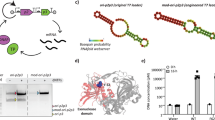
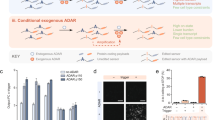
Abstract
Directed evolution in mammalian cells offers a powerful approach for advancing synthetic biology applications. However, existing mammalian-based directed evolution methods face substantial bottlenecks, including host genome interference, small library size and uncontrolled mutagenesis. Here we engineered an orthogonal alphaviral RNA replication system to evolve RNA-based devices, enabling RNA replicase-assisted continuous evolution (REPLACE) in proliferating mammalian cells. This system generates a large, continuously diversified library of replicative RNAs through replicase-limited mode of replication and inducible mutagenesis. Using REPLACE, we engineered fluorescent proteins and transcription factors. Notably, cells equipped with REPLACE can undergo Darwinian adaptation, allowing them to evolve in response to both cell-extrinsic and cell-intrinsic challenges. Collectively, this work establishes a powerful platform for advancing mammalian synthetic biology and cell engineering applications through directed evolution.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
269,00 € per year
only 22,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Raw NGS and PacBio sequencing data and corresponding processed data are available from the NCBI Gene Expression Omnibus (GEO) under accession number GSE235343. The processed Sanger Sequencing data for identified mutants are available in Supplementary Data 1. The structural data for TetR, PadR and MEK1 were obtained from the AlphaFold Protein Structure Database (TetR: AF-A0A829R067-F1-model_v4.pdb; PadR: AF-P94443-F1-model_v4.pdb; MEK1: AF-A4QPA9-F1-model_v4.pdb). The structure of the PadR small-molecule ligand ferulic acid and the structure of MEK1 allosteric inhibitor cobimetinib were sourced from the PDB under accession codes 5X14 and 7JUS. Sequences for plasmids and primers in this work are included in the Supplementary Information. Key plasmids can be obtained from WeKwikGene (https://wekwikgene.wllsb.edu.cn/, identifiers 0000673–0000676), a nonprofit plasmid repository. Source data are provided with this paper.
Code availability
An example Python script for analyzing sequencing data is included in the Supplementary Information.
References
Lienert, F., Lohmueller, J. J., Garg, A. & Silver, P. A. Synthetic biology in mammalian cells: next generation research tools and therapeutics. Nat. Rev. Mol. Cell Biol. 15, 95–107 (2014).
Church, G. M., Elowitz, M. B., Smolke, C. D., Voigt, C. A. & Weiss, R. Realizing the potential of synthetic biology. Nat. Rev. Mol. Cell Biol. 15, 289–294 (2014).
Tan, X., Letendre, J. H., Collins, J. J. & Wong, W. W. Synthetic biology in the clinic: engineering vaccines, diagnostics, and therapeutics. Cell 184, 881–898 (2021).
Weber, W. & Fussenegger, M. Emerging biomedical applications of synthetic biology. Nat. Rev. Genet. 13, 21–35 (2012).
Lim, W. A. & June, C. H. The principles of engineering immune cells to treat cancer. Cell 168, 724–740 (2017).
Sedlmayer, F., Aubel, D. & Fussenegger, M. Synthetic gene circuits for the detection, elimination and prevention of disease. Nat. Biomed. Eng. 2, 399–415 (2018).
Ruder, W. C., Lu, T. & Collins, J. J. Synthetic biology moving into the clinic. Science 333, 1248–1252 (2011).
Purnick, P. E. M. & Weiss, R. The second wave of synthetic biology: from modules to systems. Nat. Rev. Mol. Cell Biol. 10, 410–422 (2009).
Gallup, O., Ming, H. & Ellis, T. Ten future challenges for synthetic biology. Eng. Biol. 5, 51–59 (2021).
Castle, S. D., Grierson, C. S. & Gorochowski, T. E. Towards an engineering theory of evolution. Nat. Commun. 12, 3326 (2021).
Kheir Gouda, M., Manhart, M. & Balázsi, G. Evolutionary regain of lost gene circuit function. Proc. Natl Acad. Sci. USA 116, 25162–25171 (2019).
Simon, A. J., d’Oelsnitz, S. & Ellington, A. D. Synthetic evolution. Nat. Biotechnol. 37, 730–743 (2019).
Lezia, A., Csicsery, N. & Hasty, J. Design, mutate, screen: multiplexed creation and arrayed screening of synchronized genetic clocks. Cell Syst. 13, 365–375 (2022).
Cobb, R. E., Sun, N. & Zhao, H. Directed evolution as a powerful synthetic biology tool. Methods 60, 81–90 (2013).
Haseltine, E. L. & Arnold, F. H. Synthetic gene circuits: design with directed evolution. Annu. Rev. Biophys. Biomol. Struct. 36, 1–19 (2007).
Yokobayashi, Y., Weiss, R. & Arnold, F. H. Directed evolution of a genetic circuit. Proc. Natl Acad. Sci. USA 99, 16587–16591 (2002).
Morrison, M. S., Podracky, C. J. & Liu, D. R. The developing toolkit of continuous directed evolution. Nat. Chem. Biol. 16, 610–619 (2020).
Hendel, S. J. & Shoulders, M. D. Directed evolution in mammalian cells. Nat. Methods 18, 346–357 (2021).
Wang, Y. et al. Directed evolution: methodologies and applications. Chem. Rev. 121, 12384–12444 (2021).
Molina, R. S. et al. In vivo hypermutation and continuous evolution. Nat. Rev. Methods Prim. 2, 1–22 (2022).
Tian, R. et al. Establishing a synthetic orthogonal replication system enables accelerated evolution in E. coli. Science 383, 421–426 (2024).
Ravikumar, A., Arzumanyan, G. A., Obadi, M. K. A., Javanpour, A. A. & Liu, C. C. Scalable, continuous evolution of genes at mutation rates above genomic error thresholds. Cell 175, 1946–1957 (2018).
Majors, B. S., Chiang, G. G. & Betenbaugh, M. J. Protein and genome evolution in mammalian cells for biotechnology applications. Mol. Biotechnol. 42, 216–223 (2009).
Azam, M., Latek, R. R. & Daley, G. Q. Mechanisms of autoinhibition and STI-571/imatinib resistance revealed by mutagenesis of BCR-ABL. Cell 112, 831–843 (2003).
Wang, L., Jackson, W. C., Steinbach, P. A. & Tsien, R. Y. Evolution of new nonantibody proteins via iterative somatic hypermutation. Proc. Natl Acad. Sci. USA 101, 16745–16749 (2004).
Peck, S. H., Chen, I. & Liu, D. R. Directed evolution of a small-molecule-triggered intein with improved splicing properties in mammalian cells. Chem. Biol. 18, 619–630 (2011).
Hess, G. T. et al. Directed evolution using dCas9-targeted somatic hypermutation in mammalian cells. Nat. Methods 13, 1036–1042 (2016).
Ma, Y. et al. Targeted AID-mediated mutagenesis (TAM) enables efficient genomic diversification in mammalian cells. Nat. Methods 13, 1029–1035 (2016).
Berman, C. M. et al. An adaptable platform for directed evolution in human cells. J. Am. Chem. Soc. 140, 18093–18103 (2018).
Piatkevich, K. D. et al. A robotic multidimensional directed evolution approach applied to fluorescent voltage reporters. Nat. Chem. Biol. 14, 352–360 (2018).
Das, A. T. et al. Viral evolution as a tool to improve the tetracycline-regulated gene expression system. J. Biol. Chem. 279, 18776–18782 (2004).
English, J. G. et al. VEGAS as a platform for facile directed evolution in mammalian cells. Cell 178, 748–761 (2019).
Chen, H. et al. Efficient, continuous mutagenesis in human cells using a pseudo-random DNA editor. Nat. Biotechnol. 38, 165–168 (2020).
Sahin, U., Karikó, K. & Türeci, Ö. mRNA-based therapeutics—developing a new class of drugs. Nat. Rev. Drug Discov. 13, 759–780 (2014).
Paunovska, K., Loughrey, D. & Dahlman, J. E. Drug delivery systems for RNA therapeutics. Nat. Rev. Genet. 23, 265–280 (2022).
Khan, K. H. Gene expression in mammalian cells and its applications. Adv. Pharm. Bull. 3, 257–263 (2013).
Strauss, J. H. & Strauss, E. G. The alphaviruses: gene expression, replication, and evolution. Microbiol. Rev. 58, 491–562 (1994).
Frolov, I. et al. Alphavirus-based expression vectors: strategies and applications. Proc. Natl Acad. Sci. USA 93, 11371–11377 (1996).
Tian, L. et al. RNA-dependent RNA polymerase (RdRp) inhibitors: the current landscape and repurposing for the COVID-19 pandemic. Eur. J. Med. Chem. 213, 113201 (2021).
Xiong, C. et al. Sindbis virus: an efficient, broad host range vector for gene expression in animal cells. Science 243, 1188–1191 (1989).
Wolff, J. A. et al. Direct gene transfer into mouse muscle in vivo. Science 247, 1465–1468 (1990).
Zhou, X. et al. Self-replicating Semliki forest virus RNA as recombinant vaccine. Vaccine 12, 1510–1514 (1994).
Wroblewska, L. et al. Mammalian synthetic circuits with RNA binding proteins for RNA-only delivery. Nat. Biotechnol. 33, 839–841 (2015).
Jose, J., Taylor, A. B. & Kuhn, R. J. Spatial and temporal analysis of alphavirus replication and assembly in mammalian and mosquito cells. mBio 8, e02294-16 (2017).
Shirako, Y. & Strauss, J. H. Cleavage between nsP1 and nsP2 initiates the processing pathway of Sindbis virus nonstructural polyprotein P123. Virology 177, 54–64 (1990).
Frolov, I. et al. Selection of RNA replicons capable of persistent noncytopathic replication in mammalian cells. J. Virol. 73, 3854–3865 (1999).
Guo, J.-T., Zhu, Q. & Seeger, C. Cytopathic and noncytopathic interferon responses in cells expressing hepatitis C virus subgenomic replicons. J. Virol. 77, 10769–10779 (2003).
Urakova, N. et al. β-d-N4-hydroxycytidine is a potent anti-alphavirus compound that induces a high level of mutations in the viral genome. J. Virol. 92, e01965-17 (2018).
Delang, L., Abdelnabi, R. & Neyts, J. Favipiravir as a potential countermeasure against neglected and emerging RNA viruses. Antivir. Res. 153, 85–94 (2018).
Sheahan, T. P. et al. An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice. Sci. Transl. Med. 12, eabb5883 (2020).
Kabinger, F. et al. Mechanism of molnupiravir-induced SARS-CoV-2 mutagenesis. Nat. Struct. Mol. Biol. 28, 740–746 (2021).
Slotkin, W. & Nishikura, K. Adenosine-to-inosine RNA editing and human disease. Genome Med. 5, 105 (2013).
Heim, R., Prasher, D. C. & Tsien, R. Y. Wavelength mutations and posttranslational autoxidation of green fluorescent protein. Proc. Natl Acad. Sci. USA 91, 12501–12504 (1994).
Sawano, A. & Miyawaki, A. Directed evolution of green fluorescent protein by a new versatile PCR strategy for site-directed and semi-random mutagenesis. Nucleic Acids Res. 28, e78 (2000).
Stoltzfus, C. R. et al. Two-photon directed evolution of green fluorescent proteins. Sci. Rep. 5, 11968 (2015).
Hirano, M. et al. A highly photostable and bright green fluorescent protein. Nat. Biotechnol. 40, 1132–1142 (2022).
Wang, Y. et al. A versatile genetic control system in mammalian cells and mice responsive to clinically licensed sodium ferulate. Sci. Adv. 6, eabb9484 (2020).
Park, S. C., Kwak, Y. M., Song, W. S., Hong, M. & Yoon, S. Structural basis of effector and operator recognition by the phenolic acid-responsive transcriptional regulator PadR. Nucleic Acids Res. 45, 13080–13093 (2017).
Emery, C. M. et al. MEK1 mutations confer resistance to MEK and B-RAF inhibition. Proc. Natl Acad. Sci. USA 106, 20411–20416 (2009).
Khan, Z. M. et al. Structural basis for the action of the drug trametinib at KSR-bound MEK. Nature 588, 509–514 (2020).
Roskoski, R. Classification of small molecule protein kinase inhibitors based upon the structures of their drug–enzyme complexes. Pharmacol. Res. 103, 26–48 (2016).
Caunt, C. J., Sale, M. J., Smith, P. D. & Cook, S. J. MEK1 and MEK2 inhibitors and cancer therapy: the long and winding road. Nat. Rev. Cancer 15, 577–592 (2015).
Delaney, A. M., Printen, J. A., Chen, H., Fauman, E. B. & Dudley, D. T. Identification of a novel mitogen-activated protein kinase kinase activation ___domain recognized by the inhibitor PD 184352. Mol. Cell. Biol. 22, 7593–7602 (2002).
Gao, Y. et al. V211D mutation in MEK1 causes resistance to MEK inhibitors in colon cancer. Cancer Discov. 9, 1182–1191 (2019).
Adjei, A. A. Blocking oncogenic Ras signaling for cancer therapy. J. Natl Cancer Inst. 93, 1062–1074 (2001).
Stewart, S. & Guan, K.-L. The dominant negative Ras mutant, N17Ras, can inhibit signaling independently of blocking ras activation. J. Biol. Chem. 275, 8854–8862 (2000).
Treisman, R. The serum response element. Trends Biochem. Sci. 17, 423–426 (1992).
Zhang, B., Zhang, Y., Wang, Z. & Zheng, Y. The role of Mg2+ cofactor in the guanine nucleotide exchange and GTP hydrolysis reactions of Rho family GTP-binding proteins. J. Biol. Chem. 275, 25299–25307 (2000).
Feig, L. A. Dominant inhibitory Ras mutants: tools for elucidating ras function. In GTPases in Biology I (eds Dickey, B. F. & Birnbaumer, L.) (Springer, 1993).
Kwan, A. K., Piazza, G. A., Keeton, A. B. & Leite, C. A. The path to the clinic: a comprehensive review on direct KRASG12C inhibitors. J. Exp. Clin. Cancer Res. 41, 27 (2022).
Spoerner, M. et al. Conformational states of human Rat sarcoma (Ras) protein complexed with its natural ligand GTP and their role for effector interaction and GTP hydrolysis. J. Biol. Chem. 285, 39768–39778 (2010).
Pantsar, T. et al. Assessment of mutation probabilities of KRAS G12 missense mutants and their long-timescale dynamics by atomistic molecular simulations and Markov state modeling. PLoS Comput. Biol. 14, e1006458 (2018).
Harvey, W. T. et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 19, 409–424 (2021).
Sanderson, T. et al. A molnupiravir-associated mutational signature in global SARS-CoV-2 genomes. Nature 623, 594–600 (2023).
Denes, C. E. et al. The VEGAS platform is unsuitable for mammalian directed evolution. ACS Synth. Biol. 11, 3544–3549 (2022).
Liljeström, P., Lusa, S., Huylebroeck, D. & Garoff, H. In vitro mutagenesis of a full-length cDNA clone of Semliki forest virus: the small 6,000-molecular-weight membrane protein modulates virus release. J. Virol. 65, 4107–4113 (1991).
Agapov, E. V. et al. Noncytopathic Sindbis virus RNA vectors for heterologous gene expression. Proc. Natl Acad. Sci. USA 95, 12989–12994 (1998).
Varadi, M. et al. AlphaFold Protein Structure Database: massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 50, D439–D444 (2022).
Acknowledgements
We thank H. Ye (East China Normal University) for his gift of plasmids and members of the Y.L. lab, particularly S. Fan, J. Wu, Z. Gao, P. Ma, Y. Liu and B. Chen, for help and discussions. We thank the Flow Cytometry Core at the National Center for Protein Sciences at Peking University, particularly H. Yang and X. Zhang, for their technical help. We thank the Quantitative Imaging Facility at the Center for Quantitative Biology at Peking University for equipment support and the High-Performance Computing Platform of the Peking-Tsinghua Center for Life Sciences for computational support. Y.L. acknowledges grants from the National Key R&D Program of China (2020YFA0906900 and 2018YFA0900703), the National Natural Science Foundation of China (T2325002, T2321001 and 32088101) and the Key Technology R&D Program of Sichuan (2024YFHZ0064).
Author information
Authors and Affiliations
Contributions
L.M. and Y.L. conceptualized and designed the study. L.M. performed all the experiments and most of the analysis, with input on data interpretation and analysis from Y.L. L.M. and Y.L. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
L.M. and Y.L. have been granted a patent related to the tools developed in this work (patent no. CN116064597B).
Peer review
Peer review information
Nature Chemical Biology thanks G. Neely and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–32, notes 1–9 and Tables 1–4.
Supplementary Data 1
Sanger sequencing data of individual variants from different directed evolution campaigns.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ma, L., Lin, Y. Orthogonal RNA replication enables directed evolution and Darwinian adaptation in mammalian cells. Nat Chem Biol 21, 451–463 (2025). https://doi.org/10.1038/s41589-024-01783-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-024-01783-2
This article is cited by
-
REPLACE-ing RNA through evolution
Nature Chemical Biology (2025)
-
Nickase fidelity drives EvolvR-mediated diversification in mammalian cells
Nature Communications (2025)
-
A chimeric viral platform for directed evolution in mammalian cells
Nature Communications (2025)