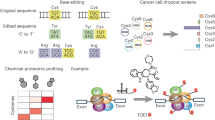
Abstract
More than half of the ~20,000 protein-encoding human genes have paralogs. Chemical proteomics has uncovered many electrophile-sensitive cysteines that are exclusive to subsets of paralogous proteins. Here we explore whether such covalent compound–cysteine interactions can be used to discover ligandable pockets in paralogs lacking the cysteine. Leveraging the covalent ligandability of C109 in the cyclin CCNE2, we substituted the corresponding residue in paralog CCNE1 to cysteine (N112C) and found through activity-based protein profiling that this mutant reacts stereoselectively and site-specifically with tryptoline acrylamides. We then converted the tryptoline acrylamide–CCNE1-N112C interaction into in vitro NanoBRET (bioluminescence resonance energy transfer) and in cellulo activity-based protein profiling assays capable of identifying compounds that reversibly inhibit both the N112C mutant and wild-type CCNE1:CDK2 (cyclin-dependent kinase 2) complexes. X-ray crystallography revealed a cryptic allosteric pocket at the CCNE1:CDK2 interface adjacent to N112 that binds the reversible inhibitors. Our findings, thus, show how electrophile–cysteine interactions mapped by chemical proteomics can extend the understanding of protein ligandability beyond covalent chemistry.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
269,00 € per year
only 22,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Proteomic data are available from ProteomeXchange with identifiers PXD048328 and PXD053077. The atomic coordinates and structure factors were deposited to the PDB (www.pdb.org) under accession codes 8VQ3 (I-198–CCNE1:CDK2 structure) and 8VQ4 (I-125A–CCNE1:CKD2 structure)). Coding sequences of the DNA constructs used in this study are provided in Supplementary Table 1. Source data are provided with this paper.
References
Ibn-Salem, J., Muro, E. M. & Andrade-Navarro, M. A. Co-regulation of paralog genes in the three-dimensional chromatin architecture. Nucleic Acids Res. 45, 81–91 (2017).
Man, O., Atarot, T., Sadot, A., Olender, T. & Lancet, D. From subgenome analysis to protein structure. Curr. Opin. Struct. Biol. 13, 353–358 (2003).
Conant, G. C. & Wolfe, K. H. Turning a hobby into a job: how duplicated genes find new functions. Nat. Rev. Genet. 9, 938–950 (2008).
Ryan, C. J., Mehta, I., Kebabci, N. & Adams, D. J. Targeting synthetic lethal paralogs in cancer. Trends Cancer 9, 397–409 (2023).
Xin, Y. & Zhang, Y. Paralog-based synthetic lethality: rationales and applications. Front. Oncol. 13, 1168143 (2023).
Muller, F. L. et al. Passenger deletions generate therapeutic vulnerabilities in cancer. Nature 488, 337–342 (2012).
Oike, T. et al. A synthetic lethality-based strategy to treat cancers harboring a genetic deficiency in the chromatin remodeling factor BRG1. Cancer Res. 73, 5508–5518 (2013).
Helming, K. C. et al. ARID1B is a specific vulnerability in ARID1A-mutant cancers. Nat. Med. 20, 251–254 (2014).
Benedetti, L., Cereda, M., Monteverde, L., Desai, N. & Ciccarelli, F. D. Synthetic lethal interaction between the tumour suppressor STAG2 and its paralog STAG1. Oncotarget 8, 37619–37632 (2017).
van der Lelij, P. et al. Synthetic lethality between the cohesin subunits STAG1 and STAG2 in diverse cancer contexts. eLife 6, e26980 (2017).
Koferle, A. et al. Interrogation of cancer gene dependencies reveals paralog interactions of autosome and sex chromosome-encoded genes. Cell Rep. 39, 110636 (2022).
De Kegel, B., Quinn, N., Thompson, N. A., Adams, D. J. & Ryan, C. J. Comprehensive prediction of robust synthetic lethality between paralog pairs in cancer cell lines. Cell Syst. 12, 1144–1159 (2021).
Parrish, P. C. R. et al. Discovery of synthetic lethal and tumor suppressor paralog pairs in the human genome. Cell Rep. 36, 109597 (2021).
Clark, J. D., Flanagan, M. E. & Telliez, J. B. Discovery and development of Janus kinase (JAK) inhibitors for inflammatory diseases. J. Med. Chem. 57, 5023–5038 (2014).
Xu, H. et al. PF-06651600, a dual JAK3/TEC family kinase inhibitor. ACS Chem. Biol. 14, 1235–1242 (2019).
Liu, C. et al. Discovery of BMS-986202: a clinical Tyk2 inhibitor that binds to Tyk2 JH2. J. Med. Chem. 64, 677–694 (2021).
Kavanagh, M. E. et al. Selective inhibitors of JAK1 targeting an isoform-restricted allosteric cysteine. Nat. Chem. Biol. 18, 1388–1398 (2022).
Freeman-Cook, K. D. et al. Discovery of PF-06873600, a CDK2/4/6 Inhibitor for the Treatment of Cancer. J. Med. Chem. 64, 9056–9077 (2021).
Hagel, M. et al. First selective small molecule inhibitor of FGFR4 for the treatment of hepatocellular carcinomas with an activated FGFR4 signaling pathway. Cancer Discov. 5, 424–437 (2015).
Borsari, C. et al. Covalent proximity scanning of a distal cysteine to target PI3Kα. J. Am. Chem. Soc. 144, 6326–6342 (2022).
Kathman, S. G. et al. Remodeling oncogenic transcriptomes by small molecules targeting NONO. Nat. Chem. Biol. 19, 825–836 (2023).
Ostrem, J. M., Peters, U., Sos, M. L., Wells, J. A. & Shokat, K. M. K-Ras(G12C) inhibitors allosterically control GTP affinity and effector interactions. Nature 503, 548–551 (2013).
Canon, J. et al. The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature 575, 217–223 (2019).
Guiley, K. Z. & Shokat, K. M. A small molecule reacts with the p53 somatic mutant Y220C to rescue wild-type thermal stability. Cancer Discov. 13, 56–69 (2023).
Vinogradova, E. V. et al. An activity-guided map of electrophile–cysteine interactions in primary human T cells. Cell 182, 1009–1026 (2020).
Lazear, M. R. et al. Proteomic discovery of chemical probes that perturb protein complexes in human cells. Mol. Cell 83, 1725–1742 (2023).
Njomen, E. et al. Multi-tiered chemical proteomic maps of tryptoline acrylamide–protein interactions in cancer cells. Nat. Chem. https://doi.org/10.1038/s41557-024-01601-1 (2024).
Chu, C., Geng, Y., Zhou, Y. & Sicinski, P. Cyclin E in normal physiology and disease states. Trends Cell Biol. 31, 732–746 (2021).
Kirman, L. C. et al. CDK2 Inhibitors and methods of using the same. WIPO patent WO2022165513A1 (2022).
Fagundes, R. & Teixeira, L. K. Cyclin E/CDK2: DNA replication, replication stress and genomic instability. Front. Cell Dev. Biol. 9, 774845 (2021).
Taylor-Harding, B. et al. Cyclin E1 and RTK/RAS signaling drive CDK inhibitor resistance via activation of E2F and ETS. Oncotarget 6, 696–714 (2015).
Tsherniak, A. et al. Defining a cancer dependency map. Cell 170, 564–576 (2017).
Kanska, J., Zakhour, M., Taylor-Harding, B., Karlan, B. Y. & Wiedemeyer, W. R. Cyclin E as a potential therapeutic target in high grade serous ovarian cancer. Gynecol. Oncol. 143, 152–158 (2016).
Machleidt, T. et al. NanoBRET—a novel BRET platform for the analysis of protein–protein interactions. ACS Chem. Biol. 10, 1797–1804 (2015).
Bachovchin, D. A., Brown, S. J., Rosen, H. & Cravatt, B. F. Identification of selective inhibitors of uncharacterized enzymes by high-throughput screening with fluorescent activity-based probes. Nat. Biotechnol. 27, 387–394 (2009).
Leung, D., Hardouin, C., Boger, D. L. & Cravatt, B. F. Discovering potent and selective reversible inhibitors of enzymes in complex proteomes. Nat. Biotechnol. 21, 687–691 (2003).
Yu, Y. et al. Accelerated discovery of macrocyclic CDK2 inhibitor QR-6401 by generative models and structure-based drug design. ACS Med. Chem. Lett. 14, 297–304 (2023).
Freeman-Cook, K. et al. Expanding control of the tumor cell cycle with a CDK2/4/6 inhibitor. Cancer Cell 39, 1404–1421 (2021).
Dietrich, C. et al. INX-315, a selective CDK2 inhibitor, induces cell cycle arrest and senescence in solid tumors. Cancer Discov. 14, 446–467 (2024).
Robers, M. B. et al. Single tracer-based protocol for broad-spectrum kinase profiling in live cells with NanoBRET. STAR Protoc. 2, 100822 (2021).
Honda, R. et al. The structure of cyclin E1/CDK2: implications for CDK2 activation and CDK2-independent roles. EMBO J. 24, 452–463 (2005).
Oruganty, K., Talathi, N. S., Wood, Z. A. & Kannan, N. Identification of a hidden strain switch provides clues to an ancient structural mechanism in protein kinases. Proc. Natl Acad. Sci. USA 110, 924–929 (2013).
Brown, N. R., Noble, M. E., Endicott, J. A. & Johnson, L. N. The structural basis for specificity of substrate and recruitment peptides for cyclin-dependent kinases. Nat. Cell Biol. 1, 438–443 (1999).
Ramharter, J. et al. One atom makes all the difference: getting a foot in the door between SOS1 and KRAS. J. Med. Chem. 64, 6569–6580 (2021).
Bock, A. & Bermudez, M. Allosteric coupling and biased agonism in G protein-coupled receptors. FEBS J. 288, 2513–2528 (2021).
Sadowsky, J. D. et al. Turning a protein kinase on or off from a single allosteric site via disulfide trapping. Proc. Natl Acad. Sci. USA 108, 6056–6061 (2011).
Jahnke, W. et al. Binding or bending: distinction of allosteric Abl kinase agonists from antagonists by an NMR-based conformational assay. J. Am. Chem. Soc. 132, 7043–7048 (2010).
Pines, J. Cell cycle: reaching for a role for the Cks proteins. Curr. Biol. 6, 1399–1402 (1996).
McGrath, D. A. et al. Cks confers specificity to phosphorylation-dependent CDK signaling pathways. Nat. Struct. Mol. Biol. 20, 1407–1414 (2013).
Al-Rawi, A., Kaye, E., Korolchuk, S., Endicott, J. A. & Ly, T. Cyclin A and Cks1 promote kinase consensus switching to non-proline-directed CDK1 phosphorylation. Cell Rep. 42, 112139 (2023).
Bourne, Y. et al. Crystal structure and mutational analysis of the human CDK2 kinase complex with cell cycle-regulatory protein CksHs1. Cell 84, 863–874 (1996).
Jafari, R. et al. The cellular thermal shift assay for evaluating drug target interactions in cells. Nat. Protoc. 9, 2100–2122 (2014).
Thorarensen, A. et al. Design of a Janus kinase 3 (JAK3) specific inhibitor 1-((2S,5R)-5-((7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino)-2-methylpiperidin-1-yl)prop-2-en-1-one (PF-06651600) allowing for the interrogation of JAK3 signaling in humans. J. Med. Chem. 60, 1971–1993 (2017).
Hanan, E. J. et al. Discovery of GDC-0077 (inavolisib), a highly selective inhibitor and degrader of mutant PI3Kα. J. Med. Chem. 65, 16589–16621 (2022).
Tokarski, J. S. et al. Tyrosine kinase 2-mediated signal transduction in T lymphocytes is blocked by pharmacological stabilization of its pseudokinase ___domain. J. Biol. Chem. 290, 11061–11074 (2015).
Erlanson, D. A. et al. Site-directed ligand discovery. Proc. Natl Acad. Sci. USA 97, 9367–9372 (2000).
Garske, A. L., Peters, U., Cortesi, A. T., Perez, J. L. & Shokat, K. M. Chemical genetic strategy for targeting protein kinases based on covalent complementarity. Proc. Natl Acad. Sci. USA 108, 15046–15052 (2011).
Kung, A. et al. A chemical-genetic approach to generate selective covalent inhibitors of protein kinases. ACS Chem. Biol. 12, 1499–1503 (2017).
Barkovich, K. J., Moore, M. K., Hu, Q. & Shokat, K. M. Chemical genetic inhibition of DEAD-box proteins using covalent complementarity. Nucleic Acids Res. 46, 8689–8699 (2018).
Gentile, D. R. et al. Ras binder induces a modified switch-II pocket in GTP and GDP states. Cell Chem. Biol. 24, 1455–1466 (2017).
Cohen, M. S., Zhang, C., Shokat, K. M. & Taunton, J. Structural bioinformatics-based design of selective, irreversible kinase inhibitors. Science 308, 1318–1321 (2005).
Hardy, J. A. & Wells, J. A. Searching for new allosteric sites in enzymes. Curr. Opin. Struct. Biol. 14, 706–715 (2004).
Nussinov, R. & Tsai, C. J. The design of covalent allosteric drugs. Annu. Rev. Pharmacol. Toxicol. 55, 249–267 (2015).
Backus, K. M. et al. Proteome-wide covalent ligand discovery in native biological systems. Nature 534, 570–574 (2016).
Bar-Peled, L. et al. Chemical proteomics identifies druggable vulnerabilities in a genetically defined cancer. Cell 171, 696–709 (2017).
Boatner, L. M., Palafox, M. F., Schweppe, D. K. & Backus, K. M. CysDB: a human cysteine database based on experimental quantitative chemoproteomics. Cell Chem. Biol. 30, 683–698 (2023).
Kuljanin, M. et al. Reimagining high-throughput profiling of reactive cysteines for cell-based screening of large electrophile libraries. Nat. Biotechnol. 39, 630–641 (2021).
Maurais, A. J. & Weerapana, E. Reactive-cysteine profiling for drug discovery. Curr. Opin. Chem. Biol. 50, 29–36 (2019).
Spradlin, J. N., Zhang, E. & Nomura, D. K. Reimagining druggability using chemoproteomic platforms. Acc. Chem. Res. 54, 1801–1813 (2021).
Bathla, P., Mujawar, A., De, A. & Sandanaraj, B. S. Development of noninvasive activity-based protein profiling–bioluminescence resonance energy transfer platform technology enables target engagement studies with absolute specificity in living systems. ACS Pharmacol. Transl. Sci. 7, 375–383 (2024).
Ostrem, J. M. & Shokat, K. M. Direct small-molecule inhibitors of KRAS: from structural insights to mechanism-based design. Nat. Rev. Drug Discov. 15, 771–785 (2016).
Hallin, J. et al. Anti-tumor efficacy of a potent and selective non-covalent KRAS(G12D) inhibitor. Nat. Med. 28, 2171–2182 (2022).
Rees, H. A. & Liu, D. R. Base editing: precision chemistry on the genome and transcriptome of living cells. Nat. Rev. Genet. 19, 770–788 (2018).
Ribeiro, A. A. & Ortiz, V. A chemical perspective on allostery. Chem. Rev. 116, 6488–6502 (2016).
Shpakov, A. O. Allosteric regulation of G-protein-coupled receptors: from diversity of molecular mechanisms to multiple allosteric sites and their ligands. Int. J. Mol. Sci. 24, 6187 (2023).
Zha, J. et al. AlloReverse: multiscale understanding among hierarchical allosteric regulations. Nucleic Acids Res. 51, W33–W38 (2023).
Schreiber, S. L. A chemical biology view of bioactive small molecules and a binder-based approach to connect biology to precision medicines. Isr. J. Chem. 59, 52–59 (2019).
Schoepfer, J. et al. Discovery of asciminib (ABL001), an allosteric inhibitor of the tyrosine kinase activity of BCR-ABL1. J. Med. Chem. 61, 8120–8135 (2018).
Rostovtsev, V. V., Green, L. G., Fokin, V. V. & Sharpless, K. B. A stepwise Huisgen cycloaddition process: copper(I)-catalyzed regioselective ‘ligation’ of azides and terminal alkynes. Angew. Chem. Int. Ed. Engl. 41, 2596–2599 (2002).
Tornoe, C. W., Christensen, C. & Meldal, M. Peptidotriazoles on solid phase: [1,2,3]-triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem. 67, 3057–3064 (2002).
Chen, P. et al. Spectrum and degree of CDK drug interactions predicts clinical performance. Mol. Cancer Ther. 15, 2273–2281 (2016).
Vonrhein, C. et al. Data processing and analysis with the autoPROC toolbox. Acta Crystallogr. D 67, 293–302 (2011).
Tickle, I. J. et al. STARANISO (Global Phasing Ltd., 2018).
McCoy, A. J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007).
Liebschner, D. et al. Macromolecular structure determination using X-rays, neutrons and electrons: recent developments in Phenix. Acta Crystallogr D 75, 861–877 (2019).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr D 66, 486–501 (2010).
Acknowledgements
This work was supported by the National Cancer Institute (R35 CA231991) and Pfizer. We thank Y.-A. He for purification of recombinant CCNE1:CDK2 complexes used for crystallography and R. A. Ferre for development of the CCNE1:CDK2 crystallization conditions. We thank T. J. Matthewson for preparing compound plates for the NanoBRET high-throughput screen. Use of the IMCA-CAT beamline 17-ID (or 17-BM) at the Advanced Photon Source was supported by the companies of the Industrial Macromolecular Crystallography Association through a contract with Hauptman-Woodward Medical Research Institute. This research used resources of the Advanced Photon Source, a US Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under contract no. DE-AC02-06CH11357.
Author information
Authors and Affiliations
Contributions
Y.Z. and B.F.C. conceptualized the study and wrote the paper. Y.Z. designed and performed the gel-ABPP, NanoBRET, ADP-Glo, IP–MS and cell biology experiments. Y.Z. and D.B. designed and performed the protein-directed ABPP experiments. O.B. and S.J.W. purified relevant CCNE1:CDK2 complexes. M.H. and E.J. led the efforts in resolving the I-125A-bound and I-198-bound CCNE1:CDK2 cocrystal structures. Z.L. and B.M. led the efforts in chemical synthesis. Y.Z., Z.L., M.H., O.B., E.J., A.N., D.B., M.D.P., J.D.M., S.N., T.V., A.M.G., M.M.H., A.E.S., A.R.N., B.M. and B.F.C. contributed to data analysis and interpretation. A.N., M.D.P., J.D.M., S.N., T.V., A.M.G., M.M.H., A.E.S. and A.R.N. provided administrative, technical or material support. All authors edited and approved the paper. B.F.C. supervised the study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemical Biology thanks Nathanael Gray, Matthew Robers and the other, anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Cancer Dependency Map data for the CCNE1 and CCNE2 genes.
Cancer Dependency Map data for the CCNE1 and CCNE2 genes, indicating the number of human cancer cell lines showing gene effect scores < −1.0, reflecting strong dependency on the gene32.
Extended Data Fig. 2 Gel-ABPP of a focused library of tryptoline acrylamides for reactivity with an N112C-CCNE1:CDK2 complex.
a, Structures of alkyne-modified tryptoline acrylamides screened for reactivity with an N112C-CCNE1:CDK1 complex. b, Gel-ABPP data showing reactivity of alkyne-modified tryptoline acrylamides (5 µM, 1 h) with purified N112C-CCNE1:CDK2 or WT-CCNE1:CDK2 complexes (1 µM). Gel-ABPP analysis was performed as described in Fig. 1d. Coomassie blue signals correspond to N112C- or WT-CCNE1. Red box marks profile of WX-02-520, which reacted with N112C-CCNE1 in a stereoselective (compared to enantiomer WX-02-521) and site-specific (compared to WT-CCNE1) manner. Data are from a single experiment.
Extended Data Fig. 3 N112C-CCNE1 and WT-CCNE2 show differential reactivity profiles with tryptoline acrylamides.
a, Gel-ABPP data showing concentration-dependent, stereoselective blockade of WX-02-520 engagement of purified N112C-CCNE1 by WX-02-308 in comparison to enantiomer WX-02-326. Purified N112C-CCNE1:CDK2 complex (1 µM) was pre-treated with the indicated concentrations of WX-02-308 or WX-02-326 (2 h) followed by WX-02-520 (1 µM) and gel-ABPP analysis. Data are from a single experiment representative of two independent experiments with similar results. b, Gel-ABPP data showing stereoselective engagement of WT-CCNE2, but not C109A-CCNE2 by WX-02-346 in comparison to enantiomer WX-02-348 or WX-02-520 and WX-02-521. Purified WT- or C109A-CCNE2:CDK2 complexes were treated with the indicated concentrations of alkyne tryptoline acrylamides followed by gel-ABPP analysis. Data are from a single experiment representative of two independent experiments with similar results.
Extended Data Fig. 4 Mass spectrometry (MS) analysis of tryptoline acrylamide stereoprobe reactivity with purified CCNE2:CDK2 and N112C-CCNE1:CDK2 complexes.
a,e, Reverse-phase liquid chromatography-MS traces for purified recombinant WT-CCNE2 (a) and N112C-CCNE1 (e) complexes with CDK2. b-d, Deconvoluted intact mass analysis of the peak mass spectrum for CCNE2 (3 µM) with no compound treatment (b), WX-02-346 (50 µM) treatment (c), and WX-02-348 (50 µM) treatment (d). Multiple phosphorylation states can be assigned to each peak based on expected mass and mass shifts ( + 80 Da / phosphate). f-h, Deconvoluted intact mass analysis of the peak mass spectrum for N112C-CCNE1 (3 µM) with no compound treatment (f), WX-02-308 (50 µM) treatment (g), and WX-02-326 (50 µM) treatment (h). For b-d and f-h, each peak is annotated with mass (top) and peak intensity (bottom). Peak intensities were used to semi-quantitatively assess relative species abundance (shown in table in i). i, Summary of results listing relative abundance of each modified protein species as percent of total across the four tested stereoprobes. Also included are results from experiments performed with control proteins (C109A-CCNE2 and WT-CCNE1) that show only minor and non-stereoselective reactivity with the tested stereoprobes. Green highlights values where substantial ( > 50%) and stereoselective ( > 3X over enantiomer) tryptoline acrylamide reactivity with the indicated CCNE protein was observed. Data are average values ± s.e.m. from three biological replicates.
Extended Data Fig. 5 Reactivity of PEG-conjugated tryptoline acrylamides with N112C-CCNE1.
a, Structures of PEG-conjugated tryptoline acrylamide stereoprobes. b, Gel-ABPP data showing stereoselective blockade of WX-02-520 engagement of purified N112C-CCNE1 by WX-02-588-conjugate in comparison to enantiomer WX-02-589-conjugate or WX-02-520-conjugate and WX-02-521-conjugate. Purified N112C-CCNE1:CDK2 complex (1 µM) was pre-treated with the indicated concentrations of PEG-conjugated stereoprobes (2 h) followed by WX-02-520 (1 µM, 1 h) and gel-ABPP analysis. Red asterisks mark decreased WX-02-520 reactivity with N112C-CCNE1 in samples pre-treated with WX-02-588-conjugate. Data are from a single experiment representative of two independent experiments with similar results.
Extended Data Fig. 6 Characterization of YZ-01-A interactions with N112C-CCNE1.
a, Gel-ABPP data showing that YZ-01-A stereoselectively engages purified N112C-CCNE1:CDK2 but not WT-CCNE1:CDK2 complexes. Purified N112C- or WT-CCNE1:CDK2 complexes were incubated with YZ-01-A or YZ-01-B (5 µM, 1 or 2 h) followed by gel-ABPP analysis. Bottom, representative gel-ABPP data. Top, quantification of data from two biological replicates. b, Gel-ABPP data showing stereoselective engagement of N112C-CCNE1, but not WT-CCNE1 by YZ-01-A in HEK293T cell lysates recombinantly co-expressing Flag-NLuc-N112C-CCNE1 (or Flag-NLuc-WT-CCNE1) and CDK2 treated with YZ-01-A or YZ-01-B (10 µM, 90 min). Whole-cell lysates were used for gel-ABPP analysis. Data are from a single experiment representative of two independent experiments with similar results. c, Concentration- and time-dependence of BRET signals for YZ-01-A interactions with N112C-CCNE1 or WT-CCNE1. Lysates of HEK293T cells expressing Flag-NLuc-N112C-CCNE1 or Flag-NLuc-WT-CCNE1 was treated with different concentrations of YZ-01-A (0.1 – 30 µM) for 60, 90, or 120 min. Data are average values ± s.e.m. from four biological replicates. d, Structures of WX-02-14 and WX-02-34. e, Comparison of gel-ABPP and NanoBRET data showing concentration-dependent, stereoselective blockade of YZ-01-A engagement of Flag-NLuc-N112C-CCNE1 by WX-02-14 in comparison to WX-02-34 (0.2 – 50 µM, 2 h). Dashed horizontal line marks background signals for NanoBRET assays performed with YZ-01-A (10 µM, 90 min) and Flag-NLuc-WT-CCNE1. CI, confidence intervals. Data are average values ± s.e.m. from three biological replicates. f, NanoBRET data showing a time-dependent increase in potency of blockade of YZ-01-A reactivity with Flag-N112C-CCNE1 by WX-02-14 and WX-02-308. Data are average values ± s.e.m. from three biological replicates.
Extended Data Fig. 7 Additional characterization of CCNE1:CDK2 2,6-diazaspiro[3.4]octane ligands.
a, Gel-ABPP data showing concentration-dependent blockade of YZ-01-A engagement of N112C-CCNE1 by I-125A or I-198 in 30 min or 90 min reactions. Experiment was performed as described in Fig. 3c. Data are from a single experiment. b, Gel-ABPP data showing that the residual YZ-01-A reactivity with N112C-CCNE1 in the presence of I-125A or I-198 (1 µM) is stereoselective and site-specific. Experiments were performed as described in Fig. 3c. Data are from a single experiment representative of two independent experiments with similar results. c, NanoBRET data showing concentration-dependent blockade of CDK2 active site-directed tracer K-10 (0.2 µM) binding to Flag-N112C-(left) or Flag-WT-CCNE1(right):CDK2-NLuc complexes by dinaciclib, but not I-125A, as performed in HEK293T cell lysates recombinantly expressing CCNE1:CDK2-NLuc complexes. Data are average values ± s.e.m. from three biological replicates. d, Gel-ABPP data showing the concentration-dependent reactivity of WX-03-348 with WT-CCNE2. Purified WT- or C109A-CCNE2:CDK2 complexes (0.2 µM each) were doped into HEK293T cell lysates (1 mg mL−1) and treated with WX-03-346 or WX-03-348 for 100 min. Right, quantification of data from two biological replicates. e, Gel-ABPP data showing that I-125A does not block the reactivity of WX-03-348 with WT-CCNE2. Purified WT- or C109A-CCNE2:CDK2 complexes (0.2 µM each) were doped into HEK293T cell lysates (1 mg mL−1) and treated with WX-03-57, WX-03-59 (both at 20 µM), or I-125A (1 or 10 µM) for 2 h followed by WX-03-348 (3 µM) for 100 min at RT. Data are from a single experiment representative of two independent experiments with similar results.
Extended Data Fig. 8 Structures of I-125A and I-198-CCNE1:CDK2 complexes.
a, Ribbon diagram structure of CCNE1:CDK2 (green: blue) complex with the I-198 compound shown in orange and the binding cavity shown as a transparent surface. b, Ribbon diagram structure of CCNE1:CDK2 (green: blue) complex with the I-125A compound shown in orange. For a, b, N112 is shown in purple and the distance from this residue to ligands indicated by dashes. c, Detailed view of the I-125A binding site, protein-ligand interactions, and the distance to N112 indicated by dashes. CCNE1 and CDK2 residues are shown in green and blue, respectively, and water molecules are in magenta. d, Protein-ligand interaction plot between CCNE1: CDK2 and I-125A. e, A global sequence alignment of CCNE1 and CCNE2. CCNE1 residues shown in Fig. 4c and Extended Data Fig. 9d are highlighted in green.
Extended Data Fig. 9 Effects of tryptoline acrylamide ligands on protein interactions of N112C-CCNE1:CDK2 complexes.
a, Western blotting of enriched CKS2 in the indicated anti-Flag IP experiments performed as described in Fig. 5d. Top, bar graph depicting quantification of CKS2 enrichment blots and represent average values ± s.e.m. from three biological replicates. Unpaired, two-sided t test with Welch’s correction was perform to test statistical significance. b, Scheme of in cellulo NanoBRET assay measuring CCNE1:CKS1B and CCNE1:CKS2 interactions. c, NanoBRET data showing stabilization of Flag-N112C-CCNE1:CKS1B and:CKS2 interactions, but not Flag-WT-CCNE1:CKS1B and:CKS2 interactions by WX-02-308 (20 µM 6 h). Also shown are NanoBRET data for three representative nuclear proteins (PCBP1, DDX21, RBBP7) not known to interact with CCNE1:CDK2 complexes as negative controls. Data represent average values ± s.e.m. from four independent replicates. Unpaired, two-sided t test with Welch’s correction was used to test statistical significance. d, NanoBRET data showing WX-02-308, but not WX-02-326 or I-125A, stabilizes Flag-N112C-CCNE1:CKS1B and:CKS2 interactions in a concentration-dependent manner. Data represent average values ± s.e.m. from four biological replicates. e, Overlay of crystal structures of CKS1B:CDK2 (PDB: 1BUH) and CCNE1:CDK2 (PDB: 5L2W) showing the relative locations of CCNE1_N112 and CKS1B binding site on CCNE1.
Extended Data Fig. 10 A pilot N112C-CCNE1 NanoBRET high-throughput screen for the discovery of CCNE1 ligands.
a, Schematic for HTS-compatible NanoBRET assay with an N112C-CCNE1:CDK2 complex. HEK293T cell lysate recombinantly expressing Flag-NLuc-N112C-CCNE1 and CDK2 (20 µL of cell lysates at 2 mg protein/µL) are mixed with vivazine (1:100 dilution) and YZ-01-A (10 µM), then dispensed into a 384-well plate pre-plated with individual compounds per well (0.05 µL, 4 mM compound DMSO stocks; final concentration of 10 µM). Compound effects on the reaction between YZ-01-A with Flag-NLuc-N112C-CCNE1:CDK2 are measured by BRET with signals from a reaction with YZ-01-A and Flag-NLuc-WT-CCNE1:CDK2 serving as a background control. b, Time-dependent assessment of signal intensity for the NanoBRET assay performed as described in c. Arrow marks the 170-min time point chosen for HTS because, at this time point, the reaction between YZ-01-A and Flag-NLuc-N112C-CCNE1:CDK2 is still incomplete and a good Z’ score is observed (0.85). Data are average values ± s.e.m. from five biological replicates. c, Time-dependent assessment of signal-to-noise (S/N, signal: Flag-NLuc-N112C-CCNE1 treated with YZ-01-A; noise: Flag-NLuc-WT-CCNE1 treated with YZ-01-A). d, NanoBRET data acquired under optimized assay conditions at 185-min time point showing concentration-dependent stereoselective blockade of YZ-01-A engagement of Flag-NLuc-N112C-CCNE1 by WX-02-308 in comparison to WX-02-326 (2 h pre-treatment) Data are average values ± s.e.m. from four biological replicates. e, NanoBRET data acquired with HTS assay conditions at 175 or 185 min showing concentration-dependent partial blockade of YZ-01-A engagement of Flag-NLuc-N112C-CCNE1 by I-125A and I-198. Data are average values ± s.e.m. from four biological replicates. f, A pilot NanoBRET screen performed as described in a of 324 compounds from the ChemBridge macrocycles collection (10 µM final concentration) alongside the indicated concentrations of I-125A. Dashed lines mark maximum signal (top, DMSO control with Flag-NLuc-N112C-CCNE1:CDK2 complex), 70% inhibition of signal (middle), and background signal (bottom, DMSO control with Flag-NLuc-WT-CCNE1:CDK2 complex).
Supplementary information
Supplementary Information
Supplementary Figs. 1–5, Table 1 and Note.
Supplementary Data 1
Unprocessed proteomic data related to Figs. 1b,c and 5d–f and Supplementary Fig. 1 a–c.
Supplementary Table 2
Coding sequences of DNA constructs used in this study.
Supplementary Data 2
Statistical source data for Supplementary Fig. 1.
Supplementary Data 3
Statistical source data for Supplementary Fig. 2.
Supplementary Data 4
Statistical source data for Supplementary Fig. 3.
Source data
Source Data Fig. 1
Unprocessed gels.
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Unprocessed gels and western blots.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Unprocessed gels and western blots.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Unprocessed gels and western blots.
Source Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Unprocessed gels.
Source Data Extended Data Fig. 3
Unprocessed gels.
Source Data Extended Data Fig. 5
Unprocessed gels.
Source Data Extended Data Fig. 6
Unprocessed gels and western blots.
Source Data Extended Data Fig. 6
Statistic source data.
Source Data Extended Data Fig. 7
Unprocessed gels and western blots.
Source Data Extended Data Fig. 7
Statistic source data.
Source Data Extended Data Fig. 9
Unprocessed gels and western blots.
Source Data Extended Data Fig. 9
Statistic source data.
Source Data Extended Data Fig. 10
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, Y., Liu, Z., Hirschi, M. et al. An allosteric cyclin E-CDK2 site mapped by paralog hopping with covalent probes. Nat Chem Biol 21, 420–431 (2025). https://doi.org/10.1038/s41589-024-01738-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-024-01738-7