). Each dot represents one technical replicate. Three technical replicates were shown for each condition. Statistics, Welch’s two-sided t-test. c. Isotope-tracing of 13C-labeled substrates in HHOs using LC-MS/MS (top) and CE-TOFMS (bottom). During gluconeogenesis, dHHOs were treated with the indicated 13C-labeled glucose substrates. Non-labeled glucose substrate-treated dHHOs are used as control. Data are shown as mean ± SD. One bar represents one experiment. (top) n = 6 replicates for each isotope for PY30, n = 5, 6, 5, 5 and 5 replicates (from the left) for PY39a, n = 5, 5, 5, 4 and 5 replicates (from the left) for PY39b. (bottom) Data of 4 (pyruvate) and 5 (non-labeled) replicates for PY12, 6 for PY30, and five replicates for PY39b. Error bar shows mean ± SD. Each dot represents one technical replicate. d. Quantification of Seahorse assay results (Related to Fig. 5f). 1) basal respiration, 2) Proton leak, 3) Maximal respiration and 4) Spare respiratory capacity were calculated. Data are from three HHOs derived from different donors. Each dot shows one technical replicate. Data and error bars show mean ± SD of three technical replicates. e. HHOs were cultured according to the workflow before analysis. Glucose and urea production (left) and CYP activity (right) of cells cultured in 3D and 2D were assessed. Data are from three HHOs derived from different donors (biological replicates), with each dot representing one technical replicate. For the gluocose data, n = 3 technical replicates for conditions 1, 2, and n = 5 replicates for condition 3, and for the urea data,n = 5 technical replicates for each condition. Data and error bars show mean ± SD. N.A., Not applicable. f. (Top) Comparison of expansion efficiency of PHHs between eHHO conditions and the protocol described by Hendriks et al. Cell numbers were estimated using passage ratios and ATP-based cell number. Each dot represents a passage event. (Bottom) Glucose production and urea synthesis under the indicated expansion condition. After expansion, both conditions were differentiated with our differentiation medium (DM) and subjected to fasting. Each dot represents one well. Data and error bars show mean ± SD of four technical replicates. Total culture days are shown in parentheses. g. The gene expression of the indicated secretory proteins in PHHs, eHHOs, and dHHOs based on RNA-seq data. h, i, j. The abundance of factor IX (h), complement C3 (i) and α1-antitrypsin (j) in the supernatant of PHHs, eHHOs, and dHHOs. Data from three HHO lines derived from independent donors (biological replicates). Each dot shows one well. Data and error bars show mean ± SD of three technical replicates.
Extended Data Fig. 10 Generation of gene knockout hepatocyte organoids.
a. Confirmation of TP53-knockout in HHOs with Sanger sequencing. Two knockout clones derived from two different donors (PY9 and PY30) were generated. b. Selection of TP53-knockout organoids by a treatment with the MDM2 inhibitor, nutlin-3. Scale bar, 500 μm. Similar results were obtained from at least three technical replications. c. Representative morphology of TP53-knockout HHOs showing their duct-like cystic structures. Scale bar, 100 μm. Identical results were obtained from observation of at least 50 organoids. d. The percentage of EdU+ cells in TP53-knockout HHOs. Culture duration from PHHs are shown on the bottom. n = 47 (218 days) and 73 (345 days) cells were analyzed. e. Albumin secretion, glucose production and urea synthesis from TP53-knockout HHOs. Culture duration from PHHs are shown on the bottom. Data from two TP53-knockout clones. Each dot shows one well. Data and error bars show mean ± SD of three technical replicates. f,g. Ornithine (f) and Urea (g) production from wild type (WT) and OTC-knockout HHOs. Each dot shows one well. Data and error bars show mean ± SD of three technical replicates. h–j. Generation of additional OTC-knockout HHOs. Confirmation of additional OTC-knockout in HHOs with Sanger sequencing (h). Three knockout clones derived from PY9 were generated. A GFP-expressing vector was introduces as a control, and three GFP-expressing clones were also generated. Protein-level confirmation of OTC knockout in OTC-WT and KO clones (i). Morphology of GFP-expressing WT clones and OTC-knockout HHOs (j). Identical results were obtained from at least 50 organoids for each clone. k. Urea synthesis from OTC-knockout HHOs compared with WT HHO clones. HHOs were incubated in gluconeogenesis assay medium without arginine and with 1 mM ornithine. Each dot shows one well. Data and error bars show mean ± SD of four technical replicates. l. The percentage of EdU+ cells in WT GFP clones and OTC-knockout HHOs. Culture duration from PHHs are shown on the bottom. n refers to the number of cells analyzed in each clonal HHO lines.
Supplementary information
Supplementary Information
Supplementary Figs. 1 and 2 and Supplementary Tables 1, 2, 5, 6 and 8.
Supplementary Table 3
Summary of comparative gene expression analyses. Top: comparison models in Extended Data Fig. 3b. Bottom: differentially expressed genes in comparison between expansion medium (n = 2) versus EM-OSM (n = 2) conditions; 903 genes (up, 366; down, 537; |log2FC| > 2, padj < 0.05).
Supplementary Table 4
STAT3 target genes identified by ChIP-seq analysis. List of 1,435 STAT3 target genes (STAT3 peaks in TSS 50 kb; q value > 5).
Supplementary Table 7
Formulas of the culture media and buffers used in the study. HHO and PHH culture medium: basal medium, expansion medium for eHHO, differentiation medium for dHHO, differentiation medium (DM) + WR for dHHO + WR, fasting medium, post-fasting assay medium, plating medium and maintenance medium. CYP assay medium: CYP induction medium and CYP substrate medium. Other reported hepatocyte model: Culture medium for ProliHH, culture medium for CLiP and culture medium for CLiP. 13C isotope tracing medium: post-fasting assay medium for non-labelled, post-fasting assay medium for 13C-pyruvate, post-fasting assay medium for 13C-lactate, post-fasting assay medium for 13C-glycerol and post-fasting assay medium for 13C-alanine. Post-fasting assay medium arginine free: post-fasting assay medium arginine free.
Supplementary Video 1
3D reconstruction of dHHO. DHHO was stained with CDH1 (blue), ZO-1 (green) and MRP2 (red). Representative cropped images are in Fig. 3d and Extended Data Fig. 7a.
Supplementary Video 2
Live imaging of CLF secretion from eHHO and dHHO. Four-dimensional (x–y–z–time) images were acquired with a 10-min interval for 3 h. 3D images were converted to .mov file.
Source data
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Igarashi, R., Oda, M., Okada, R. et al. Generation of human adult hepatocyte organoids with metabolic functions. Nature 641, 1248–1257 (2025). https://doi.org/10.1038/s41586-025-08861-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-025-08861-y