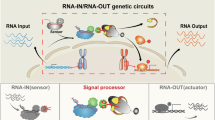
Abstract
Spatial RNA organization has a pivotal role in diverse cellular processes and diseases1,2,3,4. However, functional implications of the spatial transcriptome remain largely unexplored due to limited technologies for perturbing endogenous RNA within specific subcellular regions1,5. Here we present CRISPR-mediated transcriptome organization (CRISPR-TO), a system that harnesses RNA-guided, nuclease-dead dCas13 for programmable control of endogenous RNA localization in live cells. CRISPR-TO enables targeted localization of endogenous RNAs to diverse subcellular compartments, including the outer mitochondrial membrane, p-bodies, stress granules, telomeres and nuclear stress bodies, across various cell types. It allows for inducible and reversible bidirectional RNA transport along microtubules via motor proteins, facilitating real-time manipulation and monitoring of RNA localization dynamics in living cells. In primary cortical neurons, we demonstrate that repositioned mRNAs undergo local translation along neurites and at neurite tips, and co-transport with ribosomes, with β-actin mRNA localization enhancing the formation of dynamic filopodial protrusions and inhibiting axonal regeneration. CRISPR-TO-enabled screening in primary neurons identifies Stmn2 mRNA localization as a driver of neurite outgrowth. By enabling large-scale perturbation of the spatial transcriptome, CRISPR-TO bridges a critical gap left by sequencing and imaging technologies, offering a versatile platform for high-throughput functional interrogation of RNA localization in living cells and organisms.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
199,00 € per year
only 3,90 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The RNA-seq datasets generated and analysed during this study are available in the Gene Expression Omnibus repository under the accession number GSE278329. All RNA-seq data have been aligned to the mouse reference genome GRCm39. All data supporting the findings of this study are included in this article and Supplementary Information, and are available at https://doi.org/10.25740/rv392xg8117. Source data are provided with this paper.
Code availability
Custom scripts used for image analysis are available on GitHub (https://github.com/QilabGitHub/CRISPR-TO).
References
Das, S., Vera, M., Gandin, V., Singer, R. H. & Tutucci, E. Intracellular mRNA transport and localized translation. Nat. Rev. Mol. Cell Biol. 22, 483–504 (2021).
Leung, K. M. et al. Asymmetrical beta-actin mRNA translation in growth cones mediates attractive turning to netrin-1. Nat. Neurosci. 9, 1247–1256 (2006).
Moor, A. E. et al. Global mRNA polarization regulates translation efficiency in the intestinal epithelium. Science 357, 1299–1303 (2017).
Thelen, M. P. & Kye, M. J. The role of RNA binding proteins for local mRNA translation: implications in neurological disorders. Front. Mol. Biosci. 6, 161 (2019).
Buxbaum, A. R., Haimovich, G. & Singer, R. H. In the right place at the right time: visualizing and understanding mRNA localization. Nat. Rev. Mol. Cell Biol. 16, 95–109 (2015).
Jeffery, W. R., Tomlinson, C. R. & Brodeur, R. D. Localization of actin messenger RNA during early ascidian development. Dev. Biol. 99, 408–417 (1983).
Holt, C. E., Martin, K. C. & Schuman, E. M. Local translation in neurons: visualization and function. Nat. Struct. Mol. Biol. 26, 557–566 (2019).
Fazal, F. M. et al. Atlas of subcellular RNA localization revealed by APEX-seq. Cell 178, 473–490 (2019).
Alon, S. et al. Expansion sequencing: spatially precise in situ transcriptomics in intact biological systems. Science 371, eaax2656 (2021).
Chen, K. H., Boettiger, A. N., Moffitt, J. R., Wang, S. & Zhuang, X. Spatially resolved, highly multiplexed RNA profiling in single cells. Science 348, aaa6090 (2015).
Lubeck, E., Coskun, A. F., Zhiyentayev, T., Ahmad, M. & Cai, L. Single-cell in situ RNA profiling by sequential hybridization. Nat. Methods 11, 360–361 (2014).
Terenzio, M. et al. Locally translated mTOR controls axonal local translation in nerve injury. Science 359, 1416–1421 (2018).
Zhao, W. et al. CRISPR-Cas9-mediated functional dissection of 3′-UTRs. Nucleic Acids Res. 45, 10800–10810 (2017).
Katz, Z. B. et al. β-Actin mRNA compartmentalization enhances focal adhesion stability and directs cell migration. Genes Dev. 26, 1885–1890 (2012).
Tsuboi, T. et al. Mitochondrial volume fraction and translation duration impact mitochondrial mRNA localization and protein synthesis. eLife 9, e57814 (2020).
Abudayyeh, O. O. et al. RNA targeting with CRISPR-Cas13. Nature 550, 280–284 (2017).
Cox, D. B. T. et al. RNA editing with CRISPR-Cas13. Science 358, 1019–1027 (2017).
Konermann, S. et al. Transcriptome engineering with RNA-targeting type VI-D CRISPR effectors. Cell 173, 665–676 (2018).
Tieu, V. et al. A versatile CRISPR-Cas13d platform for multiplexed transcriptomic regulation and metabolic engineering in primary human T cells. Cell 187, 1278–1295 (2024).
Wang, H. et al. CRISPR-mediated live imaging of genome editing and transcription. Science 365, 1301–1305 (2019).
Yang, L. Z. et al. Dynamic imaging of RNA in living cells by CRISPR-Cas13 systems. Mol. Cell 76, 981–997 (2019).
Wilson, C., Chen, P. J., Miao, Z. & Liu, D. R. Programmable m6A modification of cellular RNAs with a Cas13-directed methyltransferase. Nat. Biotechnol. 38, 1431–1440 (2020).
Liang, F. S., Ho, W. Q. & Crabtree, G. R. Engineering the ABA plant stress pathway for regulation of induced proximity. Sci. Signal. 4, rs2 (2011).
Liao, P. et al. The ameliorative effects and mechanisms of abscisic acid on learning and memory. Neuropharmacology 224, 109365 (2023).
Raj, A., van den Bogaard, P., Rifkin, S. A., van Oudenaarden, A. & Tyagi, S. Imaging individual mRNA molecules using multiple singly labeled probes. Nat. Methods 5, 877–879 (2008).
White, M. R. & Garcin, E. D. The sweet side of RNA regulation: glyceraldehyde-3-phosphate dehydrogenase as a noncanonical RNA-binding protein. Wiley Interdiscip. Rev. RNA 7, 53–70 (2016).
Vercellino, I. & Sazanov, L. A. The assembly, regulation and function of the mitochondrial respiratory chain. Nat. Rev. Mol. Cell Biol. 23, 141–161 (2022).
Yoon, Y. Sharpening the scissors: mitochondrial fission with aid. Cell Biochem. Biophys. 41, 193–206 (2004).
Kannan, S. et al. Compact RNA editors with small Cas13 proteins. Nat. Biotechnol. 40, 194–197 (2021).
Ozcan, A. et al. Programmable RNA targeting with the single-protein CRISPR effector Cas7-11. Nature 597, 720–725 (2021).
Hu, W. et al. Single-base tiled screen unveils design principles of PspCas13b for potent and off-target-free RNA silencing. Nat. Struct. Mol. Biol. 31, 1702–1716 (2024).
Park, H. Y., Trcek, T., Wells, A. L., Chao, J. A. & Singer, R. H. An unbiased analysis method to quantify mRNA localization reveals its correlation with cell motility. Cell Rep. 1, 179–184 (2012).
Yang, L. Z. et al. Multi-color RNA imaging with CRISPR-Cas13b systems in living cells. Cell Insight 1, 100044 (2022).
Luo, Y., Na, Z. & Slavoff, S. A. P-bodies: composition, properties, and functions. Biochemistry 57, 2424–2431 (2018).
Yang, P. et al. G3BP1 is a tunable switch that triggers phase separation to assemble stress granules. Cell 181, 325–345 (2020).
Hubstenberger, A. et al. P-body purification reveals the condensation of repressed mRNA regulons. Mol. Cell 68, 144–157 (2017).
Munoz, P. et al. TRF1 controls telomere length and mitotic fidelity in epithelial homeostasis. Mol. Cell. Biol. 29, 1608–1625 (2009).
Biamonti, G. & Vourc’h, C. Nuclear stress bodies. Cold Spring Harb. Perspect. Biol. 2, a000695 (2010).
Canale, P., Campolo, J., Borghini, A. & Andreassi, M. G. Long telomeric repeat-containing RNA (TERRA): biological functions and challenges in vascular aging and disease. Biomedicines 11, 3211 (2023).
Guardia, C. M. et al. Reversible association with motor proteins (RAMP): a streptavidin-based method to manipulate organelle positioning. PLoS Biol. 17, e3000279 (2019).
Janicki, S. M. et al. From silencing to gene expression: real-time analysis in single cells. Cell 116, 683–698 (2004).
Xu, H. et al. TriTag: an integrative tool to correlate chromatin dynamics and gene expression in living cells. Nucleic Acids Res. 48, 13013–13014 (2020).
Safieddine, A. et al. A choreography of centrosomal mRNAs reveals a conserved localization mechanism involving active polysome transport. Nat. Commun. 12, 1352 (2021).
Ning, L. et al. A bright, nontoxic, and non-aggregating red fluorescent protein for long-term labeling of fine structures in neurons. Front. Cell Dev. Biol. 10, 893468 (2022).
Afridi, R., Tsuda, M., Ryu, H. & Suk, K. The function of glial cells in the neuroinflammatory and neuroimmunological responses. Cells 11, 659 (2022).
Denny, J. B. Molecular mechanisms, biological actions, and neuropharmacology of the growth-associated protein GAP-43. Curr. Neuropharmacol. 4, 293–304 (2006).
Powell, J. E. et al. Targeted gene silencing in the nervous system with CRISPR-Cas13. Sci. Adv. 8, eabk2485 (2022).
Pilaz, L. J. et al. Subcellular mRNA localization and local translation of Arhgap11a in radial glial progenitors regulates cortical development. Neuron 111, 839–856 (2023).
Buxbaum, A. R., Wu, B. & Singer, R. H. Single β-actin mRNA detection in neurons reveals a mechanism for regulating its translatability. Science 343, 419–422 (2014).
Xu, K., Zhong, G. & Zhuang, X. Actin, spectrin, and associated proteins form a periodic cytoskeletal structure in axons. Science 339, 452–456 (2013).
David, A. et al. Nuclear translation visualized by ribosome-bound nascent chain puromycylation. J. Cell Biol. 197, 45–57 (2012).
Wu, B., Eliscovich, C., Yoon, Y. J. & Singer, R. H. Translation dynamics of single mRNAs in live cells and neurons. Science 352, 1430–1435 (2016).
Eom, T., Antar, L. N., Singer, R. H. & Bassell, G. J. Localization of a β-actin messenger ribonucleoprotein complex with zipcode-binding protein modulates the density of dendritic filopodia and filopodial synapses. J. Neurosci. 23, 10433–10444 (2003).
Donnelly, C. J. et al. Axonally synthesized β-actin and GAP-43 proteins support distinct modes of axonal growth. J. Neurosci. 33, 3311–3322 (2013).
Gomez, T. M. & Letourneau, P. C. Actin dynamics in growth cone motility and navigation. J. Neurochem. 129, 221–234 (2014).
Wang, G. et al. Spatial organization of the transcriptome in individual neurons. Preprint at bioRxiv https://doi.org/10.1101/2020.12.07.414060 (2020).
Park, J. W., Vahidi, B., Taylor, A. M., Rhee, S. W. & Jeon, N. L. Microfluidic culture platform for neuroscience research. Nat. Protoc. 1, 2128–2136 (2006).
Thornburg-Suresh, E. J. C. & Summers, D. W. Microtubules, membranes, and movement: new roles for stathmin-2 in axon integrity. J. Neurosci. Res. 102, e25382 (2024).
Gumy, L. F. et al. Transcriptome analysis of embryonic and adult sensory axons reveals changes in mRNA repertoire localization. RNA 17, 85–98 (2011).
Zivraj, K. H. et al. Subcellular profiling reveals distinct and developmentally regulated repertoire of growth cone mRNAs. J. Neurosci. 30, 15464–15478 (2010).
Wessels, H. H. et al. Massively parallel Cas13 screens reveal principles for guide RNA design. Nat. Biotechnol. 38, 722–727 (2020).
Tsanov, N. et al. smiFISH and FISH-quant — a flexible single RNA detection approach with super-resolution capability. Nucleic Acids Res. 44, e165 (2016).
Andronov, L. et al. Nanoscale cellular organization of viral RNA and proteins in SARS-CoV-2 replication organelles. Nat. Commun. 15, 4644 (2024).
Rust, M. J., Bates, M. & Zhuang, X. Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM). Nat. Methods 3, 793–795 (2006).
Huang, B., Wang, W., Bates, M. & Zhuang, X. Three-dimensional super-resolution imaging by stochastic optical reconstruction microscopy. Science 319, 810–813 (2008).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Orengo, J. P., Bundman, D. & Cooper, T. A. A bichromatic fluorescent reporter for cell-based screens of alternative splicing. Nucleic Acids Res. 34, e148 (2006).
Vargas, D. Y., Raj, A., Marras, S. A., Kramer, F. R. & Tyagi, S. Mechanism of mRNA transport in the nucleus. Proc. Natl Acad. Sci. USA 102, 17008–17013 (2005).
Luo, B. et al. Endoplasmic reticulum stress eIF2α-ATF4 pathway-mediated cyclooxygenase-2 induction regulates cadmium-induced autophagy in kidney. Cell Death Dis. 7, e2251 (2016).
Acknowledgements
We thank all members from L.S.Q. laboratory for facilitating experiments and useful discussion; X. Wu from Harvard University for help with the code for quantifying colocalization; L. Andronov from W. E. Moerner laboratory at Stanford University for help with imaging analysis; S. Lee and D. Jiang from M. Z. Lin laboratory at Stanford University for help with primary neuron cultures and providing the Addgene plasmid 190041; Z. Feng and H. Guo from W. H. Wong laboratory at Stanford Univeristy for help with Z-test analysis; A. Gitler laboratory for valuable discussions; T. C. Südhof for helpful feedback; D. L. Spector in the Cold Spring Harbor Laboratory for the U2OS 2-6-3 cells; the Stanford University Cell Sciences Imaging Core Facility (RRID:SCR_017787) for assisting with microscopy imaging; and C. P. Caridi and the HTSKC for assistance with image analysis and use of the ImageXpress Micro Confocal system funded by SIG S10OD026899. M.H. and Y.Z. acknowledge support by the Stanford School of Medicine Dean’s Postdoctoral Fellowship. A.A.C. acknowledges the American Heart Association Postdoctoral Fellowship. L.S.Q. acknowledges support by the Chau Hoi Shuen Foundation, the National Science Foundation, and the NIH. The super-resolution imaging described was supported, in part, by award number 1S10OD034400-01A1 from the National Center for Research Resources (NCRR). This work is supported by National Science Foundation CAREER award 2046650 (to L.S.Q.), NIH Director’s Pioneer Award DP1NS137219 (to L.S.Q.), NIH grant R01CA266470 and R21HG013133 (to L.S.Q.). L.S.Q. is a Chan Zuckerberg Biohub Investigator. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCRR or the NIH.
Author information
Authors and Affiliations
Contributions
M.H. and L.S.Q. conceived the study and planned the experimental design. M.H., M.L.F., A.A.C., E.L., S.C. and L.M. conducted the experiments. M.H., Y.Z., A.A.C., M.L.F., S.C., J.B., E.L., L.M., Y.M. and L.S.Q. analysed the data. M.H., Y.Z., A.A.C., J.B., Y.M., M.L.F. and L.S.Q. plotted the figures. L.S.Q. acquired funding and supervised the study. M.H. and L.S.Q. wrote the manuscript, with input from all authors.
Corresponding author
Ethics declarations
Competing interests
M.H. and L.S.Q. are inventors on a provisional patent application (US 63/594,147, US 2024/052536) filed by Stanford University related to this work. L.S.Q. is a founder and scientific advisor to Epicrispr Biotechnologies and a scientific advisor to the Laboratory of Genomics Research. The other authors declare no competing interests.
Peer review
Peer review information
Nature thanks Gavin Knott, Mitchell O’Connell and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Optimization of dCas13-ABI construct and development of CRISPR-TO.
a, Schematic of a dual-color splicing reporter expressing dsRed and eGFP18,67. SA, splicing acceptor site. Exon inclusion leads to eGFP expression while exon skipping leads to dsRed expression. b, Left, schematic of two dCas13-ABI constructs with different protein fusions. Right, bar graph showing ratio of HEK293T cells with dominant isoforms expressing dsRed to eGFP. Cells were co-transfected with the dual-color splicing reporter, different dCas13-ABI fusion constructs, and gRNA targeting the SA site (gSA) or a non-targeting gRNA (gNT) for one day, followed by flow cytometry. Each dot represents one biological replicate. These results show a higher binding activity of Construct 1. c, Bar graph showing ratio of cells with dominant isoforms expressing dsRed to eGFP with different linkers indicated in the table. Each dot represents one biological replicate. These results show a higher binding activity of Linker 1 (L1). d, Schematic of different dCas13-ABI constructs with NLS or NES and representative microscopic images of HeLa cells expressing these constructs. Cells expressing NLS- or NES-tagged dCas13-L1-BFP-ABI show formation of large aggregates of expressed proteins. This issue is mostly solved by swapping BFP to eGFP (comparing rows 2&4 to rows 1&3). The shape of the nucleus was depicted with a white dotted line. Scale bar, 10 µm. e, Schematic illustrating the 24×GCN4 reporter mRNA with dCas13 bound on it. f, Schematic of plasmids expressing different PYL1-fusion proteins for localizing RNA to various subcellular compartments. The use of NES-dCas13 or NLS-dCas13 was indicated under each target compartment. g, Representative microscopic images showing MitoTracker staining of HeLa cells transfected with the MAVS*-PYL1 plasmid or without transfection. Same contrast and brightness were applied in the blue channel. Scale bar, 20 µm. h, Representative microscopic images showing the localization of 24×GCN4 reporter mRNA to the OMM in HeLa cells in the gGCN4+ABA group, but not in the two control groups. gGCN4 indicates the gRNA targeting GCN4 repeats. Scale bar, 20 µm. i, Swarm plots depicting the percentage of 24×GCN4 reporter mRNA and dCas13 protein localized on the OMM in individual cells. Each dot represents one cell with the bar indicating median. n indicates the number of quantified cells for each group. Two-sided, unpaired Student’s t-test. Only cells co-expressing dCas13, MAVS*-PYL1, and reporter mRNA were analyzed.
Extended Data Fig. 2 The versatility of CRISPR-TO for manipulating various endogenous mRNA localization across diverse cell types.
a, Schematic of the targeting ___location of gRNAs (gG1, gG2, and gG3, together denoted as gG123) designed for the endogenous GAPDH mRNA. b-e, Left: representative microscopic images showing the localization of endogenous ACTB, SDHC, SDHD, and NDUFS2 mRNAs to the OMM in HeLa cells under different gRNA and ABA conditions. Scale bar, 20 µm. Right: quantification of the percentage of the target mRNA and dCas13 protein localized on the OMM in individual cells. f-g, Left: representative microscopic images showing the localization of endogenous GAPDH mRNA and Actb mRNA to the OMM in HEK293T cells and Neuro-2a cells under different gRNA conditions, respectively. Scale bar, 20 µm. Right: quantification of the percentage of the target mRNA and dCas13 protein localized on the OMM in individual cells. In b-g, white dotted lines indicate the plasma membrane of the imaged cells. h, Schematic of the stable HeLa cells expressing CRISPR-TO components including MAVS*-PYL1 (located on the OMM), dCas13 (diffused in the cytoplasm), and three gRNAs targeting endogenous GAPDH mRNA (gG1, gG2, and gG3) for recruiting GAPDH mRNA to the OMM. i, Representative microscopic images of a large field of view showing endogenous GAPDH mRNA enriched on the OMM in the stable HeLa cells (shown in h) after ABA treatment. Scale bar, 50 µm. j, Quantification of the percentage of the endogenous GAPDH mRNA and dCas13 protein localized on the OMM in the stable HeLa cells (shown in h) after DMSO or ABA treatment. The dotted line indicates the maximum value of the DMSO group and the percentage of cells above the dotted line in the ABA group was indicated in the plot. For all the quantification plots, the black bar indicates the median. n indicates the number of quantified cells for each group. Two-sided, unpaired Student’s t-test.
Extended Data Fig. 3 Characterization of determinants of spatial RNA control using CRISPR-TO.
a, Representative microscopic images showing RNA FISH signals of reporter mRNA in one cell of gT+DMSO group. One region with sparse and well-separated reporter mRNA particles was selected for ThunderStorm analysis to quantify the fluorescence intensity of individual reporter mRNA particles. Scale bar of the left image, 20 µm. Scale bar of the inset image, 5 µm. b, Distribution of the fluorescence intensity of the isolated reporter mRNA particles stained with RNA FISH probes. The unimodal distribution suggests that the analyzed reporter mRNA particles were individual molecules25,68. c, Distribution of the number of reporter mRNA particles in individual cells on one z-slice for the gGCN4+ABA group. d, Scatter plot showing the percentage of dCas13 protein localized on the OMM versus the number of reporter mRNA particles in individual cells on one z-slice for the gGCN4+ABA group, related to Fig. 1f. e, Representative microscopic images showing reporter mRNA enriched on the OMM after CRISPR-TO perturbation in a HeLa cell with high reporter mRNA expression (1.2×105 mRNA particles on one z-slice). Scale bar, 20 µm. f, Schematic of reporter mRNAs with different copy numbers of GCN4 repeats. g, Box-and-whisker plots depicting the percentage of dCas13 protein localized on the OMM in individual cells after ABA treatment, related to Fig. 1g. From left to right, n = 52, 58, 60, 60, 56, 51 cells. Two-sided, unpaired Student’s t-test versus gNT control. In d and g, only cells co-expressing dCas13, MAVS*-PYL1, and reporter mRNA were analyzed. h, Box-and-whisker plots depicting the percentage of endogenous GAPDH mRNA and dCas13 protein localized on the OMM in individual cells after ABA treatment for cells transfected with dCas13, MAVS*-PYL1, and gNT or combinations of one to three targeting gRNAs, each expressed from a separate plasmid. Only cells co-expressing dCas13 and MAVS*-PYL1 were analyzed. From left to right, n = 77, 43, 42, 46, 33, 46, 45, 77 cells. Two-sided, unpaired Student’s t-test. Box-and-whisker plots: median, 25% to 75% boxes, whiskers (10–90%), and outliers indicated. i, Schematic of the targeting ___location of gRNAs designed for the endogenous mouse Actb mRNA and Gap43 mRNA. j, Representative microscopic images showing the localization of endogenous Gap43 mRNA to the OMM in ABA-treated Neuro-2a cells expressing gNT or the combination of targeting gRNAs 8, 13 and 15 (Supplementary Tables 1 and 2). Scale bar, 10 µm. White dotted lines indicate the plasma membrane of the imaged cells. k, The ___location of the targeting gRNAs and the read coverage of mouse cortical neuron RNA-seq data for mouse Actb mRNA. The color lines indicate the mutations of RNA-seq data to the Refseq Genes. l-n, The distribution of read coverage over gRNAs (l), off-target genome hits (m), and Minimum Free Energy (MFE, n) for gRNAs with different scores (Supplementary Table 5). Each set of gRNAs targeting Actb or Gap43 mRNA were arbitrarily scored from 0 to 4 based on the observed efficiency of the target mRNA relocalized to the OMM via CRISPR-TO in Neuro-2a cells: 0 (not working), 1 (probably working), 2 (working), 3 (working well), and 4 (working very well). n = 11 (score 0), 7 (score 1), 12 (score 2), 14 (score 3), 3 (score 4) sets of gRNAs. The P values were calculated using independent two-sided Mann–Whitney U test with Benjamini–Hochberg correction. In the violin plots, the embedded box represents the central 50% of the data—that is, the interquartile range (IQR) from the 25th to the 75th percentile—with the center line marking the median; the whiskers extend to the most extreme values within 1.5 times the IQR, and any points plotted beyond these whiskers are individual outliers representing values that deviate markedly from the bulk of the distribution.
Extended Data Fig. 4 Analysis of the influence of dCas13 binding on RNA stability, distribution, and translation, related to Fig. 1j.
a-f, Representative microscopic images showing dCas13 expression and RNA FISH signals of endogenous GAPDH mRNA (a), human ACTB mRNA (b), mouse Actb mRNA (c), NDUFS2 mRNA (d), SDHC mRNA (e), and SDHD mRNA (f) in HeLa cells (a,b,d,e,f) or Neuro-2a cells (c) expressing dCas13 and the corresponding targeting gRNAs compared with gNT. Calibration bar indicates the fluorescence intensity of RNA FISH signals. Scale bar, 50 µm. g-l, Violin plots quantifying the integrated intensity of RNA FISH signals of the target mRNA in individual cells expressing dCas13 and the corresponding targeting gRNAs compared with gNT. n, cell number. m-r, Violin plots quantifying the Polarization Index and Dispersion Index of the target mRNA in individual cells expressing dCas13 and the corresponding targeting gRNAs compared with gNT. n, cell number. s and v-x, Violin plots quantifying the integrated intensity of IF signals of proteins translated from the target mRNA in single HeLa cells expressing dCas13 and the corresponding targeting gRNAs compared with gNT. n, cell number. t-u, Column plots showing the mean fluorescence intensity of human ACTB protein (t) and mouse ACTB protein (u) IF signals analyzed by flow cytometry in HeLa cells (t) or Neuro-2a cells (u) expressing dCas13 and the corresponding targeting gRNAs compared with gNT. Each dot represents one biological replicate. Data presented as means ± standard deviation. n = 3 wells per group. All the P values were calculated using two-sided, unpaired Student’s t-test between gT and gNT groups.
Extended Data Fig. 5 Characterization of mismatch tolerance of gRNA for dCas13 binding efficiency in the CRISPR-TO system.
a, Schematic showing the design of 30 gRNAs with different mismatches in the spacer of gG1 which targets the endogenous GAPDH mRNA. The mutated nucleotides are shown in black. b, Quantification of percentage of RNA relocalization efficiency and percentage of dCas13 localized on the OMM in individual cells for different gRNAs. % RNA relocalization efficiency was calculated by subtracting the background % of GAPDH mRNA localized on the OMM using gNT from the % of GAPDH mRNA localized on the OMM for each gRNA. Only cells co-expressing dCas13 and MAVS*-PYL1 were analyzed. Black bar, mean. n, cell number. Two-sided, unpaired Student’s t-test between gG1 and individual groups. The dashed line indicates the mean of the gG1 group. c, Comparison of % RNA relocalization efficiency of gRNAs with single mutation (SM), double mutations (DM), and triple mutations (TM) on different ___location of the gRNA spacer. Two-sided, one sample t-test with a hypothetical value 0.1298 (indicated by the dashed line) which is the mean % RNA relocalization efficiency of the gG1 group. d, Comparison of % RNA relocalization efficiency and % dCas13 localized on the OMM between gG1 group and gG12 group which co-expresses gG1 and gG2 together. The number above each plot is the fold change between the two groups. n, number of cells analyzed per group. Black bar, mean. n, cell number. Two-sided, unpaired Student’s t-test.
Extended Data Fig. 6 Statistical analysis of microscopic images in Fig. 2 and investigation of p-body function in RNA decay.
a-b, i-j, and l, Quantification of the percentage of the target RNA (red) and dCas13 protein (green) localized at p-bodies (a), stress granules (b), telomeres (i), nuclear stress bodies (j), and the centrosome (l), related to Fig. 2b,c,e,f,i. Black bar, median. n, cell number. Two-sided, unpaired Student’s t-test. c, Representative microscopic images showing endogenous GAPDH mRNA accumulated at p-bodies in HeLa cells stably expressing dCas13, DDX6-PYL1, and gG123 after 1-day ABA treatment. Scale bar, 20 µm. Inset images represent magnification of the region surrounded by the white dotted line. Scale bar, 5 µm. d, Quantification of the relative expression level of GAPDH mRNA analyzed by qRT-PCR in HeLa cells stably expressing dCas13, DDX6-PYL1, and gG123 after 2-day treatment of ABA or DMSO. Each dot represents one biological replicate. Data presented as means ± standard deviation. n = 3 wells per group. e, Schematic of the reporter mRNA plasmid pSLQ14860_pCAGGS-TRE3G-BFP-7×GCN4-CMV-rtta construct. f, Experimental procedures for measuring RNA decay rate. g, RNA decay curves for the BFP-7×GCN4 reporter mRNA under different conditions. Mean and standard deviation values at each time point are plotted based on multiple measurements across three biological replicates. h, Parameters and results for the RNA decay curves fitted by one phase decay function. P values were calculated by Z-test of the decay constant k (Supplementary Methods). k, Percentage of cells with endogenous GAPDH mRNA (red) and dCas13 protein (green) enriched at the leading edge of cells, related to Fig. 2h. Data was presented as means ± s.e.m. Two-sided Fisher’s exact test. n, cell number. For a,b,i,j,k,l, only transfected cells were analyzed.
Extended Data Fig. 7 Additional data related to the characterization of real-time dynamics of mRNA localization by CRISPR-TO in Fig. 2j,k.
a, Box-and-whisker plots depicting the percentage of dCas13 protein (green) and endogenous GAPDH mRNA (red) localized on the OMM in individual cells. HeLa cells were transfected with dCas13, MAVS*-PYL1, and gG123 with different ABA concentrations for 4 h. Each dot represents one cell. n, cell number. Two-sided, unpaired Student’s t-test between 0 µM and indicated groups. The curves are fitted with a saturation binding curve with Hill coefficient, with assumption that the background values are the mean of the 0 µM group. Box-and-whisker plots: median, 25% to 75% boxes, whiskers (10–90%), and outliers indicated. b, Box-and-whisker plots depicting the percentage of dCas13 protein localized on the OMM over time. HeLa cells expressing dCas13, MAVS*-PYL1, and gG123 were treated with 250 µM ABA for indicated durations. Localization dynamics fitted by a two-phase exponential model; second-phase parameters (characteristic time τ, background A0, and maximum activation amplitude Am) constrained by mRNA localization data (Fig. 2j, left). n, cell number. Two-sided, unpaired Student’s t-test between 0 h and indicated groups. c, Time-course plot showing percentage of OMM-localized free (orange) and mRNA-bound (blue) dCas13 proteins over time after ABA treatment. Curves were generated by a simulation based on the two-phase model (Extended Data Fig. 7b and Supplementary Methods). d, Box-and-whisker plots depicting the percentage of dCas13 protein localized on the OMM over time. HeLa cells expressing dCas13, MAVS*-PYL1, and gG123 were treated with 250 µM ABA for 4 h followed by ABA removal for indicated durations. Rightmost group represents cells treated with DMSO for 4 h. Dissociation curve fitted by simple exponential decay model with baseline constrained to DMSO mean. n, cell number. Two-sided, unpaired Student’s t-test between 0 h and indicated groups. For a,b,d, only cells co-expressing dCas13 and MAVS*-PYL1 were analyzed. e, Schematic illustrating the MS2-tagged reporter mRNA used for real-time tracking of CRISPR-TO-mediated mRNA localization in live cells. f, Representative microscopic images showing MS2 reporter mRNA accumulation at the minus ends of microtubules (centrosome, arrows) after ABA addition. Scale bar, 20 µm. Insets (dashed outlines) show magnified views of the centrosome region (arrow). g, Quantification of the fluorescence intensity and the fraction of MS2 reporter mRNA located at the minus ends of microtubules in Extended Data Fig. 7f over time after adding ABA. h, Representative trajectories of 60 MS2 reporter mRNA particles detected in the cell shown in Extended Data Fig. 7f. The trajectories are exhibited with their traversed distance (see Supplementary Methods for calculating traversed distance) from smallest to largest. The first 20 trajectories were categorized as type I which shows confined, sub-diffusive motion of mRNA particles due to crowding cytoplasmic environment. The last 20 trajectories were categorized as type II which shows super-diffusive motion of mRNA particles corresponding to the active transport along microtubules via motor proteins. The middle 20 trajectories were likely a mixture of the two types. i, Mean squared displacement (MSD) analysis of 20 representative trajectories for two identified MS2 reporter mRNA particle populations (type I: confined/sub-diffusive; type II: active transport/super-diffusive). Lines represent linear fits of the first three MSD points. n = 20 trajectories for type I and II, respectively. Data are presented as mean values ± standard deviation. j, Distance of five representative type I and type II MS2 reporter mRNA trajectories to the centrosome (minus end of microtubules) over time.
Extended Data Fig. 8 Additional data related to the control of endogenous mRNA transport in primary mouse cortical neurons via CRISPR-TO in Fig. 3.
a, Quantification of cell viability for neurons under different treatments. b, Quantification of the ratio of ATF4 average fluorescent intensity between the nucleus and cytoplasm for neurons under different treatments to examine ER stress69. In the right diagram, neurons were transfected with CRISPR-TO components (dCas13, KIF5A*-PYL1, and different gRNAs) and CAAX-mScarlet3. Cell populations with and without dCas13 expression were analyzed. For a-b, data was presented as mean values ± standard deviation. Each dot represents one biological replicate. n = 3 or 4 wells per group. Two-sided, unpaired Student’s t-test. c, Representative microscopic images showing the dramatic decrease of astrocytes after fluorodeoxyuridine (FUDR) treatment in the in vitro mouse cortical neuron culture system. Scale bar, 400 µm. d, Representative microscopic images showing very few microglial cells in the in vitro mouse cortical neuron culture system after FUDR treatment. Scale bar, 400 µm. e-f, Representative confocal microscopic images of a single neuron in the gT+DMSO group (e) or the gNT+ABA group (f). The left image shows the shape of a neuron. Scale bar, 100 µm. Right images show magnification of dotted boxes in the left image. Scale bar, 50 µm. g, Histogram of the fluorescence intensity of Actb mRNA particles stained by RNA FISH. n = 196 mRNA particles. h, Quantification of the fluorescence intensity of endogenous Actb mRNA (red) and Gap43 mRNA (green) at neurite tips after CRISPR-TO perturbation with different gRNAs, related to Fig. 3h. n indicates the number of quantified neurite tips for each group. The black bar indicates the mean value. Two-sided, unpaired Student’s t-test. i-j, Quantification of the integrated fluorescence intensity of Actb mRNA FISH signals in the cell body of primary mouse cortical neurons expressing gT/gNT only (i) or dCas13+gT/gNT (j). k, Quantification of the integrated fluorescence intensity of Actb mRNA FISH signals and dCas13 protein signals in the cell body of primary mouse cortical neurons expressing CRISPR-TO components and treated with ABA or DMSO. Black bar, mean. n, cell body. Two-sided, unpaired Student’s t-test. The number above each group is the fold change between two groups. l, Representative microscopic images showing similar Actb mRNA level inside somas (blue arrows) of neurons in gT+DMSO and gT+ABA group, and dCas13 proteins remaining inside somas after ABA treatment in the gT+ABA group. Scale bar, 50 µm. Calibration bar indicates the fluorescence intensity of Actb mRNA FISH signals and dCas13 proteins. Inset images surrounded by the dotted line represent magnification of the region indicated by the white arrow, showing Actb mRNA enriched at neurite tips in the gT+ABA group after CRISPR-TO perturbation.
Extended Data Fig. 9 Additional data related to functional characterization of mRNA localization in Fig. 4.
a, Schematic of the plasmid of the reporter mRNA expressing Dendra2 protein with 24 copies of GCN4 repeats in the 3’ UTR. b, Quantification of the green and red fluorescence intensity of Dendra2 in primary mouse cortical neurons expressing the reporter mRNA after illuminated with 624.7 ± 62.5 mW/cm2 365 nm laser for different periods. There are 64% green Dendra2 proteins converted after 40-second illumination. c, Violin plot quantifying the green florescence recovery of Dendra2 at 70 min post UV-conversion under different conditions. Two-sided, unpaired Student’s t-test between gT+ABA (red) and each group. d, Representative confocal images showing the dual-color staining of Actb mRNA and Rn28s1 rRNA inside the neurite tip (Image 1; scale bar, 5 µm) and along the neurites (Image 2 and 3; scale bar, 10 µm) of gT+ABA group. Inset images represent magnification of the region surrounded by the dotted lines. e, Histogram of the mean intensity of Rn28s1 rRNA FISH signals at the Actb mRNA particle for all the Actb mRNA particles (red) and Actb mRNA particles not colocalized with Rn28s1 rRNA (black) in the neurite of gT+ABA group. A cutoff was set as the maximum value of the black distribution to quantify the percentage of Actb mRNA particles colocalized with Rn28s1 rRNA. f, Intensity plots of the dotted lines in the left images which are inset images shown in d. g, Histogram showing the X, Y localization precision of AF488-labeled Actb mRNA, AF647-labeled Rn28s1 rRNA, and CF583R-labeled translation sites measured using STORM microscopy in fixed neurons. h-i, Top: representative super-resolution images showing the three-color staining of Actb mRNA, ribosome, and translation site inside neurites for the gT+ABA group after CRISPR-TO perturbation. Scale bar, 1 µm (h) and 5 µm (i). Inset images represent magnification of the region pointed by the white arrow. Scale bar, 200 nm (h) and 1 µm (i). Representative Actb mRNA particles were pointed by yellow arrows. Bottom: histogram of the relative intensity of the white box region. j, Representative super-resolution images of Actb mRNA and translation sites at neurite tips for the gT+ABA group and gNT+ABA control group after CRISPR-TO perturbation. The shape of neurite tips is depicted with a white dotted line. Inset: zoom-in images of the region pointed by the white arrow. k, Schematic illustrating the SINAPS reporter mRNA and translated proteins observed in live primary mouse cortical neurons along neurites. Reporter mRNA was visualized using stdMCP-tdTomato (red), and translated SunTag protein was visualized using scFV-sfGFP (green). From top to bottom: translating mRNA (particles with colocalized green and red signals); untranslating mRNA (particles only with red signal); and diffused SunTag protein (particles only with green signal). l, Representative microscopic images of the SINAPS reporter mRNA and SunTag protein inside two neurites. Scale bar, 20 µm. Inset images represent magnification of the region pointed by white arrows. Experiment was only performed once but similar results were observed in many neurons. m, Frequency distribution of the number of endogenous Actb mRNA particles in individual neurite tips for the gT+ABA group with different ABA treatment time. P values were calculated using two-sided, unpaired Student’s t-test between no ABA treatment group and indicated groups. n, number of neurite tips. n, Representative microscopic images showing the localization of Actb mRNA particles at neurite tips with protrusions after 3-hour ABA treatment for CRISPR-TO perturbation. Scale bar, 5 µm. White dotted lines indicate the shape of the neurite tip. Experiment was only performed once but similar results were observed in many neurons. o, Schematic of neurons culturing in a microfluidic device and injured by axotomy. p, Representative microscopic images showing spatial localization of endogenous Actb mRNA in one microfluidic channel for primary mouse cortical neurons expressing CRISPR-TO components with or without ABA treatment. Scale bars, 10 µm. q, Quantification of the number of endogenous Actb mRNA particles per µm in microfluidic channels. n indicates the number of quantified segments of microfluidic channels for each group. Two-sided, unpaired Student’s t-test. r, Experimental procedures for Fig. 4k,l.
Extended Data Fig. 10 Additional data related to CRISPR-TO screens in primary neurons in Fig. 5.
a-b, Representative microscopic images (a) and quantification (b) showing the low transfection efficiency but high co-transfection efficiency in primary mouse cortical neurons transfected with plasmids expressing CAAX-mScarlet-I, dCas13, KIF5A*-PYL1, and gNT. Scale bar, 200 µm. c, Neurites and cell bodies in the raw image (left) were recognized by the Neurite Outgrowth module embedded in MetaXpress software (right). Scale bar, 200 µm. d, Plate layout for the CRISPR-TO screen in primary mouse cortical neurons. e, Quantification of the neurite length changes over time after adding ABA. Mean ± s.e.m. of different fields of views is shown for each time point. The color bar above each plot of gT group shows the P value of each time point compared with the three gNT groups (Source Data). The three color bars above the last plot show the P value of each time point compared among the three gNT groups. Data represent mean ± s.e.m. from 24 (Mtor, Tmsb4x, Dixdc1, gNT3) or 25 (all the other groups) fields of view per time point. Two-sided, unpaired Student’s t-test.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1–10, Supplementary Notes, Supplementary Methods, Supplementary Tables 1–10, and References.
Supplementary Video 1
Real-time dynamic imaging of MS2 mRNA transported towards the minus ends of microtubules (centrosome) via CRISPR-TO. The video captures the real-time dynamic imaging of MS2 mRNA (red) being transported towards the minus ends of microtubules (centrosome, white arrow) by the motor protein KIFC1*-PYL1 (green) in engineered osteosarcoma U2OS stable cells via CRISPR-TO. The video was recorded 120 seconds after ABA addition. The time stamp indicates the duration of ABA treatment. Scale bar: 20 μm.
Supplementary Video 2
Real-time dynamic tracking of individual MS2 mRNA particles transported towards the centrosome via CRISPR-TO. The video captures the real-time tracking of individual MS2 mRNA particles (red) being transported towards the centrosome (green cluster on the left) along microtubules by the motor protein KIFC1*-PYL1 (green) in engineered osteosarcoma U2OS stable cells via CRISPR-TO. The shape of microtubules is marked by KIFC1*-PYL1. The video was recorded 120 seconds after ABA addition. The time stamp indicates the duration of ABA treatment. Scale bar: 2 μm.
Supplementary Video 3
Real-time dynamic imaging of MS2 mRNA transported towards the plus ends of microtubules via CRISPR-TO. The video captures the real-time dynamic imaging of MS2 mRNA (red) being transported towards the plus ends of microtubules (the leading edge of the cell, white arrows) by the motor protein KIF5A*-PYL1 (green) in engineered osteosarcoma U2OS stable cells via CRISPR-TO. The video was recorded 60 seconds after ABA addition. The time stamp indicates the duration of ABA treatment. Scale bar: 20 μm.
Supplementary Video 4
Real-time tracking of green fluorescence recovery of Dendra2 after UV-conversion in primary mouse cortical neurons. The video captures the real-time dynamic tracking of green fluorescence recovery of Dendra2 after UV-conversion in primary mouse cortical neurons, with Dendra2 reporter mRNA transported to neurite tips by CRISPR-TO. Fluorescence recovery at neurite tips (white arrow) is faster for the ABA group where the reporter mRNA was localized to neurite tips by CRISPR-TO, compared to the DMSO control group. The video was recorded every 5 minutes after UV-conversion. Calibration bar, green fluorescence intensity of Dendra 2. Scale bar: 10 μm.
Supplementary Video 5
Real-time tracking of SINAPS reporter mRNA and translation in primary mouse cortical neurons following CRISPR-TO perturbation. The video captures real-time dynamics of SINAPS reporter mRNA (red) and translated SunTag protein (green) in primary mouse cortical neurons, where SINAPS reporter mRNA was transported to neurite tips by CRISPR-TO. Left, translating reporter mRNAs which colocalize with SunTag protein (white arrow) and exhibit slow movement. Right, untranslating reporter mRNAs not colocalizing with SunTag protein and exhibiting fast movement. Green particles not colocalizing with reporter mRNA are diffused SunTag proteins and display fast movement. Videos were captured at 0.64-second interval after 24-hour ABA treatment. Scale bar, 10 μm.
Supplementary Video 6
Real-time dynamic imaging of morphological changes at neurite tips of primary mouse cortical neurons after CRISPR-TO perturbation. The video captures the real-time dynamic imaging of morphological changes at neurite tips of primary mouse cortical neurons, where endogenous Actb mRNA was transported to neurite tips by CRISPR-TO. The neurite tip in the targeting gRNA (gT) group grew out dynamic protrusions after ABA treatment, while the nontargeting gRNA (gNT) group did not. The video was recorded 11 minutes after ABA addition. The time stamp (hours:minutes) indicates the duration of ABA treatment. Scale bar: 20 μm.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Han, M., Fu, M.L., Zhu, Y. et al. Programmable control of spatial transcriptome in live cells and neurons. Nature (2025). https://doi.org/10.1038/s41586-025-09020-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41586-025-09020-z