Abstract
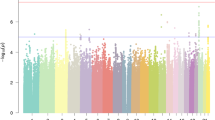
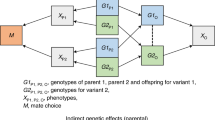
Nonrandom mating induces genome-wide correlations between unlinked genetic variants, known as gametic phase disequilibrium (GPD), whose contribution to heritability remains uncharacterized. Here we introduce the disequilibrium genome-based restricted maximum likelihood (DGREML) method to simultaneously quantify the additive contribution of SNPs to heritability and that of their directional covariances. We applied DGREML to 26 phenotypes of 550,000 individuals from diverse biobanks and found that cross-autosome GPD contributes 10–27% of the SNP-based heritability of height, educational attainment, intelligence, income, self-rated health status and sedentary behaviors. We observed a differential contribution of GPD to the heritability of height between the UK, Chinese and Japanese populations. Finally, bivariate DGREML analyses of educational attainment and height show that cross-autosome GPD contributes at least 32% of their genetic correlation. Altogether, our versatile and powerful method reveals understudied features of the genetic architecture of complex traits and informs potential mechanisms generating these features.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
209,00 € per year
only 17,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Individual-level data used in this study are available through specific application to the relevant biobanks. UK Biobank data were accessed under project 12505. Data of the Biobank Japan Project are available at the National Bioscience Database Center (NBDC) Human Database with the research ID hum0014 (https://humandbs.biosciencedbc.jp/hum0014-v26). The Data Sharing Policy of the China Kadoorie Biobank is described at www.ckbiobank.org/data-access/data-2. Specific queries about data access should be addressed to [email protected].
Code availability
GCTA software to estimate parameters is available at https://yanglab.westlake.edu.cn/software/gcta/index.html. Scripts used to run simulations and the makeDGRM program to calculate DGRM are available via Zenodo at https://doi.org/10.5281/zenodo.13831647 (ref. 48). PLINK for quality control of the data is available at https://www.cog-genomics.org/plink/1.9/ and https://www.cog-genomics.org/plink/2.0/.
References
Yang, J. et al. Common SNPs explain a large proportion of the heritability for human height. Nat. Genet. 42, 565–569 (2010).
Pasaniuc, B. & Price, A. L. Dissecting the genetics of complex traits using summary association statistics. Nat. Rev. Genet. 18, 117–127 (2017).
Wray, N. R. et al. Pitfalls of predicting complex traits from SNPs. Nat. Rev. Genet. 14, 507–515 (2013).
de Vlaming, R. et al. Meta-GWAS accuracy and power (MetaGAP) calculator shows that hiding heritability is partially due to imperfect genetic correlations across studies. PLoS Genet. 13, e1006495 (2017).
Dudbridge, F. Power and predictive accuracy of polygenic risk scores. PLoS Genet. 9, e1003348 (2013).
Bulik-Sullivan, B. K. et al. LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat. Genet. 47, 291–295 (2015).
Ning, Z., Pawitan, Y. & Shen, X. High-definition likelihood inference of genetic correlations across human complex traits. Nat. Genet. 52, 859–864 (2020).
Haseman, J. K. & Elston, R. C. The investigation of linkage between a quantitative trait and a marker locus. Behav. Genet. 2, 3–19 (1972).
Pazokitoroudi, A. et al. Efficient variance components analysis across millions of genomes. Nat. Commun. 11, 4020 (2020).
Wu, Y. & Sankararaman, S. A scalable estimator of SNP heritability for biobank-scale data. Bioinformatics 34, i187–i194 (2018).
Golan, D., Lander, E. S. & Rosset, S. Measuring missing heritability: inferring the contribution of common variants. Proc. Natl Acad. Sci. USA 111, E5272–E5281 (2014).
Yang, J., Lee, S. H., Goddard, M. E. & Visscher, P. M. GCTA: a tool for genome-wide complex trait analysis. Am. J. Hum. Genet. 88, 76–82 (2011).
Speed, D., Hemani, G., Johnson, M. R. & Balding, D. J. Improved heritability estimation from genome-wide SNPs. Am. J. Hum. Genet. 91, 1011–1021 (2012).
Speed, D. et al. Reevaluation of SNP heritability in complex human traits. Nat. Genet. 49, 986–992 (2017).
Yang, J. et al. Genetic variance estimation with imputed variants finds negligible missing heritability for human height and body mass index. Nat. Genet. 47, 1114–1120 (2015).
Lee, S. H. et al. Estimation of SNP heritability from dense genotype data. Am. J. Hum. Genet. 93, 1151–1155 (2013).
Speed, D., Hemani, G., Johnson, M. R. & Balding, D. J. Response to Lee et al.: SNP-based heritability analysis with dense data. Am. J. Hum. Genet. 93, 1155–1157 (2013).
Evans, L. M. et al. Comparison of methods that use whole genome data to estimate the heritability and genetic architecture of complex traits. Nat. Genet. 50, 737–745 (2018).
Ma, R. & Dicker, L. H. The Mahalanobis kernel for heritability estimation in genome-wide association studies: fixed-effects and random-effects methods. Preprint at https://arxiv.org/abs/1901.02936 (2019).
Schoech, A. P. et al. Quantification of frequency-dependent genetic architectures in 25 UK Biobank traits reveals action of negative selection. Nat. Commun. 10, 790 (2019).
Zeng, J. et al. Signatures of negative selection in the genetic architecture of human complex traits. Nat. Genet. 50, 746–753 (2018).
Wainschtein, P. et al. Assessing the contribution of rare variants to complex trait heritability from whole-genome sequence data. Nat. Genet. 54, 263–273 (2022).
Gimelfarb, A. A general linear model for the genotypic covariance between relatives under assortative mating. J. Math. Biol. 13, 209–226 (1981).
Nagylaki, T. The correlation between relatives with assortative mating. Ann. Hum. Genet. 42, 131–137 (1978).
Risch, H. The correlation between relatives under assortative malting for an X-linked and autosomal trait. Ann. Hum. Genet. 43, 151–165 (1979).
Kemper, K. E. et al. Phenotypic covariance across the entire spectrum of relatedness for 86 billion pairs of individuals. Nat. Commun. 12, 1050 (2021).
Border, R. et al. Assortative mating biases marker-based heritability estimators. Nat. Commun. 13, 660 (2022).
Rawlik, K., Canela-Xandri, O., Woolliams, J. & Tenesa, A. SNP heritability: what are we estimating? Preprint at bioRxiv https://doi.org/10.1101/2020.09.15.276121 (2020).
Crow, J. F. & Kimura, M. An Introduction to Population Genetics Theory (Blackburn Press, 2009).
Yengo, L. et al. Imprint of assortative mating on the human genome. Nat. Hum. Behav. 2, 948–954 (2018).
Horwitz, T. B., Balbona, J. V., Paulich, K. N. & Keller, M. C. Evidence of correlations between human partners based on systematic reviews and meta-analyses of 22 traits and UK Biobank analysis of 133 traits. Nat. Hum. Behav. 7, 1568–1583 (2023).
Wright, S. The genetical structure of populations. Ann. Eugen. 15, 323–354 (1951).
Patterson, N., Price, A. L. & Reich, D. Population structure and Eigenanalysis. PLoS Genet. 2, e190 (2006).
Robinson, M. R. et al. Genetic evidence of assortative mating in humans. Nat. Hum. Behav. 1, 0016 (2017).
Gianola, D. Assortative mating and the genetic correlation. Theor. Appl. Genet. 62, 225–231 (1982).
Border, R. et al. Cross-trait assortative mating is widespread and inflates genetic correlation estimates. Science 378, 754–761 (2022).
Walters, R. G. et al. Genotyping and population characteristics of the China Kadoorie Biobank. Cell Genom. 3, 100361 (2023).
Yamamoto, K. et al. Genetic footprints of assortative mating in the Japanese population. Nat. Hum. Behav. 7, 65–73 (2023).
Yengo, L. et al. A saturated map of common genetic variants associated with human height. Nature 610, 704–712 (2022).
Keller, M. C. et al. The genetic correlation between height and IQ: shared genes or assortative mating? PLoS Genet. 9, e1003451 (2013).
Beauchamp, J. P., Cesarini, D., Johannesson, M., Lindqvist, E. & Apicella, C. On the sources of the height-intelligence correlation: new insights from a bivariate ACE model with assortative mating. Behav. Genet. 41, 242–252 (2011).
Bai, Z. & Silverstein, J. W. Spectral Analysis of Large Dimensional Random Matrices (Springer, 2010); https://doi.org/10.1007/978-1-4419-0661-8
Niarchou, M. et al. Genome-wide association study of dietary intake in the UK biobank study and its associations with schizophrenia and other traits. Transl. Psychiatry 10, 51 (2020).
Chang, C. C. et al. Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience 4, 7 (2015).
Das, S. et al. Next-generation genotype imputation service and methods. Nat. Genet. 48, 1284–1287 (2016).
Taliun, D. et al. Sequencing of 53,831 diverse genomes from the NHLBI TOPMed Program. Nature 590, 290–299 (2021).
Cong, P.-K. et al. Genomic analyses of 10,376 individuals in the Westlake BioBank for Chinese (WBBC) pilot project. Nat. Commun. 13, 2939 (2022).
Yengo, L. Code for generating disequilibrium genetic relationship matrix and applications (examples) of the DGREML method using simulated data. Zenodo https://doi.org/10.5281/zenodo.13831647 (2024).
Acknowledgements
L.Y. is supported by the Australian Research Council (grant nos. DE200100425 and FT220100069) and the Snow Medical Research Foundation. P.M.V. is supported by the Australian Research Council (grant no. FL180100072). Y.O. was supported by JSPS KAKENHI (grant no. 22H00476) and AMED (grant nos. JP21gm4010006, JP22km0405211, JP22ek0410075, JP22km0405217 and JP22ek0109594), JST Moonshot R&D (grant nos. JPMJMS2021 and JPMJMS2024), Takeda Science Foundation and Bioinformatics Initiative of Osaka University Graduate School of Medicine. M.C.K. was supported by NIMH (grant nos. MH130448 and MH100141). The China Kadoorie Biobank (CKB) baseline survey was supported by the Kadoorie Charitable Foundation in Hong Kong. The CKB study was supported by grants from Wellcome Trust (grant nos. 212946/Z/18/Z, 202922/Z/16/Z, 104085/Z/14/Z and 088158/Z/09/Z), the National Natural Science Foundation of China (grant nos. 82192901, 82192904 and 82192900) and the National Key Research and Development Program of China (grant no. 2016YFC0900500) and by core funding from the UK Medical Research Council (grant nos. MC_UU_00017/1, MC_UU_12026/2 and MC_U137686851), Cancer Research UK (grant nos. C16077/A29186 and C500/A16896) and the British Heart Foundation (grant no. CH/1996001/9454) to the Clinical Trial Service Unit and Epidemiological Studies Unit at Oxford University. DNA extraction and genotyping were supported by GlaxoSmithKline and the UK Medical Research Council (grant nos. MC-PC-13049 and MC-PC-14135).
Author information
Authors and Affiliations
Consortia
Contributions
L.Y. and Y.Z. conceptualized the study. L.Y. and P.M.V. jointly supervised the study. Y.Z., S.S., S.M. and M.G. conducted statistical analyses of UK Biobank, Biobank Japan and China Kadoorie Biobank data with assistance or guidance from L.Y., Y.O., K.Y., R.G.W., M.C.K. and M.E.G. M.C.K., Y.O. and M.E.G. also contributed through suggestions and comments on study design, methods, analyses and their interpretation. Z.C. and L.L. have contributed to data collection, data management and scientific leadership of China Kadoorie Biobank. L.Y., Y.Z. and P.M.V. wrote the paper with the participation of all authors. All the authors approved the final version of the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Genetics thanks Xia Shen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–39 and Notes 1–9.
Supplementary Code 1
This archive contains multiple *.R files and *.RData files to generate Figs. 1–3.
Supplementary Tables
Supplementary Tables 1–10.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, Y., Sakaue, S., Morris, S. et al. The contribution of gametic phase disequilibrium to the heritability of complex traits. Nat Genet 57, 1418–1425 (2025). https://doi.org/10.1038/s41588-025-02192-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41588-025-02192-4