Abstract
Eukaryotic transposon-encoded Fanzor proteins hold great promise for genome-engineering applications as a result of their compact size and mechanistic resemblance to TnpB. However, the unmodified Fanzor systems show extremely low activity in mammalian cells. Guided by the predicted structure of a Fanzor2 complex using AlphaFold3, we engineered the NlovFz2 nuclease and its cognate ωRNA to create an evolved enNlovFz2 system, with an expanded target-adjacent motif (TAM) recognition scope (5′-NMYG) and a substantially improved genome-editing efficiency, achieving an 11.1-fold increase over the wild-type NlovFz2, comparable to two previously reported IS200 or IS605 transposon-encoded TnpBs and two CRISPR–Cas12f1 nucleases. Notably, enNlovFz2 efficiently mediated gene disruption in mouse embryos and restored dystrophin expression in a humanized Duchenne muscular dystrophy mouse model with single adeno-associated virus delivery. Our findings underscore the potential of eukaryotic RNA-guided Fanzor2 nucleases as a versatile toolbox for both biological research and therapeutic applications.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
269,00 € per year
only 22,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
NGS data are available at the National Center for Biotechnology Information’s Sequence Read Archive database under the BioProject accession no. PRJNA1147587. Source data are provided with this paper.
References
Makarova, K. S. et al. Evolutionary classification of CRISPR-Cas systems: a burst of class 2 and derived variants. Nat. Rev. Microbiol. 18, 67–83 (2020).
Marraffini, L. A. CRISPR-Cas immunity in prokaryotes. Nature 526, 55–61 (2015).
Wright, A. V., Nuñez, J. K. & Doudna, J. A. Biology and applications of CRISPR systems: harnessing nature’s toolbox for genome engineering. Cell 164, 29–44 (2016).
Karvelis, T. et al. Transposon-associated TnpB is a programmable RNA-guided DNA endonuclease. Nature 599, 692–696 (2021).
Sasnauskas, G. et al. TnpB structure reveals minimal functional core of Cas12 nuclease family. Nature 616, 384–389 (2023).
Nakagawa, R. et al. Cryo-EM structure of the transposon-associated TnpB enzyme. Nature 616, 390–397 (2023).
Meers, C. et al. Transposon-encoded nucleases use guide RNAs to promote their selfish spread. Nature 622, 863–871 (2023).
Altae-Tran, H. et al. The widespread IS200/IS605 transposon family encodes diverse programmable RNA-guided endonucleases. Science 374, 57–65 (2021).
Schuler, G., Hu, C. & Ke, A. Structural basis for RNA-guided DNA cleavage by IscB-omegaRNA and mechanistic comparison with Cas9. Science 376, 1476–1481 (2022).
Hirano, S. et al. Structure of the OMEGA nickase IsrB in complex with omegaRNA and target DNA. Nature 610, 575–581 (2022).
Saito, M. et al. Fanzor is a eukaryotic programmable RNA-guided endonuclease. Nature 620, 660–668 (2023).
Jiang, K. et al. Programmable RNA-guided DNA endonucleases are widespread in eukaryotes and their viruses. Sci. Adv. 9, eadk0171 (2023).
Xu, P. et al. Structural insights into the diversity and DNA cleavage mechanism of Fanzor. Cell 187, 5238–5252.e5220 (2024).
Zetsche, B. et al. Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR-Cas system. Cell 163, 759–771 (2015).
Fonfara, I., Richter, H., Bratovič, M., Le Rhun, A. & Charpentier, E. The CRISPR-associated DNA-cleaving enzyme Cpf1 also processes precursor CRISPR RNA. Nature 532, 517–521 (2016).
Harrington, L. B. et al. Programmed DNA destruction by miniature CRISPR-Cas14 enzymes. Science 362, 839–842 (2018).
Bao, W. & Jurka, J. Homologues of bacterial TnpB_IS605 are widespread in diverse eukaryotic transposable elements. Mob. DNA 4, 12 (2013).
Li, Z. et al. Engineering a transposon-associated TnpB-ωRNA system for efficient gene editing and phenotypic correction of a tyrosinaemia mouse model. Nat. Commun. 15, 831 (2024).
Marquart, K. F. et al. Effective genome editing with an enhanced ISDra2 TnpB system and deep learning-predicted ωRNAs. Nat. Methods 21, 2084–2093 (2024).
Xiang, G. et al. Evolutionary mining and functional characterization of TnpB nucleases identify efficient miniature genome editors. Nat. Biotechnol. 42, 745–757 (2024).
Su, M. et al. Molecular basis and engineering of miniature Cas12f with C-rich PAM specificity. Nat. Chem. Biol. 20, 180–189 (2024).
Kong, X. et al. Engineered CRISPR-OsCas12f1 and RhCas12f1 with robust activities and expanded target range for genome editing. Nat. Commun. 14, 2046 (2023).
Kim, D. Y. et al. Efficient CRISPR editing with a hypercompact Cas12f1 and engineered guide RNAs delivered by adeno-associated virus. Nat. Biotechnol. 40, 94–102 (2022).
Han, D. et al. Development of miniature base editors using engineered IscB nickase. Nat. Methods 20, 1029–1036 (2023).
Chen, W. et al. Cas12n nucleases, early evolutionary intermediates of type V CRISPR, comprise a distinct family of miniature genome editors. Mol. Cell 83, 2768–2780.e6 (2023).
Wu, Z. et al. Structure and engineering of miniature Acidibacillus sulfuroxidans Cas12f1. Nat. Catal. 6, 695–709 (2023).
Nishimasu, H. et al. Engineered CRISPR-Cas9 nuclease with expanded targeting space. Science 361, 1259–1262 (2018).
Walton, R. T., Christie, K. A., Whittaker, M. N. & Kleinstiver, B. P. Unconstrained genome targeting with near-PAMless engineered CRISPR-Cas9 variants. Science 368, 290–296 (2020).
Zhang, H. et al. An engineered xCas12i with high activity, high specificity, and broad PAM range. Protein Cell 14, 538–543 (2023).
Yang, Y. et al. Highly efficient and rapid detection of the cleavage activity of Cas9/gRNA via a fluorescent reporter. Appl. Biochem. Biotechnol. 180, 655–667 (2016).
Leenay, R. T. et al. Identifying and visualizing functional PAM diversity across CRISPR-Cas systems. Mol. Cell 62, 137–147 (2016).
Bae, S., Park, J. & Kim, J. S. Cas-OFFinder: a fast and versatile algorithm that searches for potential off-target sites of Cas9 RNA-guided endonucleases. Bioinformatics 30, 1473–1475 (2014).
Liu, Y. et al. PEM-seq comprehensively quantifies DNA repair outcomes during gene-editing and DSB repair. STAR Protoc. 3, 101088 (2022).
Olson, E. N. Toward the correction of muscular dystrophy by gene editing. Proc. Natl Acad. Sci. USA 118, e2004840117 (2021).
Min, Y. L. et al. CRISPR-Cas9 corrects Duchenne muscular dystrophy exon 44 deletion mutations in mice and human cells. Sci. Adv. 5, eaav4324 (2019).
Long, C. et al. Correction of diverse muscular dystrophy mutations in human engineered heart muscle by single-site genome editing. Sci. Adv. 4, eaap9004 (2018).
Boyer, M. et al. Giant Marseillevirus highlights the role of amoebae as a melting pot in emergence of chimeric microorganisms. Proc. Natl Acad. Sci. USA 106, 21848–21853 (2009).
Xu, X. et al. Engineered miniature CRISPR-Cas system for mammalian genome regulation and editing. Mol. Cell 81, 4333–4345.e4 (2021).
Han, L. et al. Engineering miniature IscB nickase for robust base editing with broad targeting range. Nat. Chem. Biol. 20, 1629–1639 (2024).
Yan, H. et al. Assessing and engineering the IscB-ωRNA system for programmed genome editing. Nat. Chem. Biol. 20, 1617–1628 (2024).
Wang, Y. et al. Guide RNA engineering enables efficient CRISPR editing with a miniature Syntrophomonas palmitatica Cas12f1 nuclease. Cell Rep. 40, 111418 (2022).
Wang, D., Zhang, F. & Gao, G. CRISPR-based therapeutic genome editing: strategies and in vivo delivery by AAV vectors. Cell 181, 136–150 (2020).
Raguram, A., Banskota, S. & Liu, D. R. Therapeutic in vivo delivery of gene editing agents. Cell 185, 2806–2827 (2022).
Komor, A. C., Kim, Y. B., Packer, M. S., Zuris, J. A. & Liu, D. R. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature 533, 420–424 (2016).
Gaudelli, N. M. et al. Programmable base editing of A•T to G•C in genomic DNA without DNA cleavage. Nature 551, 464–471 (2017).
Anzalone, A. V. et al. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature 576, 149–157 (2019).
Kong, X. et al. Precise genome editing without exogenous donor DNA via retron editing system in human cells. Protein Cell 12, 899–902 (2021).
Nuñez, J. K. et al. Genome-wide programmable transcriptional memory by CRISPR-based epigenome editing. Cell 184, 2503–2519.e2517 (2021).
Clement, K. et al. CRISPResso2 provides accurate and rapid genome editing sequence analysis. Nat. Biotechnol. 37, 224–226 (2019).
Yin, J. et al. Optimizing genome editing strategy by primer-extension-mediated sequencing. Cell Discov. 5, 18 (2019).
Acknowledgements
We appreciate the support from the Gene Editing Scientific Teaching (NWAFU-GEST), High-Performance Computing and Life Science Research Core Service platforms (K. R. Huang, X. R. Liu, L. Chen, M. Zhou and L. Q. Li) at Northwest A&F University (NWAFU). We also thank the members of HuidaGene Therapeutics Co., Ltd. for their contributions in supplying experimental materials and insightful discussions. This work is supported by the Biological Breeding-Major Projects (grant nos. 2023ZD04074 to K.X., 2023ZD04051 to Y.W. and 2022ZD04014 to X.W.), the National Natural Science Foundation of China (grant nos. 32441080 and 32301251 to Y.W., 22207074 to Z.W. and 32272848 to X.W.), the National Science and Technology Major Project of China (grant no. 2023ZD0500500 to Z.W.), the China Agricultural Research System (grant no. CARS-39-03 to X.W.) and local grants (grant nos. QCYRCXM-2023-104 and Z1013023006 to Y.W., 22YF1428100 to Z.W. and 2023A02011-2-4 and 2022GDTSLD-46 to X.W.).
Author information
Authors and Affiliations
Contributions
Y.W. and X.W. conceived the project. Y.W., Z.W. and K.X. designed the experiments. Y.W., P.G., S.L. and G.L. performed data analysis. Z.W. and D.P. conducted the structural prediction and biochemical assays. P.G., Y.F.C., H.J., Y.Y., S.L., Z.W., Z.L. and M.Z. performed cell transfection and FACS. Y.W. and G.L. performed animal experiments. Y.W. and Z.W. wrote the manuscripts. Y.W., X.W., Z.W., Y.L.C. and K.X. supervised the project.
Corresponding authors
Ethics declarations
Competing interests
Y.W., X.W. and K.X. have filed a patent application related to this work through NWAFU (patent no. 202411627205.2). The other authors declare no competing interests.
Peer review
Peer review information
Nature Chemical Biology thanks Chunyi Hu, Shahid Mansoor and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
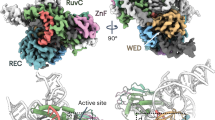
Extended Data Fig. 1 Structural comparisons of the Fanzor1-ωRNA, Fanzor2-ωRNA, TnpB-ωRNA, and CRISPR-Cas12f1 systems.
a, Experimental or predicted structures of SpuFz1-ωRNA (PDB: 8GKH), NlovFz2-ωRNA (AlphaFold3), ISDra2-ωRNA (PDB: 8EXA), and AsCas12f1-sgRNA (PDB: 7WJU), along with their topological schematics. b, Experimental or predicted structures of SpuFz1 (PDB: 8GKH), NlovFz2 (AlphaFold3), ISDra2 (PDB: 8EXA), and AsCas12f1 (PDB: 7WJU).
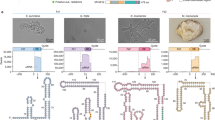
Extended Data Fig. 2 Engineering NlovFz2 ωRNA and protein to enhance editing efficiency in human cells.
a, Experimental workflow for detecting NlovFz2 genome-editing activity at the endogenous B2M locus by optimizing the structure of the ωRNA scaffold in HEK293T cells. b, Second round of ωRNA optimization involves combining high-efficiency variants of A-U and G-U base pair substitutions, building upon the results from the first round. Fold-change represents the ratio of the editing efficiency of ωRNA variants to that of WT-ωRNA. Values and error bars are expressed as mean ± SEM (n = 3 independent biological replicates). c, Schematics illustrating the detection of indel activity based on the fluorescence signal of the EGFP reporter activation in HEK293T cells. d, EGFP activation efficiency was evaluated by combining single NlovFz2 mutations (P6R, Q44R, E64R, T178R, Q285R) with an ωRNA variant (S3Loop-Δ4U + UA97CG). The dashed line indicates the cleavage activity of WT-NlovFz2.
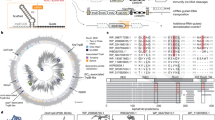
Extended Data Fig. 3 Comprehensive validation of TAM preferences of WT-NlovFz2 and enNlovFz2 at endogenous loci in human cells.
a, Schematics illustrating the detection of nuclease genome-editing activity at endogenous loci in HEK293T cells. b-e, Comparing the genome-editing efficiency of WT-NlovFz2 and enNlovFz2 with different TAM preferences (5′-NCCG, b; 5′-NCTG, c; 5′-NACG, d; 5′-NATG, e) at endogenous loci in human cells. Values and error bars are expressed as mean ± SEM (n = 3 independent biological replicates).
Extended Data Fig. 4 Comparison of genome-editing efficiency among WT-NlovFz2, enNlovFz2, IS200/IS605 transposon-encoded TnpBs, enCnCas12f1, and enRhCas12f1 in human cells.
a, Comparison of genome-editing activity at 9 human endogenous gene loci targeted by WT-NlovFz2, enNlovFz2, enISDra2 TnpB, TnpBmax, enCnCas12f1, and enRhCas12f1 in HEK293T cells. Adjusted P (Padj) values are 0.0001, 0.7090, 0.5794, 0.1856, 0.0002, respectively. b, Comparison of gene editing activity at 9 human endogenous gene loci targeted by WT-NlovFz2, enNlovFz2, ISAba30 TnpB, enCnCas12f1, and enRhCas12f1 in HEK293T cells. Adjusted P (Padj) values are 0.0176, 0.3798, 0.6279, 0.0513, respectively. Values and error bars are expressed as mean ± SEM (n = 3 independent biological replicates). P values were derived using a two-sided Student’s t-test. The top 30% of mCherry+ cells were sorted by FACS to evaluate the genome-editing efficiency of NlovFz2, TnpBs, and Cas12f1 nucleases at endogenous genomic loci.
Extended Data Fig. 5 The sgRNA/ωRNA-dependent off-target effects of enNlovFz2, enCnCas12f1, and enRhCas12f1 at in-silico predicted off-target sites were determined by targeted-amplicon sequencing.
The left, middle, and right panels represent the on-target and top10 off-target sites of enNlovFz2, enCnCas12f1, and enRhCas12f1 targeting DYRK1A, HPRT1, and DMD-guide 4 genes, respectively. Values and error bars are expressed as mean ± SEM (n = 3 independent biological replicates).
Extended Data Fig. 6 Establishment and characterization of the DMDΔE44 mdx mouse model.
a, Strategy for generating the DMDΔE44 mdx mouse model. The human DMD exon44 was deleted using the traditional CRISPR-Cas9 system. The DMDΔE44 mdx mice were generated by mating humanized DMDΔE44 mice with mdx mice. b, Dystrophin immunofluorescence in indicated muscles of wild-type (WT) and DMDΔE44 mdx mice. WT mice were generated by crossing STOCK Tg (DMD) 72Thoen/J mice (#018900) with mdx mice, which carry a c.2977 C > T, p.Gln993* mutation in exon 23 on Chr.X. The staining of dystrophin (Abcam) and spectrin (Millipore) proteins is shown in green and red, respectively. c, Western blotting was employed to confirm the absence of dystrophin (Sigma) in heart, diaphragm (DI), and tibialis anterior (TA) muscles of DMDΔE44 mdx mice. d, HE staining and Sirius red staining were performed on the TA, DI, and heart muscles of WT and DMDΔE44 mdx mice. e, Serum CK, a muscle damage and membrane leakage marker, was measured in WT and DMDΔE44 mdx mice (n = 6 independent biological replicates). The Padj value is 0.0002. f, WT and DMDΔE44 mdx mice underwent forelimb grip strength testing to assess muscle performance (n = 6 independent biological replicates). The Padj value is 0.000001. All mice were 4 weeks old at the time of the experiment. Data are presented as mean ± SEM. Each dot represents an individual mouse. P values were derived using a two-sided Student’s t-test. Scale bar: 100 μm.
Supplementary information
Supplementary Information
Supplementary Figs 1–7 and Tables 1–5.
Supplementary Data 1
Source data for Supplementary Figures.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Unprocessed western blots.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 5
Unprocessed western blots.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 6
Unprocessed western blots.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wei, Y., Gao, P., Pan, D. et al. Engineering eukaryotic transposon-encoded Fanzor2 system for genome editing in mammals. Nat Chem Biol (2025). https://doi.org/10.1038/s41589-025-01902-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41589-025-01902-7