Abstract
Identifying highly specific T cell receptors (TCRs) or antibodies against epitopic peptides presented by class I major histocompatibility complex (MHC I) proteins remains a bottleneck in the development of targeted therapeutics. Here, we introduce targeted recognition of antigen–MHC complex reporter for MHC I (TRACeR-I), a generalizable platform for targeting peptides on polymorphic HLA-A*, HLA-B* and HLA-C* allotypes while overcoming the cross-reactivity challenges of TCRs. Our TRACeR–MHC I co-crystal structure reveals a unique antigen recognition mechanism, with TRACeR forming extensive contacts across the entire peptide length to confer single-residue specificity at the accessible positions. We demonstrate rapid screening of TRACeR-I against a panel of disease-relevant HLAs with peptides derived from human viruses (human immunodeficiency virus, Epstein–Barr virus and severe acute respiratory syndrome coronavirus 2), and oncoproteins (Kirsten rat sarcoma virus, paired-like homeobox 2b and New York esophageal squamous cell carcinoma 1). TRACeR-based bispecific T cell engagers and chimeric antigen receptor T cells exhibit on-target killing of tumor cells with high efficacy in the low nanomolar range. Our platform empowers the development of broadly applicable MHC I-targeting molecules for research, diagnostic and therapeutic applications.
Similar content being viewed by others
Main
Immunotherapy is an attractive strategy for treating autoimmunity, cancer and infectious diseases but its success depends on the ability to specifically target diseased cells. It has become increasingly evident that existing immunotherapies would benefit from targeting multiple distinct antigens1, as escape mutations are frequently observed under selective pressure from a singularly targeted treatment2. Monoclonal antibodies (mAbs) are widely used in this context because they can be developed against disease-associated cell surface antigens; however, surface antigens uniquely expressed in diseased cells are sparse3. By contrast, a less explored realm of therapeutically targetable antigens is that derived from intracellular proteins. Cancer cells accumulate a large collection of aberrant protein variants including tumor-associated antigens and neoantigens4; similarly, pathogen-infected cells express intracellular foreign proteins, which could also be leveraged. Presentation of peptides from the intracellular processing of these proteins by the class I major histocompatibility complex (MHC I) offers a natural mechanism to identify diseased cells, and forms the basis for immune surveillance by T cells and adaptive immunity5,6,7. MHC I molecules present 8–12-residue peptides derived from proteasomal and lysosomal degradation of cellular proteins on the cell surface8. The ability to read these cellular state ‘barcodes’ can provide a path to expand the repertoire of targetable cellular antigens9.
Such tools can be developed from donor-derived T cell receptors (TCRs). TCR binding to peptide MHC (pMHC) is achieved through the docking of six combinatorically diversified complementarity-determining region (CDR) loops onto the pMHC I (ref. 10) using a canonical binding topology11 while also allowing for a wide range of binding orientations to account for antigen diversity12,13,14 (Fig. 1a and Supplementary Appendix 1). Current efforts to engineer specific and high-affinity MHC I binders typically implement directed evolution mutagenesis on the CDRs of known TCRs that were isolated from T cells15. However, there are three intrinsic properties of TCRs that are difficult to overcome.
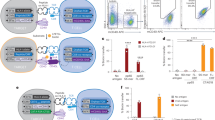
a. Summary of TCR and TCR-mimicking antibodies with pMHC-binding modes (PDB identifiers in Supplementary Appendix 1). TCRs, white ribbons; antibodies, magenta. Two edge-case TCRs are rendered as tan and purple surfaces. b, TRACeR-II platform. The N-terminal flexible region on TRACeR-II binders is defined as the ARE. The ARE loop and the scaffold form a concave surface for MHC II engagement. c, Comparison of the different peptide conformations and TRACeR (in yellow) orientations on MHC I and II. The bulged conformation of MHC I peptides requires a parallel binding mode while MHC II peptides can be engaged in a perpendicular orientation relative to the MHC. d, Schematic of our TRACeR-I development approach. In step 1, global docking was performed with PatchDock-seeded RifDock (Supplementary Appendix 2). In step 2, iterative RosettaDock and FastDesign cycles were performed to generate sequence diversity on the binding interface (Supplementary Appendices 3 and 4). Output models were evaluated by binding energy, binding energy to antigen, shape complementarity, buried unsatisfied hydrogen bonds and contact area (Supplementary Appendix 5). In step 3, the diverse sequence suggested from step 2 was implemented into a combinatorial library (library 1) for screening with FACS. In step 4, library 2 enabled the development of specific TRACeRs for different targets. Peptide-focused MHC I binders bind their cognate antigens but have minimal cross-reactivity with irrelevant pMHCs. Staining concentration, 50 nM tetramer concentration. e, Titration curves of TRACeRs with different monomeric pMHC targets under a concentration range from 0.1 nM to 50 μM. Binding signals are shown as the MFI ± s.d. (n = 3 technical replicates). f, Binding kinetics of TRACeRs determined by BLI. Binding kinetics and fitting quality are summarized in Supplementary Table 2.
First, the development of TCRs from a low-affinity starting point generally involves laborious screening, which is time consuming and resource intensive, often requiring iterative rounds of testing and optimization to achieve the desired affinity and specificity. Second, optimizing a TCR’s specificity is challenging because of its structural mechanism. The inherent polyspecificity of TCRs16, which enables them to recognize a wide array of peptides through flexible CDR loops and diverse docking angles, is an essential feature to enable immune surveillance of the prodigious possible set of epitopes derived from emerging pathogens using a relatively limited TCR repertoire17,18,19. However, this cross-reactivity, which is beneficial in a physiological context, presents challenges for using TCRs as highly specific therapeutic agents. A given TCR interacts with a limited number of exposed ‘hotspot’ residues without engaging the full length of the peptide target, which generally adopts a bulged structure with the N and C termini buried into the MHC I groove20,21 (Fig. 1a). Lastly, the human leucocyte antigen (HLA) genes encoding human MHC I peptides are highly polymorphic, with more than 38,000 known allotypes22,23. As a result, a TCR that has been identified to bind a specific antigen or MHC target is restricted to recognize a narrow set of HLA allotypes, limiting wide adaptation across persons of diverse genetic backgrounds.
Adopting TCRs across persons of different HLA genotypes is not only challenging but also has the risk of off-target alloreactivity24. Selection of mAbs with greater CDR diversity than TCRs has been proposed to overcome some of these limitations25,26,27. However, unlike TCRs, which have the capacity to recognize soluble proteins in principle but are generally restricted to recognize peptides in the context of the MHC I molecules because of thymic selection, antibodies are not biased toward epitopes displayed by MHC I (ref. 28). As a result, ensuring a peptide-focused binding mode during the selection process is challenging and engineered antibodies may deviate substantially from the optimal antigen-focused binding orientation (Fig. 1a, magenta). These factors render the engineering of TCRs and mAbs into robust targeting agents a daunting task29,30,31. Although achievable32, creating these biologics requires careful consideration of each pMHC I target separately, limiting the streamlined development of targeting modalities.
From a molecular engineering perspective, we postulated that there are alternative strategies that can be unlocked by protein design and directed evolution to target MHC I antigens, beyond the established TCR or antibody framework. We made the observation that, despite the large sequence diversity of presented peptides, they only adopt a limited set of discrete backbone conformations20. Furthermore, our previous structure-based analysis revealed conserved MHC I surface features that can be leveraged to achieve a generalizable docking mode that covers most common HLA allomorphs33. These structural traits point to a possible invariant docking orientation that binders can leverage to interact with MHC I, simplifying the antigen recognition problem to a confined sampling space. Here, we set out to develop a de novo platform to generate pMHC I binders that implements the following features: an invariant docking mode covering the full span of the antigen and that has compatibility with HLA-A*, HLA-B* and HLA-C* allotypes, a malleable surface adaptable to the physiochemistry of a wide variety of peptides to define high-level specificity and a robust system that can cost-effectively produce binders in a matter of days. This platform, which we name targeted recognition of antigen–MHC complex reporter for MHC I (TRACeR-I), exhibits a binding mode that, to our knowledge, does not exist in nature. We used crystallography to reveal a set of eight residues that offer a solution to the challenging pMHC I recognition problem. This simplistic approach allows the rapid and facile creation of peptide-focused pMHC binders for a wide range of antigens. Proof-of-concept implementation into T cell-activating modalities showed highly specific on-target killing. Our platform provides a robust, multiallelic approach to ultimately enable the de novo development of diagnostic and therapeutic modalities that can cover the breadth of naturally occurring pMHC I antigen repertoires.
Results
Exploring an unconventional MHC I recognition mode
To develop an invariant binding mode to engage pMHC I, the choice and design of a suitable scaffold are essential. We drew inspiration from our peptide-focused pMHC II-binding platform, TRACeR-II (ref. 34), based on redesigning the superantigen Mycoplasma arthritidis mitogen (MAM), to develop this MHC I counterpart (Fig. 1b). In the TRACeR-II design scheme, the N-terminal flexible loop (residues 1–20), which we called the antigen recognition element (ARE), functions as a single CDR equivalent to achieve specific target recognition. Despite their overall fold similarity, MHC I and II molecules are sufficiently divergent that TRACeR-II binders cannot cross-react with MHC I. The most distinctive difference is in the conformation of the peptide. MHC I peptides generally adopt bulged conformations with a convex antigen surface, in contrast to MHC II’s extended peptide structures (Fig. 1c). TRACeR-II consists of a small helical bundle scaffold with an unusual surface loop, creating a convenient concave surface, like a grappling hook, that docks perpendicularly to the peptide-binding groove (Fig. 1b). We sought to exploit this unique structural feature to accommodate the bulged peptide conformation and repurpose the scaffold to be compatible with MHC I. However, the bulged peptide on MHC I necessitates that the scaffold should engage the peptide-binding groove in a parallel orientation because the accessible ledge on MHC II becomes obstructed by the peptide. (Fig. 1c, surface-rendered protein in yellow).
The adaptation to MHC I targets is best optimized through library-based engineering. Specifically, we aimed to mutate the TRACeR-II scaffold to create an interface to engage MHC I antigens through combinatorial library screening. We used computational modeling to explore different docking orientations to guide the library design35 (workflow summarized in Fig. 1d; scripts in Supplementary Appendices 2–5). Starting from an available pMHC I crystal structure to guide the modeling and design (Protein Data Bank (PDB) 1S9W (ref. 36)), we used results from a low-resolution docking algorithm, Patchdock37, to seed the search trajectories of a high-resolution algorithm, RifDock35. A total of 30 models from RifDock were designed using the Rosetta modeling suite38,39,40. The selected interface positions and their corresponding sequences were integrated to create a combinatorial library using Swiftlib41, which optimizes degenerate codon coverage by taking into account a given set of allowed amino acids at each position. From this combinatorial library (library 1), we isolated a sequence that is compatible with the MHC I surface using yeast surface display with fluorescence-activated cell sorting (FACS) (Supplementary Table 1 and Supplementary Fig. 1).
ARE provides a malleable element that forms the concave antigen recognition surface, as demonstrated by the complete loss of binding upon deletion of this region (Supplementary Fig. 2). Therefore, we created an antigen-directed library (library 2) by keeping constant the scaffold but maximally diversifying the combinatorial sequence in a subset of positions within the ARE region. While we adopted the term ‘ARE’ from the TRACeR-II system to refer to the specific loop region, it is not meant to serve as a direct ARE in the MHC I-binding context. We used library 2 to optimize the binding mode and accommodate an expanded pMHC I target set. We randomized seven positions within the ARE (residues 10–16), which makes closest contact with the pMHC I target as suggested by the docking models, using NNK degenerate codons and screened the library against three divergent pMHC I targets: HLA-A*02:01/NY-ESO-1 (New York esophageal squamous cell carcinoma 1)157–165(V) (SLLMWITQV), HLA-A*02:01/EBV (Epstein–Barr virus) latent membrane protein 2 (LMP2)426–434 (CLGGLLTMV) and HLA-B*08:01/SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) M protein148–156 (HLRIAGHHL). We included here MHCs covering the divergent A*02 and B*08 supertypes42, in addition to the peptides, to test the scaffold’s potential compatibility with different MHC I surfaces. Specifically, HLA-B*08:01 has a distinct preference for peptides with charged residues (arginine or lysine) at position P5 and a different TCR-interacting surface compared to HLA-A*02:01 (ref. 43) (Supplementary Fig. 3). Because the NY-ESO-1 and EBV epitopic peptides share the same HLA-A*02:01 restriction, achieving specific and exclusive binding to each of them would suggest the presence of a peptide-focused binding interface, in which the shared HLA background does not dominate the combined pMHC I specificity. With this setup, we carried out four rounds of sorting, followed by next-generation sequencing (NGS) to identify the most enriched clone for each target (Supplementary Fig. 4). We achieved peptide-focused binding to all three targets with minimal cross-reactivity up to 50 µM pMHC I monomer concentration as probed by TRACeRs expressed on the yeast cell surface (Fig. 1d,e; hereafter, we refer to an individual binder as a TRACeR, and the platform as TRACeR-I). The ability to generate targeting moieties for peptides presented by divergent HLA allotypes indicated a plausible path for creating a generalized, multiallelic targeting platform.
In the TRACeR-II design, we introduced a disulfide anchor to restrict the flexibility of the ARE. We observed that the presence of the disulfide remains crucial for TRACeR-I and substituting the involved cysteines to serines abolished all binding signals on yeast (Supplementary Fig. 2). To avoid intermolecular disulfide crosslinking during the recombinant protein purification, we also introduced a circular permutation scheme that shifts the disulfide-stabilized flexible region to an internal section of the protein, leading to a protein structure with different connectivity but similar overall 3D shape (Supplementary Fig. 5). We expressed the circular-permuted sequences for each target as recombinant proteins to perform more detailed characterization. All designs were soluble and readily purifiable by Ni-NTA and size-exclusion chromatography (SEC) in a homodimeric form, which retained their pMHC I-binding function. The binding affinity for each target was assessed by biolayer interferometry (BLI) with KD values ranging from 22 nM to 3.70 µM (Fig. 1f, Supplementary Fig. 6 and Supplementary Table 2). The circular dichroism (CD) spectra denoted properly conformed, stable helical structures with melting temperatures of approximately 60 °C (Supplementary Fig. 7).
Defining the breadth of peptide-binding specificity
As an initial assessment for potential cross-reactivity with unrelated peptides, we first used a library of 50 HLA-A*02:01 tetramers, presenting a range of epitopic peptides previously identified from an analysis of The Cancer Genome Atlas (TCGA) peptide library44. We observed no cross-reactivity for any TRACeR with this irrelevant peptide library (Supplementary Fig. 8). Then, we focused on our binder for HLA-A*02:01/NY-ESO-1157–165(V) as a model system to further evaluate the TRACeR-I platform. NY-ESO-1 is a well-known cancer testis antigen that is expressed in a variety of tumors, including the rare and aggressive soft-tissue cancers, myxoid round cell liposarcoma (94%) and synovial sarcoma (70%)45. NY-ESO-1157–165(V) (SLLMWITQV) is a heteroclitic peptide of wild-type NY-ESO-1 (SLLMWITQC), as described previously46. Multiple TCRs and TCR-like antibodies have been developed for this target47,48,49,50, allowing for a systematic comparison to TRACeR-I.
To further explore the breadth of specificity of our binder and to obtain a low-resolution mapping of the ternary complex interface, we performed site-saturated mutational (SSM) scans on the NY-ESO-1 epitopic peptide using fluorescently labeled MHC I tetramers prepared using our previously developed chaperone-mediated peptide exchange methodology51,52. This system also allowed us to confirm the loading of each peptide to yield stable pMHC I complexes, as confirmed by differential scanning fluorimetry (Supplementary Table 3 and Supplementary Appendix 6), as opposed to cell-based peptide pulsing assays. We first substituted each of the eight nonanchor peptide residues to alanine to define the overall TRACeR footprint (Fig. 2a). We observed substantially reduced binding signals using MHC I tetramers carrying the S1A, M4A, W5A and T7A peptide substitutions, while binding to other variants remained unchanged (Fig. 2b,c). This readout is consistent with the peptide conformation in the crystal structure of the HLA-A*02:01/NY-ESO-1 complex (PDB 1S9W) where residues 1, 4, 5 and 8 are the solvent-facing positions and residue 7 is partially solvent exposed. The sensitivity to alanine point substitutions suggests that \({\rm{TRACeR}}_{\rm{MHC}\;{\rm{I}},\,{\rm{A02}}}^{{\rm{NY}}-{\rm{ESO}}-1}\) functions as a molecular imprinted binder, requiring the coordination of multiple peptide residues to fit its binding interface. To further validate this hypothesis, we independently tested all 19 other possible substitutions at each of the solvent-exposed residues and compared the specificity pattern to the established TCR 1G4 (ref. 50), consistent with prior reports53 (Fig. 2d). \({\rm{TRACeR}}_{\rm{MHC}\;{\rm{I}},\,{\rm{A02}}}^{{\rm{NY}}-{\rm{ESO}}-1}\) exhibits markedly higher specificity on peptide residue 1 (P1; this notation is used for peptide residues hereafter), indicating a distinct binding mechanism that allows atypical access to the peptide’s N terminus, which is buried in the MHC I groove. At P1, we observed cross-reactivity when the native serine was substituted with threonine, suggesting a likely hydrogen-bonding interaction to those residues. Overall, \({\rm{TRACeR}}_{\rm{MHC}\;{\rm{I}},\,{\rm{A02}}}^{{\rm{NY}}-{\rm{ESO}}-1}\) was more specific for P1 and P4, while 1G4 TCR was more selective for P7 and P8. Both were highly specific for the known TCR tryptophan ‘hotspot’ at P5, similarly to other previously developed TCRs14. When we expanded the peptide saturation mutagenesis screening to random combinations of triplets (84 × 203; 672,000 variants) using pMHC I expression by yeast surface display54,55 (Supplementary Fig. 9), the enriched per-position residue frequencies recapitulated our MHC I tetramer SSM results (Fig. 2e and Supplementary Fig. 10). The top 50 most enriched peptide sequences from the triplet scanning experiment revealed different peptide recognition patterns between 1G4 and \({\rm{TRACeR}}_{\rm{MHC}\;{\rm{I}},\,{\rm{A02}}}^{{\rm{NY}}-{\rm{ESO}}-1}\), as analyzed by clustering the cross-reactive peptide sequences based on their BLOSM62 transition frequencies56. At a hierarchical clustering threshold distance of 75, the peptide sequences formed five clusters and those favoring TRACeR versus TCR were distinct, with TRACeR binding sequences in cluster 3 being most similar and closer than TCR-binding sequences to the wild-type NY-ESO-1157–165(V) (Fig. 2f), further supporting the presence of a cognate peptide-defined interface on \({\rm{TRACeR}}_{\rm{MHC}\;{\rm{I}},\,{\rm{A02}}}^{{\rm{NY}}-{\rm{ESO}}-1}\).
a, Structure of MHC-presented NY-ESO-1 peptide. Residue positions in yellow are solvent exposed and those in blue have side chains buried in the MHC I groove. T7 is partially solvent exposed. b, Alanine scan of the NY-ESO-1 peptide showing that TRACeR binding is sensitive to S1, M4, W5 and T7 substitutions, representing four of five solvent-exposed positions. The binding signal is shown as the MFI ± s.d. (n = 3 technical replicates). c, Binding signal for alanine scan substitutions on flow cytometry. d, MHC I tetramer SSM on the five key peptide residues responding to TRACeR (top) and 1G4 TCR (bottom). The S1V peptide failed to synthesize by the vendor (filled with gray). e, Normalized enrichment ratio showing enriched peptide sequences binding to TRACeR (top) and 1G4 TCR (bottom) from the randomized triplet library (n = 3 technical replicates). f, Clusters of the combined top 50 peptide sequences binding to TRACeR and 1G4 (based on enrichment ratio). Average distance of NY-ESO-1 peptide to each cluster: cluster 1, 33.5; cluster 2, 33.8; cluster 3, 24.9; cluster 4, 32.2; cluster 5,30.7. g, In vitro tumor cell cytotoxicity assays with \({\rm{TRACeR}}_{\rm{MHC}\;{\rm{I}},\,{\rm{A02}}}^{{\rm{NY}}-{\rm{ESO}}-1}\) anti-CD3 BiTEs. The percentage of live tumor cells was normalized to PBS-treated control cells (n = 6 replicates using T cells from two independent donors).
As not all substituted peptide sequences are biologically relevant, we further examined possible cross-reactive peptides identified through two complementary approaches. First, to evaluate cases of cross-reactivity through structural mimicry, we used our established method and database of pHLA structures to search for HLA-A*02:01-restricted epitopes, with a similar peptide backbone conformation and identical hotspot residues to NY-ESO-1, with serine, threonine or cysteine for P1, methionine for P4 and tryptophan for P5. HLA-A*02:01/CINMWCWTV (PDB 3MRI) was the closest match to the search pattern and it had a peptide backbone root-mean-square deviation of 1.47 Å to NY-ESO-1 (Supplementary Fig. 11). \({\rm{TRACeR}}_{\rm{MHC}\;{\rm{I}},\,{\rm{A02}}}^{{\rm{NY}}-{\rm{ESO}}-1}\) did not bind to this possible cross-reactive peptide, despite the high similarity of the peptide conformation and hotspot residues. In addition, we examined peptides expresses in normal tissue from the HLA Ligand Atlas (https://hla-ligand-atlas.org) using a more extensive sequence-based search that further allowed for leucine, methionine, glutamine or threonine at P4. Using this process, we identified five possible cross-reactive peptides (KQIQWFWQV, YMDQWVPVI, NLMQWVPSL, SLAQWGASV and TLQTWLVQA) and showed that none of these could be recognized by our binder in analogous tetramer staining experiments (Supplementary Fig. 11).
\({\mathbf{TRACeR}}_{{\mathbf{MHC}}\;{\mathbf{I}},\,{\mathbf{A02}}}^{{\mathbf{NY}}-{\mathbf{ESO}}-{\mathbf{1}}}\) demonstrates tolerable immunogenicity and on-target cytotoxicity against tumor cells
The specific pMHC I recognition properties of TRACeR-I suggest that it can be used as a binding module for a variety of targeting applications, including therapeutic modalities. Given that TRACeRs are engineered from a protein of bacterial origin, there is the possibility that they may elicit immune responses or harbor cytotoxicity. We characterized the immunogenic profile of TRACeRs in vivo by treating mice with \({\rm{TRACeR}}_{\rm{MHC}\;{\rm{I}},\,{\rm{A02}}}^{{\rm{NY}}-{\rm{ESO}}-1}\) and compared them to mice treated with mouse IgG (mIgG) or human IgG (hIgG). Mice were treated with a frequent dosing regimen (13 intravenous injections over 30 days), such that mice would have continuous access to \({\rm{TRACeR}}_{\rm{MHC}\;{\rm{I}},\,{\rm{A02}}}^{{\rm{NY}}-{\rm{ESO}}-1}\) antigen over the testing period. Treated animals developed significantly lower antibody titers against \({\rm{TRACeR}}_{\rm{MHC}\;{\rm{I}},\,{\rm{A02}}}^{{\rm{NY}}-{\rm{ESO}}-1}\) than hIgG. Notably, \({\rm{TRACeR}}_{\rm{MHC}\;{\rm{I}},\,{\rm{A02}}}^{{\rm{NY}}-{\rm{ESO}}-1}\)-specific antibodies could only be detected after 4 weeks, while antibodies against hIgG could be detected as early as 2 weeks, suggesting only a very mild immunogenic response (Supplementary Fig. 12a). Moreover, none of the mice developed any weight loss, which would be expected if our \({\rm{TRACeR}}_{\rm{MHC}\;{\rm{I}},\,{\rm{A02}}}^{{\rm{NY}}-{\rm{ESO}}-1}\) retained mitogenic activity (Supplementary Fig. 12b). To further investigate whether any mitogenic activity57 could be detected and to characterize the antigen-specific response, splenocytes were isolated for analysis of CD4:CD8 ratios, T effector and memory phenotypes, changes in Vβ TCR repertoire and degree of anergy or exhaustion (Supplementary Fig. 12c–i). Overall, we did not detect notable differences in T cell populations in animals treated with \({\rm{TRACeR}}_{\rm{MHC}\;{\rm{I}},\,{\rm{A02}}}^{{\rm{NY}}-{\rm{ESO}}-1}\). Lastly, to quantify the degree of antigen-specific responses, \({\rm{TRACeR}}_{\rm{MHC}\;{\rm{I}},\,{\rm{A02}}}^{{\rm{NY}}-{\rm{ESO}}-1}\)-treated, control IgG-treated and naive splenocytes were incubated overnight with whole recombinant protein (Supplementary Fig. 13). We detected no antigen-specific response or any residual mitogenic activity arising from the \({\rm{TRACeR}}_{\rm{MHC}\;{\rm{I}},\,{\rm{A02}}}^{{\rm{NY}}-{\rm{ESO}}-1}\) MAM scaffold in these assays nor any notable changes in cell activation or cytokine production from splenocytes isolated from \({\rm{TRACeR}}_{\rm{MHC}\;{\rm{I}},\,{\rm{A02}}}^{{\rm{NY}}-{\rm{ESO}}-1}\)-treated mice and compared to control IgG-treated mice. These data show a tolerable TRACeR-I immunogenicity profile in animals, further supporting its potential for developing both soluble and cell-based therapeutics.
Next, to show proof of concept that TRACeR-I can be deployed in a therapeutic context as a biologic against cancer, we implemented \({\rm{TRACeR}}_{\rm{MHC}\;{\rm{I}},\,{\rm{A02}}}^{{\rm{NY}}-{\rm{ESO}}-1}\) in a bispecific T cell engager (BiTE) format, in which the TRACeR was fused to a humanized anti-CD3 single-chain antibody fragment58. We tested the ability of \({\rm{TRACeR}}_{\rm{MHC}\;{\rm{I}},\,{\rm{A02}}}^{{\rm{NY}}-{\rm{ESO}}-1}\) to elicit T cell-mediated killing of several patient-derived diffuse large B cell lymphoma (DLBCL) cell lines characterized by physiologically low MHC antigen density59. To test for on-target killing, we used DLBCL lines expressing combinations of HLA-A*02:01 and/or NY-ESO-1. T cells incubated with the TRACeR BiTE constructs upregulated CD69 and 4-1BB, suggesting effective T cell activation (Supplementary Fig. 14). Effective on-target killing was observed in both HBL-1 and HLY-1 cell lines, which natively express both HLA-A*02:01 and NY-ESO-1, with a 50% effective concentration (EC50) of approximately 75 nM). We confirmed a lack of off-target killing of SUDHL4 cells lacking the epitopic peptide (HLA-A*02:01+NY-ESO-1−) or SUDHL5 cells lacking the HLA allotype (HLA-A*02:01−NY-ESO-1−) (Fig. 2g). This inability of TRACeR anti-CD3 BiTEs to induce off-targeting killing of SUDLH4 and SUDHL5 cells was not because of intrinsic resistance to cell-mediated killing, as these cells were potently killed by anti-CD19 and anti-CD3 BiTEs (Supplementary Fig. 15). Our findings highlight that TRACeR anti-CD3 BiTEs selectively kill target cells only when the epitopic peptide and MHC are matched to the TRACeR’s specificity. These results demonstrate that TRACeRs can be deployed in a BiTE format with highly specific target recognition to induce potent T cell activation and on-target tumor cell killing with low nanomolar-rage efficacy.
Cocrystal structure of the \({\mathbf{TRACeR}}_{{\boldsymbol{MHC}}\;{\boldsymbol{I}},\,{\boldsymbol{A02}}}^{{\boldsymbol{NY}}-{\boldsymbol{ESO}}-{\boldsymbol{1}}}\)–HLA-A*02:01/NY-ESO-1 complex reveals a unique pMHC I recognition mechanism
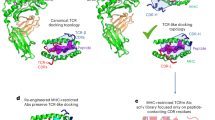
To investigate the origin of TRACeR specificity, we determined the co-crystal structure of \({\rm{TRACeR}}_{\rm{MHC}\;{\rm{I}},\,{\rm{A02}}}^{{\rm{NY}}-{\rm{ESO}}-1}\) and HLA-A*02:01/NY-ESO-1 at 2.3-Å resolution (Supplementary Table 4). In the asymmetric unit, \({\rm{TRACeR}}_{\rm{MHC}\;{\rm{I}},\,{\rm{A02}}}^{{\rm{NY}}-{\rm{ESO}}-1}\) forms a ___domain-swapped dimer interacting with two pMHC I molecules (Fig. 3a and Supplementary Video 1). This ___domain-swapped dimer is the native state and not a crystallization artifact, as suggested by the 2:2 stoichiometric complex during complex purification (Supplementary Fig. 16). The dimer results from swapping two of the three helices in the scaffolding helical bundle. Despite the ___domain swapping, each half of the resulting helical bundle showed good agreement with the equivalent regions in the wild-type MAM structure (Supplementary Fig. 17a). The binding mode also aligned with the general orientation suggested by RifDock predictions (Supplementary Fig. 17b). The two TRACeR subunits are nearly perpendicular to each other, with each half resembling two interlocking U-shaped clamps (the variable ARE region at the base turn of the ‘U’ and helical bundle portions as the arms). This spatial arrangement positions the AREs outward relative to the dimer core, creating a cradling binding pocket for the epitopic peptide in conjunction with the MHC I-facing residues on the TRACeR scaffolding helical bundle (Fig. 3b). In the co-crystal structure, the ARE regions of the dimeric \({\rm{TRACeR}}_{\rm{MHC}\;{\rm{I}},\,{\rm{A02}}}^{{\rm{NY}}-{\rm{ESO}}-1}\) turn into five-turn ɑ-helices that act as keystones in supporting the wedge between the remaining stacking helices from TRACeR and MHC I (Supplementary Fig. 18). The C terminus of the ARE ɑ-helix gains access to the peptide N terminus through an opening between the projection angles of the ɑ1 and ɑ2 helices (Fig. 3b).
a, The asymmetric unit of \({\rm{TRACeR}}_{\rm{MHC}\;{\rm{I}},\,{\rm{A02}}}^{{\rm{NY}}-{\rm{ESO}}-1}\) engaging two pMHC I molecules. Domain-swapped TRACeR, yellow and green; MHC I heavy chain, gray; MHC I light chain, orange; NY-ESO-1 peptide, cyan. b, \({\rm{TRACeR}}_{\rm{MHC}\;{\rm{I}},\,{\rm{A02}}}^{{\rm{NY}}-{\rm{ESO}}-1}\) helices binding to pMHC I, with the ___location of the ARE. c, Footprint of \({\rm{TRACeR}}_{\rm{MHC}\;{\rm{I}},\,{\rm{A02}}}^{{\rm{NY}}-{\rm{ESO}}-1}\) on pMHC I. pMHC I interface atoms within 5-Å distance from TRACeR, red; small hydrophobic residues on MHC groove that TRACeR docks on, yellow. Sequence conservation for the TRACER-contacting MHC I residues on common HLA alleles are shown as a sequence logo. d, Open-book view of MHC I–TRACeR interface showing a high level of shape complementarity. e, The interacting residues between \({\rm{TRACeR}}_{\rm{MHC}\;{\rm{I}},\,{\rm{A02}}}^{{\rm{NY}}-{\rm{ESO}}-1}\) and NY-ESO-1 peptide. TRACeR residues, green and yellow; peptide, cyan. f, Refined set of interfacial residue positions to achieve better specificity for P7 and P8. Positions included in the original library containing AREs in library 2 are shown as yellow spheres; new positions are shown in salmon. g, SSM on the five key peptide residues corresponding to the refined TRACeR specificity. The S1V peptide failed to synthesize and was not included in the library (filled with gray).
The crystal structure shows a unique binding mechanism that allows \({\rm{TRACeR}}_{\rm{MHC}\;{\rm{I}},\,{\rm{A02}}}^{{\rm{NY}}-{\rm{ESO}}-1}\) to form extensive complementary contacts throughout the entire length of the peptide, leaving few specific side-chain contacts and mostly ‘knobs-into-holes’ backbone interactions with the MHC I helices (Fig. 3b–d). As a result, the sequence specificity determinants of the TRACeR–pMHC I interaction largely arise from the peptide surface of the MHC I structure (Fig. 3b,c). Notably, for its interactions with MHC I, \({\rm{TRACeR}}_{\rm{MHC}\;{\rm{I}},\,{\rm{A02}}}^{{\rm{NY}}-{\rm{ESO}}-1}\) takes advantage of openings on the MHC ɑ1 and ɑ2 helices where either splayed side chains or alanines and glycines are present; the helices from TRACeR are docked along these specific locations with the closest contacts (Cα–Cα within 6 Å) being G62MHC, A150MHC, A158MHC and G162MHC (Fig. 3c and Supplementary Video 2). Furthermore, these three TRACeR helices also stack complementary to the unique ridge formed by the ɑ2 helices on the MHC to meet the peptide-presenting face of the MHC I structure (Fig. 3d). The interface with the MHC I helices is mediated through shape complementarity with a set of residues that are relatively oligomorphic among different HLAs (Fig. 3c, sequence logo, and Supplementary Fig. 17c), further supporting our finding that individual TRACeR binders can accommodate peptides restricted by divergent HLA allotypes (Fig. 1d).
Because the peptide in MHC I adopts a bulged conformation, interactions leading to peptide-focused recognition comprise a hydrogen-bonding network of both side-chain-mediated and backbone-mediated contacts. For NY-ESO-1, five positions (P1, P4, P5, P7 and P8) have accessible side chains and every residue except for P1, P7 and P9 has at least one available backbone hydrogen-bonding donor or acceptor to be accounted for (the P1, P7 and P9 backbone amide and carbonyl groups are already hydrogen-bonded to MHC groove residues). In making the side-chain-specific contacts, a hydrogen-bonding triad mediated by N76TRACeR confers the specificity to S1peptide on the N-terminal side of the peptide and the same geometry tolerates S1Tpeptide substitution. For M4peptide and W5peptide selectivity, several TRACeR residues form a hydrophobic pocket to accommodate the P4 and P5 hotspot residues: F13, L16, W17, F20, Y72, F80 and Y112 (Fig. 3e and Supplementary Video 3). For interactions with the peptide backbone, P2 carbonyl is involved in a hydrogen-bonding network involving N76TRACeR, P3 amide is satisfied by Y72TRACeR; P4 amide is satisfied by Y72TRACeR and its carbonyl is satisfied by Y112TRACeR, P5 and P6 backbones are solvated and P8 amide is satisfied by a water-mediated hydrogen bond involving N10TRACeR (Supplementary Video 4). For reference, the peptide-contacting residues for the 1G4 TCR are shown in Supplementary Fig. 19b. A comparison between the two interfaces (Fig. 3e and Supplementary Fig. 19) provides the rationale behind the observed binding specificity patterns from our SSM results in Fig. 2d. Although \({\rm{TRACeR}}_{\rm{MHC}\;{\rm{I}},\,{\rm{A02}}}^{{\rm{NY}}-{\rm{ESO}}-1}\) can cover the entire length of the peptide, the residues that would have provided specific interactions for T7antigen and N8antigen were not sampled in library 2, from which this binder was isolated, because only the ARE region was diversified in the original library.
The X-ray structure allowed us to define a more focused set of residues in the TRACeR binding interface to optimize contacts with T7 and N8 on the NY-ESO-1 antigen. A new round of selection was performed on a library that included eight TRACeR positions defining a peptide selectivity ‘box’ (9, 13, 17, 20, 72, 76, 80 and 112) (Fig. 3f), as all amino acids but cysteine yielded enhanced specificity to P7 and P8. We found that the substitution of aromatic residues with aspartates allowed a new interfacial sequence (DDWGYNYY, changed from FFWFYNFY) to significantly improve the binding specificity with respect to T7antigen and N8antigen, as assessed using our pMHC tetramer-based assay (Fig. 3g and Supplementary Fig. 20).
Monomeric TRACeR engineering for compatibility with chimeric antigen receptor T cells (CAR-T) and broad HLA generalizability
While the ___domain-swapped dimer TRACeR is highly stable, we sought to develop a monomeric construct to expand the compatibility of our platform with other applications such as CAR-T. Using the crystal structure as a guide, we identified a scheme to rewire the ___domain-swapped dimer into a monomer comprising two connected three-helix bundles (Fig. 4a,b and Supplementary Fig. 21). The resulting monomeric NY-ESO-1 binder retained high affinity (KD = 114 nM), as determined by BLI, and was compatible with CARs (monomer specificity, Supplementary Fig. 22; CAR-T, Fig. 4c and Supplementary Fig. 23; BLI, Fig. 4d and Supplementary Table 5). CAR-T studies using primary T cells from multiple donors consistently showed highly specific on-target killing of HBL-1 and HLY-1 cell lines, in agreement with our results using soluble BiTEs in Fig. 2g (Supplementary Fig. 24).
a, Rewiring scheme to connect ___domain-swapped TRACeR into a monomer. b, Model of rewired TRACeR on MHC I. c, In vitro tumor cell cytotoxicity assays with \({\rm{TRACeR}}_{\rm{MHC}\;{\rm{I}},\,{\rm{A02}}}^{{\rm{NY}}-{\rm{ESO}}-1}\)CAR-T cells derived from primary CD8+ T cells from two donors. Percentage of live tumor cells cocultured with engineered T cells were normalized to those cocultured with untransduced T cells. Each data point represents technical replicates from five experiments, plotted as the mean ± s.d. d. BLI affinity measurements of the rewired \({\rm{TRACeR}}_{\rm{MHC}\;{\rm{I}},\,{\rm{A02}}}^{{\rm{NY}}-{\rm{ESO}}-1}\). e, Open-book view of the TRACeR interface with library positions colored in salmon and the peptide colored in cyan. f, TRACeR-I binding to a panel of diverse disease-relevant epitopic peptides presented by diverse HLAs. The final enrichment round before NGS is shown; multiple sequences were present in the pool. Staining concentration: on target, 25 nM tetramer concentration; off target, 100 nM tetramer concentration. g, Rosetta models of the most enriched TRACeR clone from NGS for each pMHC I target.
We observed that F80TRACeR and Y112TRACeR are spatially opposite to G62MHC and A69MHC, respectively; thus, by allowing F80TRACeR and Y112TRACeR to randomize in a combinatorial library, it may allow TRACeRs to adapt further to MHC allelic variations. The successful enhancement in specificity for the dimeric TRACeR confirmed that this interface extensively interacts with the peptide antigen and can be further modified to fine-tune this interaction. Therefore, as we moved forward to generate individual TRACeRs against a broader panel of pMHC I targets using the redesigned monomeric TRACeR scaffold, we varied the equivalent box of eight residues, assuming that the ___domain-swapped (dimeric) and monomeric TRACeRs would adopt the same pMHC I binding mode (mapping of residues: 9→152, 13→156, 17→160, 20→163, 72→96, 76→100, 80→104 and 112→136, stated as the residue index in dimer construct→residue index in the monomer construct) (Fig. 4e). To explore divergent allelic and antigen compatibilities, we chose a wide array of disease-relevant epitopic peptides presented by common, divergent HLA allotypes, including HLA-A*11:01/KRAS-G12V (VVVGAVGVGK)60, HLA-A*24:02/HIV Nef (RYPLTFGWCF)61 and HLA-C*07:02/PHOX2B (QYNPIRTTF)25, in addition to the three peptides used previously, HLA-A*02:01/NY-ESO-1, HLA-B*08:01/SARS-CoV-2 M and HLA-A*02:01/EBV LMP2. After a single round of binding with magnetic beads and four rounds of FACS (Supplementary Fig. 25), we enriched pooled TRACeR sequences with on-target binding signals and no cross-reactivity across the full panel of targets, as shown using orthogonal pMHC I tetramer staining experiments (Fig. 4f, Supplementary Fig. 26 and Supplementary Table 6). The most enriched single clone for HLA-A*11:01/KRAS-G12V could discriminate the point substitution from wild-type KRAS while that for HLA-C*07:02/PHOX2B could differentiate the R6A point substitution of the key hotspot residue62 (Supplementary Fig. 27). Lastly, a more extensive SSM study using a library of KRAS/A11 tetramers prepared using our chaperone-mediated peptide exchange methodology51 revealed a highly specific antigen recognition pattern (Supplementary Fig. 28), further supporting that the specificity of our platform is maintained across TRACeRs developed for divergent antigens.
These results together depict several important aspects about the TRACeR platform. Firstly, peptide selectivity could be achieved through a minimalist set of box residues in an invariant binding mode. For each pMHC I target, the interfacial residues formed a distinct binding pattern, showing clear complementarity with the physiochemical features of the peptide in structural models (Fig. 4g). Secondly, with a predefined binding mode, sequences could converge efficiently in a few rounds of yeast selection steps to reach on-target specificity. This process was rapid and a full panel of specific binders could be isolated in a matter of days. Thirdly and most importantly, the platform is compatible with common HLA-A*, HLA-B* and HLA-C* allotypes, without requiring any additional modification. Further analysis comparing the sequence variance in the 24 MHC I residues located within 5 Å to TRACeR in our cocrystal structure (Fig. 3c, all yellow and red positions) for (1) all HLA alleles included in our set and (2) all common alleles in the US population (>0.05% allelic frequency in the Be The Match database; https://my.bethematch.org/) revealed a highly similar sequence pattern (Supplementary Fig. 29). This analysis showed that the TRACeR contacts observed in our X-ray structure are located over oligomorphic regions on HLAs and that the polymorphic positions are well accommodated by the TRACeR-I platform. Thus, our TRACeR-I platform provides a toolkit for deriving binding modalities that can cover a wide range of HLA allotypes, enabling applications across different population groups.
Discussion
Our TRACeR-I platform unlocks the potential for targeting disease-associated pMHC I antigens. This setup overcomes several inherent challenges that have limited the broader development of pMHC I-targeting modules. Considering the high peptide-focused specificity, broad HLA allelic compatibility and accelerated and simpler development attainable through TRACeR-I, we present tools to greatly expand the accessibility of targetable pMHC I antigens. Highly desirable features for future therapeutics, such as broad populational coverage, multiantigen targeting for enhanced specificity and addressing antigen-related escape mechanisms, can potentially be implemented by leveraging our platform.
As a proof of principle, we developed an NY-ESO-1/A02-specific binder into BiTEs and CAR-T proof-of-concept modalities and observed desired functional outcomes that are compatible with both delivery modalities. Affinity tuning for optimal function can be achieved through a library selection process that produces binders with a broad range of affinities. Thus, TRACeRs can be adapted for optimal performance in a range of settings. There are, however, several limitations of the study that warrant further investigation. The first is to address any remaining immunogenicity concerns during preclinical development, given the bacterial superantigen origin of our scaffold. In our animal studies, we observed tolerable antidrug antibody and T cell responses when administering with high levels of proteins and saw no systemic T cell activation (Supplementary Figs. 12 and 13). However, further studies using animal models, human mixed cell cultures and organoid-based systems are required to conclusively confirm the immunogenicity profile of our molecules. Second, our redesigned monomeric constructs generally have lower binding affinity than their ___domain-swapped dimeric counterparts. Although sufficient for certain applications, such as CAR-T, these observations suggest that there is certainly room for improvement. Third, while our results provide a proof of concept for TRACeRs in both BiTE and CAR-T formats, we acknowledge that these experiments are only an initial step in assessing the technique’s utility. For example, while the BiTEs tested in this study show good efficacy in vitro, they could also benefit from further optimization. More extensive studies, including long-term in vivo evaluation and functional-based efficacy optimization, are required to fully explore the therapeutic potential of TRACeRs.
On the molecular level, we recognize that the common structure shared among pMHC complexes27,55 may present a more general binding solution to simplify the engineering process. On the TCR-facing side of MHC I, the α1 and α2 helices form a unique structural ridge and, by leveraging this feature to develop a complementary binding protein to it, the peptide-focused MHC targeting problem can be greatly reduced to a small number of residues surrounding the peptide. In retrospect, we initially hypothesized (and observed) that substitutions on ARE alone could produce peptide-specific binders but the revelation of a ___domain-swapped X-ray structure unexpectedly provided a far superior platform. The ARE, which was originally inherited from TRACeR-II as a loop, reconfigures into a helical structure in TRACeR-I and is docked optimally by leveraging contacts with oligomorphic residues on the MHC’s α1 and α2 helices, hovering over the peptide N terminus. This resulting short helix and two other scaffold helices on TRACeR envelop the bulging peptide within this helical triangle. This allows a minimalist set of box residues surrounding the peptide to be leveraged for highly specific antigen recognition. When this engineered specificity was expanded to a larger set of pMHC I antigens, the results supported the notion that TRACeR interfacial residues are modular, with each residue spatially positioned to interact with a specific region of the peptide. In agreement with our assumption of a generalizable antigen recognition mechanism, the enriched sequences for each antigen target converge to a distinct pattern with good structural complementarity upon structural modeling. While the full range of peptide sequences that the minimalistic set of contacting residues on a TRACeR can recognize remains unknown, for the NY-ESO-1 and KRAS-G12V TRACeRs reported here, our SSM studies revealed a highly specific binding profile and lack of cross-reactivity with any peptides that can be presented in healthy tissue.
The manner in which the TRACeR helices intersect with MHC α1 and α2 helices led us to investigate the platform’s HLA allelic compatibility. Among our initial set of proof-of-concept targets, we had instances of divergent HLA-A*, HLA-B* and HLA-C* allotypes. By comparing exposed residues between HLAs that we had confirmed to bind relative to common allotypes, the similarity of the patterns in the interfacial residues supports TRACeR-I’s compatibility with a broad range of HLAs (Supplementary Fig. 29). It is worth noting that, while TCRs can confer peptide-specific interactions with HLA-C-restricted epitopes63, no peptide-specific antibodies have been developed toward HLA-C targets to date. This multiallelic compatibility of our platform allows targeting of public epitopes presented across divergent HLA allotypes, providing a promising avenue for developing therapeutics25,62 that can reach large patient cohorts.
In conclusion, through structure-guided engineering, we created a protein platform for rapid development of peptide-focused MHC I binders with high specificity. The TRACeR-I platform is uniquely poised to both accelerate the discovery of disease-relevant MHC I immunopeptidomes and expand the repertoire of targetable antigens toward a wide range of applications.
Methods
Computational modeling
We modeled TRACeR-I in four steps. In the initial step, we use global docking algorithms to model the TRACeR–MHC I interaction in silico at low-resolution, aiming to identify potential docking poses that align with the TRACeR backbone structure. Starting from the available crystal structure to guide the modeling and design (PDB 1S9W (ref. 36)), we used a low-resolution docking algorithm, Patchdock37, to seed the search trajectories of a high-resolution algorithm, RifDock35. From the RifDock output, a cluster of 30 compatible docking modes with large footprint on the peptide antigen between TRACeR and MHC I were identified. In contrast to TRACeR-II’s binding mode, in which the helical bundle binds perpendicular to the antigen groove, RifDock models favored alternative orientations in which the helical bundle lies in parallel to the groove. We then carried out calculations to design the sequences to these models using an iterative protocol established in the Rosetta modeling suite38,39,40. An iterative Rosetta high-resolution docking and interface sequence design protocol was applied on top of the RifDock outputs39,40, with the intention of identifying diverse amino acid substitutions on the interface that are compatible with a given docking pose. Outputs that passed all filtering metrics were ranked on the basis of binding energy (ΔΔG). The top 1,000 outputs were selected and implemented for experimental test in a combinatorial library. The selected interface positions and their corresponding sequences were integrated to create a combinatorial library using Swiftlib41, which optimizes degenerate codon coverage by taking into account a given set of allowed amino acids at each position. Docking and design scripts and evaluation metrics are included in Supplementary Appendices 1–6.
Library design and production
Combinatorial DNA libraries (libraries 1 and 2) were constructed from assembly PCR using Ultramer oligonucleotides (Integrated DNA Technologies) to encode the variable region. Library sequences are listed in Supplementary Table 1.
For library transformation, Saccharomyces cerevisiae yeast EBY100 cells were transformed with insert DNA and linearized pCTCON2 plasmid using an established protocol64. After transformation, cells were grown overnight in SDCAA medium at 30 °C, passaged once and stored in 20% glycerol solution at −80 °C.
Yeast display and library screening
Transformed yeast cells were grown in SDCAA medium. For induction of expression, yeast cells were centrifuged at 2,000g for 5 min and resuspended in SGCAA medium supplemented with 0.2% glucose at a cell density of 1 × 107 cells per ml and induced at 30 °C for 16–24 h. Cells were washed with PBSA (PBS with 0.5% BSA) and labeled with pMHC I tetramer or monomers, together with anti-c-Myc fluorescein isothiocyanate (FITC; Miltenyi Biotech). After incubation for ~1 h at room temperature (RT), cells were washed twice, resuspended in PBSA and then run on a Sony SH800 cell sorter. NGS of final enriched pools was performed with the Azenta Amplicon-EZ service.
The theoretical complexity for screening out initial binders in this background library (library 1) was 1.3 × 1011, but our experimental screening coverage sampled a subset of the theoretical library with 1 × 109 transformed variants. Nonetheless, after five rounds of FACS against HLA-A*02:01/NY-ESO-1, one single clone was enriched from the initial pool of a binding population. The theoretical complexity for the ARE library (library 2) was 1.28 × 109; for each target, 1 × 108 cells were sorted through FACS. For the master library derived from the monomeric TRACeR design, the theoretical library size was 198 = 1.7 × 1010 (eight randomized positions; each position had 19 amino acids without cysteine). For each target, 2 × 109 cells were processed during the first round of magnetic-activated cell sorting.
Binder protein expression and purification
Genes encoding the designed protein sequence were synthesized and cloned into pET-24a(+) Escherichia coli plasmid expression vectors (Genscript, C-terminal 6xHis-tag). Plasmids were then transformed into chemically competent BL21(DE3) E. coli (Zymo Research). The cells were cultured in 2xYT medium at 37 °C until the optical density (OD) reached 0.6–0.8. Protein expression was then induced with 1 mM IPTG at 16 °C. After overnight expression, cells were collected and resuspended with 50 mM Tris buffer pH 8.0 and 300 mM NaCl and frozen at −80 °C until extraction and purification. The cell pellet was thawed and sonicated and purified by nickel affinity followed by SEC (Superdex 75 10/300GL, GE Healthcare). All protein samples were characterized by SDS–PAGE. Protein concentrations were determined by absorbance at 280 nm measured with a Nanodrop spectrophotometer (Thermo Fisher Scientific) using the predicted extinction coefficient.
CD
CD spectra were measured on a JASCO CD spectrophotometer in a 1-mm-pathlength cuvette (Hellma). Protein samples were at ~0.2 mg ml−1 in the 50 mM Tris buffer. Melting temperature ranged from 20 to 95 °C and the absorption signal was monitored at 222 nm in 1 °C increments per minute, with 10 s of equilibration time and 1 s of digital integration time. Wavelength scans (200–260 nm) were collected at 20 and 95 °C and again at 20 °C after fast refolding.
BLI
BLI binding data were collected on an Octet QK (ForteBio) and processed using the instrument’s integrated software. For binding assays, biotinylated pMHC Is were loaded onto streptavidin-coated biosensors (ForteBio) at 1.25 μg ml−1 in the kinetic buffer (ForteBio) for 1,800 s. Analyte proteins were diluted from concentrated stocks into the binding buffer. After baseline measurement in the binding buffer alone, the binding kinetics were monitored by dipping the biosensors in wells containing the target protein at the indicated concentration (association step) and then dipping the sensors back into baseline or buffer (dissociation). The association time was 1,800 s and dissociation time was 1,200 s, with a shaking speed of 1,000 rpm. Data were analyzed and processed using ForteBio Data Analysis software 9.1.0.
Peptide libraries
All peptide sequences are given as standard single letter codes. Peptides were purchased from Genscript or Mimotope at a purity of >90%. The placeholder peptide gTAX (LFGYPVYV) was purchased from Genscript at a purity of 98%. Peptides were solubilized in a solution of less than 5% v/v DMSO at a concentration of 100 μM. All peptide solutions were centrifuged at 14,000 rpm for 15 min and filtered before use.
Recombinant MHC protein expression, refolding and purification
Plasmid DNA encoding the BirA substrate peptide (BSP, LHHILDAQKMVWNHR)-tagged luminal ___domain of the MHC I heavy chains and human β2-microglobulin (β2m) were provided by the National Institutes of Health (NIH) tetramer facility (Emory University) and transformed into E. coli BL21(DE3) cells (Novagen). BSP-tagged MHC I proteins were expressed in Luria–Bertani medium and inclusion bodies were collected and purified using a standard protocol51,52. In vitro refolding of BSP-tagged pMHC I molecules was performed by slowly diluting a 200-mg mixture of BSP-tagged MHC I and β2m at a 1:3 molar ratio over 24 h in refolding buffer (0.4 M l-arginine, 100 mM Tris pH 8, 2 mM EDTA, 4.9 mM reduced glutathione and 0.57 mM oxidized glutathione) containing 10 mg of the placeholder peptide. BSP-tagged pMHC I refolding proceeded for 96 h and was followed by SEC for protein purification.
Biotinylation and tetramer library preparation
Biotinylation of various pMHC Is and tetramer library preparation using gTAX/HLA-A*02:01 protein were performed as previously described52. The BSP-tagged pMHC I proteins were biotinylated using the BirA biotin–protein ligase bulk reaction kit (Avidity) according to the manufacturer’s instructions and prepared at a final concentration of 2 mg ml−1 monomer. The level of biotinylation was evaluated by SDS–PAGE gel shift assay in the presence of excess streptavidin.
For tetramer library preparation, biotinylated gTAX/HLA-A*02:01 were mixed with TAPBPR (7:1 molar ratio of gTAX-loaded HLA-A*02:01 to TAPBPR) and individual peptides from TCGA peptide library or NY-ESO positions 1, 4, 5, 7 and 8 full-scanning peptide libraries (1:10 molar ratio of gTAX-loaded HLA-A*02:01 to peptide). Each reaction was incubated 2 h at RT and the peptide exchange reactions were confirmed by differential scanning fluorimetry. Meanwhile, streptavidin–(R)-phycoerythrin (PE) or streptavidin–allophycocyanin (APC) (Agilent Technologies) at a 4:1 molar ratio of monomer to streptavidin was added to HLA-A*02:01 proteins in the presence of excess peptides every 10 min over ten intervals at RT in the dark. Tetramerized molecules upon peptide exchange were washed using Amicon Ultra centrifugal filter units with a 100-kDa membrane cutoff to remove excess peptides and TAPBPR with a 1:1,000 dilution of PBS buffer. Biotinylated pMHC I proteins, which did not require exchange for TCGA and NY-ESO-1 positions 1, 4, 5, 7 and 8 full-scanning peptide libraries, were prepared by directly adding streptavidin–PE or streptavidin–APC at a 4:1 molar ratio of monomer to streptavidin every 10 min over ten intervals at RT in the dark. The resulting tetramers could be stored at 4 °C for up to 4 weeks.
Differential scanning fluorimetry was used to access the thermal stabilities of the peptide-exchanged pMHC I molecules and validate the peptide loading. Individual peptide-exchanged HLA-A*02:01 protein was mixed with 10× Sypro orange dye in a buffer of 150 mM NaCl and 20 mM sodium phosphate pH 7.4 to a final volume of 70 µl. Samples were loaded into MicroAmp Optical 384-well plate. The experiment was performed on a QuantStudio 5 real-time PCR machine with excitation and emission wavelengths set to 470 nm and 569 nm. The temperature was incrementally increased at a rate of 1 °C per minute between 25 and 95 °C. Data analysis and fitting were performed in GraphPad Prism 9.
SSM scan on NY-ESO-1157–165(V) peptide using pMHC I tetramers
TRACeR was expressed on the yeast cell surface and double-stained with anti-C-myc FITC (Miltenyi Biotech) and MHC tetramer PE. Furthermore, 1G4 TCR was expressed on the T cell surface and double-stained with anti-TCR antibody AlexaFluor 488 and MHC tetramer PE. We used the chaperone-mediated peptide exchange method to load individual antigen variants into HLA-A*02:01 conjugated to fluorescently labeled tetramers51,52 and detected binding patterns by flow cytometry. After 30 min of incubation, cells were washed with PBSA twice and analyzed on flow cytometry. The median fluorescence intensity (MFI) of TRACeR was calculated on the basis of the FITC-positive population. The MFI of 1G4 TCR was calculated on the basis of the TCR-positive population.
Sequence-based selection of cross-reactive peptides
We searched the Immune Epitope Database65 for linear peptides that were positive for binding to HLA-A*02:01 (72,900 peptides) with the SMW motif using the following regular expression: ‘[SCT].MW’. Three peptides (FTIAMWLLL, CINMWCWTV and TSDMWLYR) had known protein origin and the SMW motif in the middle of the peptide and were selected for further analysis.
Triplet NNK library on yeast displayed pMHC I with NY-ESO-1 peptide
NY-ESO-1/HLA-A*02:01 was expressed on the surface of YVH10 yeast using a single-chain format consisting of peptide, B2M and the MHC I alpha chain ecotodomain, separated by GS linkers. A V5 tag was included at the C terminus of the MHC I alpha chain for detection of surface display. A peptide SSM scanning library was generated by diversifying one to three amino acid positions using degenerate NNK codons spanning the length of the NY-ESO-1 peptide. The library was built using homologous recombination of peptide oligo pools (Integrated DNA Technologies) with a vector encoding the B2M and MHC I alpha chain using electroporation conditions described previously66. The scanning library had a theoretical nucleotide diversity of 6.7 × 105.
Transformed yeast were grown in SDCAA uracil dropout medium. Yeast cells were induced for pMHC expression by inoculating freshly passaged cells at an OD at 600 nm of 0.1 per ml in SGCAA medium, ensuring coverage of the nucleotide diversity by at least tenfold. Cells were induced at 30 °C with 200 rpm shaking overnight. To label yeast with TRACeR or 1G4 TCR, cells were washed with staining buffer (1× PBS with 1% BSA) before incubating with biotinylated TRACeR or 1G4 tetramer for 1 h at RT. Tetramers were generated by combining soluble 1G4 TCR expressed recombinantly with a C-terminal SpyTag and recombinant SpyCatcher-Fc fusion at a 4:1 molar ratio for 20 min at RT. Cells were washed twice using staining buffer before labeling with 1 µg ml−1 of AlexaFluor 405-conjugated anti-V5 antibody and either 1:500 dilution of streptavidin–APC-conjugated (Invitrogen) or 1:100 dilution of streptavidin–PE-conjugated anti-human Fc antibody (Biolegend) for TRACeR or 1G4, respectively, in staining buffer for 20 min on ice. Next, cells were washed twice, resuspended in staining buffer and sorted on the FACS Melody (BD Biosciences). Yeast cells expressing HLA-A*02:01/NY-ESO-1 were first stained with biotinylated TRACeR in titrating concentrations ranging from 0.25 to 1,000 nM to interpolate an EC80 binding concentration of 156 nM to use for FACS selections. Triplet scanning yeast libraries were stained with 156 nM TRACeR or 100 nM 1G4 tetramer for all sorts. Deep sequencing of sorted libraries was performed as previously described67. Briefly, plasmids were extracted from yeast and a ~150-bp stretch of DNA spanning the peptide was PCR-amplified. DNA libraries were barcoded with xGen UDI primers (Integrated DNA Technologies) in a second round of PCR and prepared for sequencing on the Element AVITI (Element Biosciences).
Immunogenicity assay
Mice were maintained in a specific-pathogen-free facility at the University of Pennsylvania. Experiments and procedures were performed in accordance with the Institutional Animal Care and Use Committee (IACUC) of the University of Pennsylvania (protocol 803042). Female BALB/c mice from Jackson Labs, aged 5–7 weeks (n = 8 per group), were randomly assigned to different groups. They were anesthetized and administered Tris buffer through retro-orbital injection. The injection included 50 mM Tris buffer at pH 8 or mAbs mIgG (Innovative IR-MSBC-GF) or hIgG (Innovative IR-HU-GF-ED) at a dose of 0.2 mg kg−1. Injections were administered every 2–3 days over the period of 4 weeks on days 0, 2, 4, 7, 9, 11, 14, 16, 18, 21, 23, 25 and 28. Blood samples were collected on days 0, 8, 15, 22 and 29 through retro-orbital bleeding using micro-hematocrit capillary tubes (Fisher).
Preparation of mouse tissues
Spleens were collected in PBS, pressed through a 70-μm cell strainer and washed with 10 ml of PBS with 1% FBS and 2 mM EDTA. Organs were then subjected to ACK lysis and passed through a 40-μm cell strainer to prepare the final single-cell suspension.
Interferon-γ (IFNγ) ELISpot
The IFN-γ ELISpot was performed according to manufacturer protocols (BD Biosciences). For each spleen and condition, 3 × 105 bulk splenocytes were plated in RPMI (supplemented with 10% FBS, 2 mM l-glutamine, penicillin and streptomycin) in duplicate wells and either incubated with (1) no antigen or stimulus; (2) 4.5 µg ml−1 protein antigen (mIgG, hIgG or TRACeR); or (3) phorbol myristate acetate (PMA)–ionomycin at working concentrations of 50 ng ml−1 and 1 µg ml−1, respectively. Cultures were incubated at 37 °C for 16 h and subsequently developed according to manufacturer protocols. IFNγ spots were imaged and counted on a CTL ImmunoSpot S6 universal analyzer.
Flow cytometry
In all cases of analysis of primary splenocytes ex vivo or following in vitro restimulation, we blocked nonspecific antibody binding using 10 µg ml−1 anti-CD16 and anti-CD32 (clone 2.4G2, BioXCell). For viability staining, we used both a live–dead fixable Aqua dead cell stain kit (1:500 dilution; Thermo Fisher Scientific) and a Zombie UV fixable viability kit (1:1,000 dilution; Biolegend). The following antibodies were used to stain primary cells: anti-CD44 BUV395 (clone IM7, 1 μg ml−1, BD Biosciences), anti-Ly-108 BUV737 (clone 13G3, 2 μg ml−1, BD Biosciences), antiCD127 BV421 (clone A7R34, 2 μg ml−1, Biolegend), anti-CD27 BV510 (clone LG.3A10, 2 μg ml−1, Biolegend), anti-CX3CR1 BV605 (clone SA011F11, 2 μg ml−1, Biolegend), anti-CD4 BV785 (clone RM4-5, 2 μg ml−1, Biolegend), anti-KLRG1 FITC (clone 2F1, 2 μg ml−1, Biolegend), anti-CD8 PE and PerCP-Cy5.5 (clone 53-6.7, 1 μg ml−1, Biolegend), anti-CD69 PE/Cy7 (clone H1.2F3, 2 μg ml−1, Biolegend), anti-CD3 AF700 (clone 17A2, 5 μg ml−1, Biolegend), anti-CD62L APC-eFluor 780 (clone MEL-14, 1.25 μg ml−1, Invitrogen), anti-Vβ 8.1, 8.2 TCR FITC and PE (clone MR5-2, 10 μg ml−1, BD Biosciences), anti-Vβ 8.3 TCR FITC and PE (clone 1B3.3, 10 μg ml−1, BD Biosciences), anti-CD107a BUV395 (clone 1D4B, 2 μg ml−1, BD Biosciences), anti-IFNγ BV605 (clone XMG1.2, 0.5 μg ml−1, Biolegend), anti-Ki-67 FITC (clone 11F6, 2 μg ml−1, Biolegend), anti-inteleukin 2 (IL-2) PE (clone JES6-5H4, 0.5 μg ml−1, Biolegend) and anti-tumor necrosis factor APC (clone MP6-XT22, 0.5 μg ml−1, Biolegend). For intracellular staining, we permeabilized the cells using the BD Cytofix/Cytoperm fixation/permeabilization kit according to the manufacturer’s protocols (BD Biosciences). To collect data, we used the CytoFLEX LX (Beckman Coulter) and BD LSRFortessa X-50; FlowJo 10.10 was used for analysis.
In vitro restimulation and intracellular staining
In a 96-well U-bottom plate, 2 × 106 bulk splenocytes were plated and subsequently incubated with (1) no antigen; (2) 4.5 μg ml−1 protein antigen (mIgG, hIgG or TRACeR); or (3) PMA–ionomycin at working concentrations of 50 ng ml−1 and 1 μg ml−1, respectively. After 1 h of incubation at 37 °C, brefeldin A and monensin were added at final concentrations of 5 μg ml−1 and 2 μM, respectively. Additionally, we included BUV395 anti-mouse CD107a at a final concentration of 2 μg ml−1. Cultures were subsequently incubated at 37 °C for 10 h. After incubation, cells were subjected to surface and intracellular flow cytometric staining.
BiTE expression and purification
Protein expression of the \(\rm{{TRACeR}}_{{MHC}\;I,{A}02}^{{NY}-{ESO}-1}\) anti-CD3 fusion in the Drosophila melanogaster S2 cell line was performed as previously described for an analogous protein68. A DNA construct encoding the anti-CD3 fused with the \(\rm{{TRACeR}}_{{MHC}\;I,{A}02}^{{NY}-{ESO}-1}\) His-tagged at the C terminus was cloned in pMT vector and the S2 cells were stably transfected. The cultures were induced with 1 mM CuSO4 and, after 4 days, the supernatant was collected. The secreted protein was purified using a high-density metal affinity agarose resin (Agarose Bead Technologies) and was further purified by SEC using a HiLoad 16/600 Superdex 200-pg column at a flow rate of 1 ml min−1 in 150 mM NaCl and 20 mM sodium phosphate buffer (pH 7.4).
DLBCL culture
DLBCL cell lines were either directly received from the American Type Culture Collection (ATCC) or verified by ATCC short tandem repeat profiling. Cells were cultured at 37 °C in a 5% CO2 incubator with advanced RPMI (Gibco), 5% heat-inactivated FBS (Gibco), Glutamax (Gibco) and penicillin–streptomycin (Gibco). Cells were maintained between 200,000 and 1 million cells per ml and split every 2 days. Lines were routinely tested for Mycoplasma contamination using the universal Mycoplasma detection kit (ATCC).
Primary human T cell isolation and activation
For bispecific-induced cytotoxic assays, blood was collected from healthy, consenting donors by the Human Immunology Core at the University of Pennsylvania and total CD3+ T cells were enriched by magnetic separation. Cells were cultured in advanced RPMI (Gibco), 10% heat-inactivated FBS (Gibco), Glutamax (Gibco), penicillin–streptomycin (Gibco) and 10 mM HEPES (Quality Biological), supplemented with 300 U per ml recombinant IL-2 (National Cancer Institute (NCI) Biological Resources Branch (BRB)). T cells were cultured at 1 million cells per ml and activated with a 1:1 ratio of Dynabeads human T-activator CD3 and CD28 beads (Gibco) for 72 h, after which they were debeaded and expanded in IL-2-containing medium.
For generating CAR-T cells, primary CD8+ T cells were isolated from fresh leukopacks of anonymous donors (AllCells, LP, FR, 5B) by negative selection using the EasySep human CD8+ T cell isolation kit (STEMCELL Technologies, 17953). Isolated CD8+ T cells were cryopreserved in CELLBANKER 1 (AMSBIO, 11910). After thawing, T cells were cultured in human T cell medium consisting of X-VIVO 15 (Lonza, 04-418Q), 5% human AB serum and 10 mM neutralized N-acetyl l-cysteine (Sigma-Aldrich, A9165) supplemented with 30 U per ml of IL-2 (NCI BRB preclinical repository) for all experiments. After 24 h in culture, T cells were stimulated with human T-activator CD3 and CD28 Dynabeads (Life Technologies, 11131D) at a 1:1 ratio of cells to beads.
Bispecific-induced cytotoxicity assays
Tumor cells were labeled with CellTrace CFSE (Invitrogen) at 1:1,000 in PBS for 15 min at 37 °C, following by extensive washout. Cells were counted and plated at 50,000 cells per well of a flat bottom 96-well plate. Primary human T cells were washed and 100,000 T cells were plated in each well. Bispecific antibody stocks were made by serial dilution and spiked into each well as indicated by dose curves. Where indicated, CD19–CD3-bispecific antibodies or CD19–βGal-bispecific antibodies were used as positive and negative controls (Invivogen). After ~18 h of coculture, Sytox blue (Invitrogen) viability stain was added to each well. Cells were analyzed by the high-throughput sampler of a LSR Fortessa (BD Biosciences). Tumors were gated from immune cells and the live fraction of each well was normalized to wells that received no antibody.
T cells incubated with off-target tumor cell lines SUDHL4 and SUDHL5 also displayed some activation in the presence of our TRACeR BiTE, albeit at a lower level (Supplementary Fig. 10). This is likely because of the activity of dimerized anti-CD3 scFv, which can facilitate crosslinking of CD3 on T cells and facilitate T cell activation independent of target recognition and killing.
X-ray crystallography and structure determination
The HLA-A*2:01/NY-ESO-1–MAM–TRACeR complex was prepared by mixing MAM–TRACeR and HLA-A*2:01/NY-ESO-1 at a 1:1.3 molar ratio; the mixture was incubated at 4 °C for 1 h. Unbound proteins were separated by SEC using a Superdex200 increase 10/300 GL column, run at 0.5 mg min−1 flow rate with running buffer, 25 mM Tris pH 8.0 and 150 mM NaCl. The complex was concentrated to 10.4 mg ml−1 for crystallography and the sample was mixed at a 1:1 ratio with reservoir well solution and incubated at 20 °C. Crystals were obtained in 200 mM sodium sulfate and 20% w/v PEG 3350 using the sitting drop vapor diffusion method. Crystals were harvested after transferring to cryogenic solution containing reservoir solution and 20% v/v glycerol. Crystals were screened and data were collected on the National Synchrotron Light Source (FMX, 17-ID-2) at Brookhaven National Laboratory (BNL) using a Dectris Eiger 16M detector. Data were processed using XDS69 and the structure of HLA-A*02:01/NY-ESO-1–MAM–TRACeR complex was solved by molecular replacement method using the Molrep70 and REFMAC 5 (ref. 71) software of the CCP4 package suite72. The structure of HLA-A*02:01/NY-ESO-1 (PDB 1S9W)35 and a structural model of MAM–TRACeR were used as search models. The complex structure was refined using BUSTER (Global Phasing) and PHENIX73 and Coot74 were used for model building.
CAR-T cell cytotoxicity assays
CD8+ Primary human T cells expressing constitutive \(\rm{{TRACeR}}_{{MHC}\;I,{A}02}^{{NY}-{ESO}-1}\) CAR were counted and stained with CellTrace yellow dye (Invitrogen, C34567) at a concentration of 2.5 μM while otherwise following the manufacturer’s protocol. Similarly, DLBCL tumor cell lines were counted and stained with CellTrace violet dye (Thermo Fisher Scientific, C34571) at a concentration of 2.5 μM. After staining, cells were counted, resuspended in their respective media and adjusted to a concentration of 2 × 105 cells per ml for T cells and 105 cells per ml for each DLBCL tumor cell line. Then, 100 μl of T cells and a 100 μl of target cells were combined in a in a 96-well round bottom plate, for an effector-to-target ratio of 2. T cells and DLBCL tumor targets were cocultured at 37 °C with 5% CO2. After 18 h, cultures were analyzed for specific lysis of target tumor cells by flow cytometry. All cells were stained with live–dead fixable near-infrared dead cell stain kit at 1:500 dilution (Invitrogen, L10119) and human anti-CD8 FITC antibody (1:100 dilution; BD Biosciences, 347313) for 30 min in ice. After washing in flow buffer, cells were resuspended and acquired in a NovoCyte Quanteon flow cytometer (Agilent). After cells were gated by size and doublets were excluded, only live events were considered. T cells were differentiated of target cells by CellTrace and CD8 expression. The percentage of specific lysis of target cells was determined by comparing the number of target cells alive in the coculture with TRACeR CAR to the average number of target cells alive in the coculture with untransduced T cells. All flow cytometry data analysis was performed in FlowJo X software (TreeStar) and GraphPad Prism 9 (Dotmatic). The following equation was used to calculate the percentage of specific lysis:
Sequence conservation analysis
American HLA haplotype (allelic) frequencies were obtained from the BeTheMatch registry75 across five broad race groups (AFA, API, etc.) and converted to diploid (population) frequencies assuming a Hardy–Weinberg equilibrium. A combined American population frequency for each allele was obtained by normalizing values according to the racial distribution followed by the 2020 US Census. ‘Common’ alleles were defined as those with a haploid frequency greater than 0.05% in at least one of the five broad race groups for a total of 215 HLA alleles across HLA-A, HLA-B, and HLA-C loci. HLA allele sequences were obtained from the IMGT/HLA database22 and a pairwise sequence alignment was performed on the HLA-A*02:01 heavy chain sequence (consisting of 180 residues from the N terminus). Relevant HLA contact residues were identified by visual inspection and a sequence logo was created using the logomaker (version 0.8)76 Python package. Visualizations were produced with PyMOL (version 2.5.2).
TRACeR–pMHC I structural modeling
For structural modeling, all target TRACeR sequences were threaded onto the original TRACeR–NY-ESO-1 crystal structure. Then, the target TRACeR model along with the target pHLA-I complex were superimposed onto the original TRACeR–NY-ESO-1 crystal structure on the basis of Ca atoms and the original TRACeR–pHLA complex was deleted using PyMOL (version 2.5.2). Finally, the modeled complex was optimized using the FastRelax protocol77 in Rosetta78 (version 2020.08) and the relaxed model with the lowest total ref2015 score was selected as the final TRACeR–pHLA model.
The pHLA-I complexes were obtained as follows. For Nef, the highest-resolution pHLA-I crystal structure was identified from HLA3DB (ref. 20) as PDB 3VXN (ref. 79). For KRAS-G12V, the HLA-A*11:01 sequence was threaded onto the Fab-bound KRAS-G12V/A*03:01 complex (PDB 7STF (ref. 60)) using the RosettaRemodel38 application from the Rosetta (version 2020.08)4 suite of programs and the Fab and β2m were removed. For PRAME and SARS-CoV-2, the pMHC I complex was modeled using RepPred, a state-of-the-art homology modeling method20.
Ethics statement
The animal studies received approval from the IACUC of the Office of Animal Welfare at the University of Pennsylvania (protocol 803042). Blood-related research was conducted under biosafety project approval by the Environmental Health and Safety Office at Stanford and under approval from the University of Pennsylvania review board. Written informed consent in this study was provided by all participants.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All protein sequences are included in Supplementary Table 1. The crystal structure was deposited to the PDB under accession code 9DL1. Source data are provided with this paper.
Code availability
The design workflow and code are available in Supplementary Appendices 1–6. As the code was adopted and modified on the basis of a previous publication, the source of the code was also cited in the Supplementary Information.
References
Spiegel, J. Y. et al. CAR T cells with dual targeting of CD19 and CD22 in adult patients with recurrent or refractory B cell malignancies: a phase 1 trial. Nat. Med. 27, 1419–1431 (2021).
Xue, J. Y. et al. Rapid non-uniform adaptation to conformation-specific KRAS(G12C) inhibition. Nature 577, 421–425 (2020).
Dang, C. V., Reddy, E. P., Shokat, K. M. & Soucek, L. Drugging the ‘undruggable’ cancer targets. Nat. Rev. Cancer 17, 502–508 (2017).
Schumacher, T. N. & Schreiber, R. D. Neoantigens in cancer immunotherapy. Science 348, 69–74 (2015).
Croft, N. P. et al. Most viral peptides displayed by class I MHC on infected cells are immunogenic. Proc. Natl Acad. Sci. USA 116, 3112–3117 (2019).
Neefjes, J., Jongsma, M. L., Paul, P. & Bakke, O. Towards a systems understanding of MHC class I and MHC class II antigen presentation. Nat. Rev. Immunol. 11, 823–836 (2011).
Pishesha, N., Harmand, T. J. & Ploegh, H. L. A guide to antigen processing and presentation. Nat. Rev. Immunol. 22, 751–764 (2022).
Mendes, M. et al. IEDB-3D 2.0: structural data analysis within the Immune Epitope Database. Protein Sci. 32, e4605 (2023).
Klebanoff, C. A., Chandran, S. S., Baker, B. M., Quezada, S. A. & Ribas, A. T cell receptor therapeutics: immunological targeting of the intracellular cancer proteome. Nat. Rev. Drug Discov. 22, 996–1017 (2023).
Rudolph, M. G., Stanfield, R. L. & Wilson, I. A. How TCRs bind MHCs, peptides, and coreceptors. Annu. Rev. Immunol. 24, 419–466 (2006).
Zareie, P. et al. Canonical T cell receptor docking on peptide-MHC is essential for T cell signaling. Science 372, abe9124 (2021).
Hennecke, J. & Wiley, D. C. T cell receptor–MHC interactions up close. Cell 104, 1–4 (2001).
Garcia, K. C. & Adams, E. J. How the T cell receptor sees antigen—a structural view. Cell 122, 333–336 (2005).
Coles, C. H. et al. TCRs with distinct specificity profiles use different binding modes to engage an identical peptide–HLA complex. J. Immunol. 204, 1943–1953 (2020).
Richman, S. A. & Kranz, D. M. Display, engineering, and applications of antigen-specific T cell receptors. Biomol. Eng. 24, 361–373 (2007).
Wucherpfennig, K. W. et al. Polyspecificity of T cell and B cell receptor recognition. Semin. Immunol. 19, 216–224 (2007).
Bentzen, A. K. & Hadrup, S. R. T-cell-receptor cross-recognition and strategies to select safe T-cell receptors for clinical translation. Immunooncol. Technol. 2, 1–10 (2019).
Spear, T. T., Evavold, B. D., Baker, B. M. & Nishimura, M. I. Understanding TCR affinity, antigen specificity, and cross-reactivity to improve TCR gene-modified T cells for cancer immunotherapy. Cancer Immunol. Immunother. 68, 1881–1889 (2019).
Sewell, A. K. Why must T cells be cross-reactive? Nat. Rev. Immunol. 12, 668–677 (2012).
Gupta, S., Nerli, S., Kandy, S. K., Mersky, G. L. & Sgourakis, N. G. HLA3DB: comprehensive annotation of peptide/HLA complexes enables blind structure prediction of T cell epitopes. Nat. Commun. 14, 6349 (2023).
T, R. R. & Smith, J. C. Structural patterns in class 1 major histocompatibility complex-restricted nonamer peptide binding to T-cell receptors. Proteins 90, 1645–1654 (2022).
Barker, D. J. et al. The IPD-IMGT/HLA database. Nucleic Acids Res. 51, D1053–D1060 (2023).
Marsh, S. G. et al. Nomenclature for factors of the HLA system, 2010. Tissue Antigens 75, 291–455 (2010).
Macdonald, W. A. et al. T cell allorecognition via molecular mimicry. Immunity 31, 897–908 (2009).
Yarmarkovich, M. et al. Targeting of intracellular oncoproteins with peptide-centric CARs. Nature 623, 820–827 (2023).
Douglass, J. et al. Bispecific antibodies targeting mutant neoantigens. Sci. Immunol. 6, eabd5515 (2021).
Sengupta, S. et al. TCR-mimic bispecific antibodies to target the HIV-1 reservoir. Proc. Natl Acad. Sci. USA 119, e2123406119 (2022).
Tikhonova, A. N. et al. αβ T cell receptors that do not undergo major histocompatibility complex-specific thymic selection possess antibody-like recognition specificities. Immunity 36, 79–91 (2012).
Holler, P. D., Chlewicki, L. K. & Kranz, D. M. TCRs with high affinity for foreign pMHC show self-reactivity. Nat. Immunol. 4, 55–62 (2003).
Linette, G. P. et al. Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood 122, 863–871 (2013).
Cameron, B. J. et al. Identification of a titin-derived HLA-A1-presented peptide as a cross-reactive target for engineered MAGE A3-directed T cells. Sci. Transl. Med. 5, 197ra103 (2013).
Nathan, P. et al. Overall survival benefit with tebentafusp in metastatic uveal melanoma. N. Engl. J. Med. 385, 1196–1206 (2021).
Gupta, S. & Sgourakis, N. G. A structure-guided approach to predict MHC-I restriction of T cell receptors for public antigens. Preprint at bioRxiv https://doi.org/10.1101/2024.06.04.597418 (2024).
Du, H. et al. A general system for targeting MHC class II-antigen complex via a single adaptable loop. Nat. Biotechnol. https://doi.org/10.1038/s41587-024-02466-y (2024).
Cao, L. X. et al. Design of protein-binding proteins from the target structure alone. Nature 605, 551–560 (2022).
Webb, A. I. et al. Functional and structural characteristics of NY-ESO-1-related HLA A2-restricted epitopes and the design of a novel immunogenic analogue. J. Biol. Chem. 279, 23438–23446 (2004).
Schneidman-Duhovny, D., Inbar, Y., Nussinov, R. & Wolfson, H. J. PatchDock and SymmDock: servers for rigid and symmetric docking. Nucleic Acids Res. 33, W363–W367 (2005).
Huang, P. S. et al. RosettaRemodel: a generalized framework for flexible backbone protein design. PLoS ONE 6, e24109 (2011).
Lyskov, S. & Gray, J. J. The RosettaDock server for local proteinprotein docking. Nucleic Acids Res. 36, W233–W238 (2008).
Bender, B. J. et al. Protocols for molecular modeling with Rosetta3 and RosettaScripts. Biochemistry 55, 4748–4763 (2016).
Jacobs, T. M., Yumerefendi, H., Kuhlman, B. & Leaver-Fay, A. SwiftLib: rapid degenerate-codon-library optimization through dynamic programming. Nucleic Acids Res. 43, e34 (2015).
Sidney, J., Peters, B., Frahm, N., Brander, C. & Sette, A. HLA class I supertypes: a revised and updated classification. BMC Immunol. 9, 1 (2008).
Papadaki, G. F. et al. Decoupling peptide binding from T cell receptor recognition with engineered chimeric MHC-I molecules. Front. Immunol. 14, 1116906 (2023).
Sun, Y. et al. Universal open MHC-I molecules for rapid peptide loading and enhanced complex stability across HLA allotypes. Proc. Natl Acad. Sci. USA 120, e2304055120 (2023).
Gnjatic, S. et al. NY-ESO-1: review of an immunogenic tumor antigen. Adv. Cancer Res. 95, 1–30 (2006).
Chen, J. L. et al. Identification of NY-ESO-1 peptide analogues capable of improved stimulation of tumor-reactive CTL. J. Immunol. 165, 948–955 (2000).
Stewart-Jones, G. et al. Rational development of high-affinity T-cell receptor-like antibodies. Proc. Natl Acad. Sci. USA 106, 5784–5788 (2009).
Chen, J. L. et al. Structural and kinetic basis for heightened immunogenicity of T cell vaccines. J. Exp. Med. 201, 1243–1255 (2005).
Zhao, Y. et al. High-affinity TCRs generated by phage display provide CD4+ T cells with the ability to recognize and kill tumor cell lines. J. Immunol. 179, 5845–5854 (2007).
Robbins, P. F. et al. Single and dual amino acid substitutions in TCR CDRs can enhance antigen-specific T cell functions. J. Immunol. 180, 6116–6131 (2008).
Sun, Y. et al. Xeno interactions between MHC-I proteins and molecular chaperones enable ligand exchange on a broad repertoire of HLA allotypes. Sci. Adv. 9, eade7151 (2023).
Overall, S. A. et al. High throughput pMHC-I tetramer library production using chaperone-mediated peptide exchange. Nat. Commun. 11, 1909 (2020).
Foldvari, Z. et al. A systematic safety pipeline for selection of T-cell receptors to enter clinical use. NPJ Vaccines 8, 126 (2023).
Zhao, X. et al. Tuning T cell receptor sensitivity through catch bond engineering. Science 376, eabl5282 (2022).
Yang, X. et al. Facile repurposing of peptide-MHC-restricted antibodies for cancer immunotherapy. Nat. Biotechnol. 41, 932–943 (2023).
Pearson, W. R. Selecting the right similarity-scoring matrix. Curr. Protoc. Bioinformatics 43, 3.5.1–3.5.9 (2013).
Cole, B. C., Kartchner, D. R. & Wells, D. J. Stimulation of mouse lymphocytes by a mitogen derived from Mycoplasma arthritidis (MAM). VIII. Selective activation of T cells expressing distinct V β T cell receptors from various strains of mice by the ‘superantigen’ MAM. J. Immunol. 144, 425–431 (1990).
Beverley, P. C. et al. Distinctive functional characteristics of human 'T' lymphocytes defined by E rosetting or a monoclonal anti-T cell antibody. Eur. J. Immunol. 11, 329–334 (1981).
McCormack, E. et al. Bi-specific TCR–anti CD3 redirected T-cell targeting of NY-ESO-1- and LAGE-1-positive tumors. Cancer Immunol. Immunother. 62, 773–785 (2013).
Wright, K. M. et al. Hydrophobic interactions dominate the recognition of a KRAS G12V neoantigen. Nat. Commun. 14, 5063 (2023).
Sun, X. et al. Superimposed epitopes restricted by the same HLA molecule drive distinct HIV-specific CD8+ T cell repertoires. J. Immunol. 193, 77–84 (2014).
Sun, Y. et al. Structural principles of peptide-centric chimeric antigen receptor recognition guide therapeutic expansion. Sci. Immunol. 8, eadj5792 (2023).
Sim, M. J. W. et al. T cells discriminate between groups C1 and C2 HLA-C. eLife 11, e75670 (2022).
Chao, G. et al. Isolating and engineering human antibodies using yeast surface display. Nat. Protoc. 1, 755–768 (2006).
Vita, R. et al. The Immune Epitope Database (IEDB): 2018 update. Nucleic Acids Res. 47, D339–D343 (2019).
Zhao, F. Z. et al. Broadening a SARS-CoV-1-neutralizing antibody for potent SARS-CoV-2 neutralization through directed evolution. Sci. Signal. 16, eabk3516 (2023).
Khalek, I. S. et al. Synthetic development of a broadly neutralizing antibody against snake venom long-chain α-neurotoxins. Sci. Transl. Med. 16, eadk1867 (2024).
Morozov, G. I. et al. Interaction of TAPBPR, a tapasin homolog, with MHC-I molecules promotes peptide editing. Proc. Natl Acad. Sci. USA 113, E1006–E1015 (2016).
Kabsch, W. XDS. Acta Crystallogr. D Biol. Crystallogr. 66, 125–132 (2010).
Vagin, A. & Teplyakov, A. MOLREP: an automated program for molecular replacement. J. Appl. Crystallogr. 30, 1022–1025 (1997).
Winn, M. D., Murshudov, G. N. & Papiz, M. Z. Macromolecular TLS refinement in REFMAC at moderate resolutions. Method Enzymol. 374, 300–321 (2003).
Winn, M. D. et al. Overview of the CCP4 suite and current developments. Acta Crystallogr. D Biol. Crystallogr. 67, 235–242 (2011).
Afonine, P. V. et al. Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallogr. D Biol. Crystallogr. 68, 352–367 (2012).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Gragert, L., Madbouly, A., Freeman, J. & Maiers, M. Six-locus high resolution HLA haplotype frequencies derived from mixed-resolution DNA typing for the entire US donor registry. Hum. Immunol. 74, 1313–1320 (2013).
Tareen, A. & Kinney, J. B. Logomaker: beautiful sequence logos in Python. Bioinformatics 36, 2272–2274 (2020).
Leaver-Fay, A., Snoeyink, J. & Kuhlman, B. On-the-fly rotamer pair energy evaluation in protein design. In Bioinformatics Research and Applications (eds Măndoiu, I., Sunderraman, R. & Zelikovsky, A.) (2008).
Leaver-Fay, A. et al. Rosetta3: an object-oriented software suite for the simulation and design of macromolecules. Methods Enzymol. 487, 545–574 (2011).
Shimizu, A. et al. Structure of TCR and antigen complexes at an immunodominant CTL epitope in HIV-1 infection. Sci. Rep. 3, 3097 (2013).
Acknowledgements
We thank E. Mellins (Stanford) and N. Roan (University of California, San Francisco) for their feedback on the manuscript. We thank A. Muselman and E. G. Engleman (Stanford) for assistance with animal experiments. H.D. is supported by a Stanford Bio-X Graduate Fellowship. J.L. is supported by a Stanford Graduate Fellowship Award. B.B. is supported by the NIH Biotechnology Training Program (T32GM141819). R.G., D.D. and A.J.M. are supported by The Mark Foundation for Cancer Research. D. Hoces is supported by the postdoc mobility fellowship from the Swiss National Science Foundation. This work was further supported by the American Cancer Society (ACS134055-IRG-218, P.-S.H), Stanford School of Medicine, Discovery Innovation Fund (P.-S.H.), NIH grants R01 AI143997 (N.G.S.), R35 GM125034 (N.G.S.) and U01 DK112217 (N.G.S), the Children’s Hospital of Philadelphia Cell and Gene Therapy Collaborative (N.G.S.) and the Asplundh foundation (N.G.S.). This research used resources (FMX, 17-ID-2) of the National Synchrotron Light Source II, a US Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by BNL under contract no. DE-SC0012704. The Center for BioMolecular Structure is supported by the NIH National Institute of General Medical Sciences through a Center Core P30 Grant (P30GM133893) and by the DOE Office of Biological and Environmental Research (KP1607011). R.A.H.-L. holds a Career Award at the Scientific Interface from Burroughs Welcome Fund and is a Chan-Zuckerberg Biohub San Francisco investigator. We thank the NIH Tetramer Core Facility (contract no. 75N93020D00005) for providing NY-ESO-1/A02, EBV/A02, SARS-CoV-2/B08 and tetramers. We thank GenScript for providing the precision mutant library in kind support.
Author information
Authors and Affiliations
Contributions
Project conceptualization, H.D., N.G.S. and P.-S.H. TRACeR design, H.D. and P.-S.H. TRACeR experimental development and characterization, H.D., W.B., J.L., H.Y., B.B. and K.D.G. X-ray crystallography, L.M. Structural analysis and modeling, L.M., S.G., H.D., G.E.N., N.G.S. and P.-S.H. Triplet NNK antigen specificity scanning, C.K., C.A.C., E.Z. and J.G.D. MHC I tetramer generation, Y.S. and H.A.T.P. Recombinant protein expression and purification, H.D., J.L., B.B., H.Y., L.M., Y.S., D. Hwang, M.C.Y. and G.F.P. BiTEs killing assays, D. Hwang and D.D. CAR-T design and killing assays, D. Hoces, R.H.L., S.M.S. and D. Hwang Animal immunogenicity studies, D. Hwang, R.G., S.D.C., L.C.E. and A.J.M. Contribution to data, data analysis, experimental design and manuscript feedback, all authors.
Corresponding authors
Ethics declarations
Competing interests
H.D., N.G.S. and P.-S.H. are listed as coinventors on provisional patent applications related to this work. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Biotechnology thanks Michael Birnbaum and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–29, Tables 1–6 and Appendices 1–6.
Supplementary Video 1
Structure overview video.
Supplementary Video 2
Video showing MHC contacts.
Supplementary Video 3
Video showing TRACeR hotspot contacts.
Supplementary Video 4
Video showing hydrogen-bonding interactions between TRACeR and peptide.
Supplementary Data
Statistical source data for supplementary figures.
Supplementary Data
Raw gel image data for Supplementary Fig. 7b.
Source data
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Du, H., Mallik, L., Hwang, D. et al. Targeting peptide antigens using a multiallelic MHC I-binding system. Nat Biotechnol (2024). https://doi.org/10.1038/s41587-024-02505-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41587-024-02505-8