Abstract
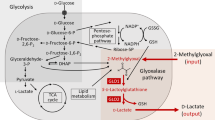
Methylglyoxal (MG) is a reactive metabolite involved in diabetes and aging through the formation of protein adducts. Less is known about the extent that MG and its metabolic product S-d-lactoylglutathione (LGSH) form adducts with cell metabolites. Using a ‘symmetric’ isotope-labeled and reactivity-based metabolomics approach in living cells, we found over 200 adducts and, surprisingly, discovered that 10 of the most abundant are lactoylated amino acids mainly derived from LGSH. The most abundant adduct d-Lac-Cys is formed rapidly between LGSH and cysteine, whereas the diastereoisomer l-Lac-Cys is formed directly from MG and cysteine, assigning cysteine with both glyoxalase 1-like and glyoxalase 2-like activity. Cellular cysteine and MG dynamically regulate d-Lac-Cys and l-Lac-Cys levels and the adducts are increased in diabetes, suggesting their use as novel biomarkers. Lastly, cysteine amides, as proxies for protein cysteines, also undergo lactoylation by MG and LGSH, suggesting the existence of two additional pathways for nonenzymatic lactoylation of proteins.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
269,00 € per year
only 22,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The metabolomics datasets generated during the current study were deposited to the MetaboLight repository with dataset identifier MTBLS6287. All other datasets generated and analyzed during the current study are presented in the manuscript or Supplementary Information. Source data are provided with this paper.
References
Kold-Christensen, R. & Johannsen, M. Methylglyoxal metabolism and aging-related disease: moving from correlation toward causation. Trends Endocrinol. Metab. 31, 81–92 (2020).
Gaffney, D. O. et al. Non-enzymatic lysine lactoylation of glycolytic enzymes. Cell Chem. Biol. 27, 206–213 (2020).
Lamprea-Montealegre, J. A. et al. Plasma levels of advanced glycation endproducts and risk of cardiovascular events: findings from 2 prospective cohorts. J. Am. Heart Assoc. 11, e024012 (2022).
Coukos, J. S. & Moellering, R. E. Methylglyoxal forms diverse mercaptomethylimidazole crosslinks with thiol and guanidine pairs in endogenous metabolites and proteins. ACS Chem. Biol. 16, 2453–2461 (2021).
Salomon, T. et al. Ketone body acetoacetate buffers methylglyoxal via a non-enzymatic conversion during diabetic and dietary ketosis. Cell Chem. Biol. 24, 935–943 (2017).
Corbett, A. J. et al. T-cell activation by transitory neo-antigens derived from distinct microbial pathways. Nature 509, 361–365 (2014).
Li, V. L. et al. An exercise-inducible metabolite that suppresses feeding and obesity. Nature 606, 785–790 (2022).
Nemet, I. et al. A cardiovascular disease-linked gut microbial metabolite acts via adrenergic receptors. Cell 180, 862–877 (2020).
Mossad, O. et al. Gut microbiota drives age-related oxidative stress and mitochondrial damage in microglia via the metabolite N6-carboxymethyllysine. Nat. Neurosci. 25, 295–305 (2022).
Sharma, R. et al. Circulating markers of NADH-reductive stress correlate with mitochondrial disease severity. J. Clin. Invest. 131, e136055 (2021).
Poulsen, M. W. et al. Advanced glycation endproducts in food and their effects on health. Food Chem. Toxicol. 60, 10–37 (2013).
Sibbersen, C. & Johannsen, M. Dicarbonyl derived post-translational modifications: chemistry bridging biology and aging-related disease. Essays Biochem. 64, 97–110 (2020).
Hacker, S. M. et al. Global profiling of lysine reactivity and ligandability in the human proteome. Nat. Chem. 9, 1181–1190 (2017).
Weerapana, E. et al. Quantitative reactivity profiling predicts functional cysteines in proteomes. Nature 468, 790–795 (2010).
Lin, W., Conway, L. P., Vujasinovic, M., Löhr, J. M. & Globisch, D. Chemoselective and highly sensitive quantification of gut microbiome and human metabolites. Angew. Chem. Int. Ed. Engl. 60, 23232–23240 (2021).
Aćimović, J. M., Stanimirović, B. D., Todorović, N., Jovanović, V. B. & Mandić, L. M. Influence of the microenvironment of thiol groups in low molecular mass thiols and serum albumin on the reaction with methylglyoxal. Chem. Biol. Interact. 188, 21–30 (2010).
Edwards, L. G. & Thornalley, P. J. Prevention of S-d-lactoylglutathione-induced inhibition of human leukaemia 60 cell growth by uridine. Leuk. Res. 18, 717–722 (1994).
Edwards, L. G., Adesida, A. & Thornalley, P. J. Inhibition of human leukaemia 60 cell growth by S-d-lactoylglutathione in vitro. Mediation by metabolism to N-d-lactoylcysteine and induction of apoptosis. Leuk. Res. 20, 17–26 (1996).
Sumner, L. W. et al. Proposed minimum reporting standards for chemical analysis. Metabolomics 3, 211–221 (2007).
Stipanuk, M. H., Dominy, J. E., Lee, J.-I. & Coloso, R. M. Mammalian cysteine metabolism: new insights into regulation of cysteine metabolism. J. Nutr. 136, 1652S–1659S (2006).
Rabbani, N. & Thornalley, P. J. Measurement of methylglyoxal by stable isotopic dilution analysis LC–MS/MS with corroborative prediction in physiological samples. Nat. Protoc. 9, 1969–1979 (2014).
Hall, S. S., Doweyko, A. M. & Jordan, F. Glyoxalase I enzyme studies. 4. General base catalyzed enediol proton transfer rearrangement of methyl- and phenylglyoxalglutathionylhemithiol acetal to S-lactoyl- and S-mandeloylglutathione followed by hydrolysis. A model for the glyoxalase enzyme system. J. Am. Chem. Soc. 100, 5934–5939 (1978).
Weber, A. L. Formation of the thioester, N-acetyl, S-lactoylcysteine, by reaction of N-acetylcysteine with pyruvaldehyde in aqueous solution. J. Mol. Evol. 18, 354–359 (1982).
Dawson, P. E., Muir, T. W., Clark-Lewis, I. & Kent, S. B. H. Synthesis of proteins by native chemical ligation. Science 266, 776–779 (1994).
Burke, H. M., McSweeney, L. & Scanlan, E. M. Exploring chemoselective S-to-N acyl transfer reactions in synthesis and chemical biology. Nat. Commun. 8, 15655 (2017).
Thornalley, P. J., Yurek-George, A. & Argirov, O. K. Kinetics and mechanism of the reaction of aminoguanidine with the α-oxoaldehydes glyoxal, methylglyoxal, and 3-deoxyglucosone under physiological conditions. Biochem. Pharmacol. 60, 55–65 (2000).
Coukos, J. S., Lee, C. W., Pillai, K. S., Shah, H. & Moellering, R. E. PARK7 catalyzes stereospecific detoxification of methylglyoxal consistent with glyoxalase and not deglycase function. Biochemistry 62, 3126–3133 (2023).
Jansen, R. S. et al. N-lactoyl-amino acids are ubiquitous metabolites that originate from CNDP2-mediated reverse proteolysis of lactate and amino acids. Proc. Natl Acad. Sci. USA 112, 6601–6606 (2015).
Adesida, A., Edwards, L. G. & Thornalley, P. J. Facile synthesis of (R)N-2-hydroxyacyl-l-cysteine derivatives: (R)N-2-hydroxyacyl transfer from enzymatically-synthesized (R)S-2-hydroxyacylglutathione derivatives to l-cysteine. Amino Acids 9, 185–189 (1995).
Bracher, P. J., Snyder, P. W., Bohall, B. R. & Whitesides, G. M. The relative rates of thiol–thioester exchange and hydrolysis for alkyl and aryl thioalkanoates in water. Orig. Life Evol. Biosph. 41, 399–412 (2011).
Bizzozero, O. A., Bixler, H. A. & Pastuszyn, A. Structural determinants influencing the reaction of cysteine-containing peptides with palmitoyl-coenzyme A and other thioesters. Biochim. Biophys. Acta 1545, 278–288 (2001).
Saito, F., Noda, H. & Bode, J. W. Critical evaluation and rate constants of chemoselective ligation reactions for stoichiometric conjugations in water. ACS Chem. Biol. 10, 1026–1033 (2015).
Banjac, A. et al. The cystine/cysteine cycle: a redox cycle regulating susceptibility versus resistance to cell death. Oncogene 27, 1618–1628 (2007).
Sibbersen, C. et al. Profiling of methylglyoxal blood metabolism and advanced glycation end-product proteome using a chemical probe. ACS Chem. Biol. 13, 3294–3305 (2018).
Kanikarla-Marie, P., Micinski, D. & Jain, S. K. Hyperglycemia (high-glucose) decreases l-cysteine and glutathione levels in cultured monocytes and blood of Zucker diabetic rats. Mol. Cell. Biochem. 459, 151–156 (2019).
He, Y. et al. Glyoxalase system: a systematic review of its biological activity, related-diseases, screening methods and small molecule regulators. Biomed. Pharmacother. 131, 110663 (2020).
Luengo, A. et al. Reactive metabolite production is a targetable liability of glycolytic metabolism in lung cancer. Nat. Commun. 10, 5604–5604 (2019).
Sutton, T. R. et al. A robust and versatile mass spectrometry platform for comprehensive assessment of the thiol redox metabolome. Redox Biol. 16, 359–380 (2018).
Zhao, S. & Li, L. Chemical derivatization in LC–MS-based metabolomics study. TrAC, Trends Anal. Chem. 131, 115988 (2020).
Chen, Y. J. et al. Lactate metabolism is associated with mammalian mitochondria. Nat. Chem. Biol. 12, 937–943 (2016).
James, A. M. et al. The causes and consequences of nonenzymatic protein acylation. Trends Biochem. Sci. 43, 921–932 (2018).
Akhmadi, A. et al. DJ-1 protects proteins from acylation by catalyzing the hydrolysis of highly reactive cyclic 3-phosphoglyceric anhydride. Nat. Commun. 15, 2004 (2024).
Ko, Y. et al. S-lactoyl modification of KEAP1 by a reactive glycolytic metabolite activates NRF2 signaling. Proc. Natl Acad. Sci. Usa. 120, e2300763120 (2023).
Bollong, M. J. et al. A metabolite-derived protein modification integrates glycolysis with KEAP1–NRF2 signalling. Nature 562, 600–604 (2018).
Coukos, J. S., Lee, C. W., Pillai, K. S., Liu, K. J. & Moellering, R. E. Widespread, reversible cysteine modification by methylglyoxal regulates metabolic enzyme function. ACS Chem. Biol. 18, 91–101 (2022).
Parvez, S., Long, M. J. C., Poganik, J. R. & Aye, Y. Redox signaling by reactive electrophiles and oxidants. Chem. Rev. 118, 8798–8888 (2018).
Sánchez-Gómez, F. J. et al. Detoxifying enzymes at the cross-roads of inflammation, oxidative stress, and drug hypersensitivity: role of glutathione transferase P1-1 and aldose reductase. Front. Pharmacol. 7, 237 (2016).
Farrera, D. O. & Galligan, J. J. The human glyoxalase gene family in health and disease. Chem. Res. Toxicol. 35, 1766–1776 (2022).
Sibbersen, C. et al. Development of a chemical probe for identifying protein targets of α-oxoaldehydes. Chem. Commun. 49, 4012 (2013).
Brauch, S. et al. Fast and efficient MCR-based synthesis of clickable rhodamine tags for protein profiling. Org. Biomol. Chem. 10, 958–965 (2012).
Smith, C. A., Want, E. J., O’Maille, G., Abagyan, R. & Siuzdak, G. XCMS: processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching, and identification. Anal. Chem. 78, 779–787 (2006).
Berthold, M. R. et al. KNIME: the Konstanz information miner. In Data Analysis, Machine Learning and Applications (eds Preisach, C., Burkhardt, H., Schmidt-Thieme, L. & Decker, R.) (Springer, 2008).
Tautenhahn, R., Böttcher, C. & Neumann, S. Highly sensitive feature detection for high resolution LC/MS. BMC Bioinformatics 9, 504 (2008).
Prince, J. T. & Marcotte, E. M. Chromatographic alignment of ESI-LC–MS proteomics data sets by ordered bijective interpolated warping. Anal. Chem. 78, 6140–6152 (2006).
Kuhl, C., Tautenhahn, R., Böttcher, C., Larson, T. R. & Neumann, S. CAMERA: an integrated strategy for compound spectra extraction and annotation of liquid chromatography/mass spectrometry data sets. Anal. Chem. 84, 283–289 (2012).
Tesch, G. H. & Allen, T. J. Rodent models of streptozotocin‐induced diabetic nephropathy (methods in renal research). Nephrology 12, 261–266 (2007).
Acknowledgements
This work was generously supported by Ingeborg og Leo Dannins Legat (J.nr. 10017-1, to M.J.), the Novo Nordisk Foundation (NNF20OC0065548, to M.J.), the Graduate School of Health, Aarhus University (to M.D.O.) and the Danish Diabetes Academy (PhD001-19, to S.B.O.). This project received funding from the European Research Council under the European Union’s Horizon 2020 research and innovation program (grant agreement 865738, to T.B.P.). Financial support was provided by National Institutes of Health grants: R35 GM137910 and R01 DK133196 to J.J.G.
Author information
Authors and Affiliations
Contributions
M.J. conceptualized and, together with J.H., supervised the study. M.D.O. and M.B.S. performed the reactivity-based metabolomics experiments. C.B.N. performed preprocessing of the metabolomics data and T.W. assisted in the identification of isotopic pairs. C.B.N., M.D.O., M.B.S. and L.V.B. performed the feature identification and MS2 fragmentations. J.H. and M.B.S. designed, performed and analyzed the cystine-based cell experiments. J.H. and M.B.S. performed the chemoproteomic experiment and analyzed the data. M.D.O. and L.V.B. performed and analyzed the in vitro experiments with isolated amino acids and LGSH or MG. L.V.B. and J.H. performed the kinetic analysis involving LGSH, AcSCoA, MG and cysteine and D.B. performed the computational analysis of the reaction rate for the GLO1-like reaction. C.B.H. designed and performed the in vivo study with the diabetes mouse model and carried out the creatinine and blood glucose measurements under the supervision of J.A.Ø. M.B.S. and C.B.N. performed the urine sample extraction and MRM-based analysis. M.B.S. and K.F. synthesized the lactoylated amino acids, S.B.O. synthesized the isotopically labeled MG and A.M synthesized the d-Lac-Cys and l-Lac-Cys dimers under the supervision of T.B.P. J.M.S. performed the mouse d-Lac experiment under the supervision of R.R.N. J.J.G. generated the GLO2 KO cell line. K.L.N. conducted the initial analysis to identify the lactoylated amino acids. M.J., in collaboration with M.D.O., J.H. and M.B.S., wrote the manuscript and prepared the figures with contributions from T.B.P. and input from all other authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemical Biology thanks Rebecca Scheck and the other, anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Volcano plot of reactivity-based metabolomics data in positive ionization mode.
Volcano plot comparing the ‘light’ (12C3) and ‘heavy’ (13C3) treatment groups from the experiment depicted in Fig. 2a in ESI+ mode (n = 5)(left). The y-axis represents the log10-transformed p-values calculated by an unpaired t-test. The x-axis shows the log10-transformed feature ratio 12C3-MG sample/13C3-MG sample. MG-pairs were considered significant at a ratio cutoff of <0.8 or >1.2 and a p-value < 0.05 (unpaired t-test; equal, or unequal variance based on an F-test; p-values provided in source data). The numbers indicate matching pairs of identified features. Pie chart showing MG pairs discovered in both ionization modes (yellow), pairs selectively found in positive ionization mode (ESI+; red) and pairs selectively found in negative ionization mode (ESI−;blue)(right).
Extended Data Fig. 2 Glyoxalase 1 like mechanism of formation of D- and L-Lac-Cys from MG and cysteine.
(a) Extracted ion chromatogram (EIC) of D-Lac-Cys, L-Lac-Cys and of in vitro reaction mixture of 50 μM MG and 150 μM cysteine (24h) demonstrating that D-Lac-Cys and L-Lac-Cys are formed directly from MG and cysteine (MG + Cys)(left). MS/MS spectra of peaks corresponding to D-Lac-Cys and L-Lac-Cys in reaction mixture (right). MS/MS spectra can be compared to MS/MS spectra of D-Lac-Cys and L-Lac-Cys in Fig. 2. (b) Methylglyoxal (MG, 1.3%) is in a fast equilibrium with a monohydrate (MG(H2O) 28.8%), dihydrate (MG(H2O)2, 69.9%) and a hemithioacetal (HTA, 0.2%) when mixed with 150 μM cysteine in an aqueous buffer. After mixing, HTA rearranges into initially S,D/L-Lac-Cys by a glyoxalase 1 like rearrangement followed by a S-to-N lactoyl transfer to finally form D- and L-Lac-Cys in a ratio of 1:2 and with a first order rate constant k of 10−3-10−4 s−1. For details of computational calculations see Supplementary Note 1.
Extended Data Fig. 3 Cysteine amides undergo glyoxalase 1 reactions to form hydrolytically instable thioesters.
(a) Methylglyoxal (MG, 80 µM) incubated with glutathione (GSH, 0.15 or 2 mM) in PBS at 37 °C and the non-enzymatic formation of S,D-lactoylglutathione (LGSH) was followed in a 24-hour period. The Glo1 enzyme catalyzed reaction was investigated by quantifying LGSH before and after adding Glo1 (10 µM) to a reaction between MG (80 µM) and GSH (2 mM) in PBS at 37 °C. (b) The stability of LGSH in PBS at 37 °C. (c) Lactate formation from LGSH in the reactions described in (a and b). (d) Lactate formation from MG (2 mM) in PBS or H2O at 37 °C. The effects of adding cysteine (Cys, 0.2 or 2 mM) or N-acetylcysteine (NAC, 0.2 or 2 mM) to MG in PBS was studied in additional reactions. All reactions were performed with three replicates and the mean values +/− SD for all timepoints are shown. LGSH and lactate were quantified by LC-MS/MS as described in the methods and material section.
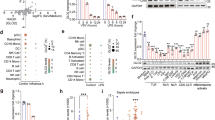
Extended Data Fig. 4 Preferential formation of D-lactoylated metabolites in GLO2 KO cells.
(a) Immunoblot of GLO2 and GAPDH in WT and GLO2 KO cells. Shown are protein data from 3 independent cultures of each cell type analyzed on the same blot. (b) Volcano plot comparing the ‘light’ (12C3) and ‘heavy’ (13C3) treatment groups from the experiment depicted in Fig. 3 in ESI+ mode (n = 5)(left). The y-axis represents the log10-transformed p-values calculated by an unpaired t-test. The x-axis shows the log10-transformed feature ratio 12C3-MG sample/13C3-MG sample. MG-pairs were considered significant at a ratio cutoff of <0.8 or >1.2 and a p-value <0.05 (unpaired t-test; equal, or unequal variance based on an F-test; p-values provided in source data). Pale colored circles (red and blue) show matching MG-pairs (20) also observed in the WT experiment (see Fig. 2a,b). Darker colored circles (red and blue) indicate additional MG pairs observed in GLO2 KO cells (112). Pie chart showing MG pairs discovered in both ionization modes (yellow), pairs selectively found in positive ionization mode (ESI+; red) and pairs selectively found in negative ionization mode (ESI− ;blue)(right).
Extended Data Fig. 5 LGSH trans-acylates around 200 times faster than AcASCoA with cysteine as acceptor.
(a) Yield of D-Lac-Cys and elimination of LGSH monitored as function of time for the reaction between 1 µM LGSH and 50-250 µM cysteine (Cys) under simulated physiological conditions (top). Yield of D-Lac-Cys and elimination of LGSH from a single data series using 1 µM LGSH and 100 µM cysteine (Cys)(bottom left). Elimination of LGSH at the five different levels of cysteine (bottom middle). Rate of reaction (k = 10 M−1S−1) determined as slope of curve from plot of observed rate constants (k’) at the five different cysteine concentrations (M)(bottom right). All reaction time courses were performed three independent times and mean values ± SD (n = 3) are shown. See method section and source data for further details and calculations. (b) Yield of N-acetyl cysteine (NAC) monitored as function of time for the reaction between 10 µM AcSCoA and 50-250 µM cysteine (Cys) under simulated physiological conditions (top). Yield of NAC as function of time for a single data series using 10 µM AcSCoA and 50–100 µM cysteine (bottom left). Formation of NAC over time at the five levels of cysteine (bottom middle). Rate of reaction (k = 0.05 M−1 S−1) determined as slope of curve from plot of observed rate constants (k’) at the four different cysteine concentrations (M)(bottom right). All reaction time courses were performed three independent times and mean values ± SD (n = 3) are shown. See method section for further experimental details and source data for further calculations.
Extended Data Fig. 6 Cysteine impact on AGEs (MG-H1 and CEA) and Lac-Cys kinetics in cells.
(a) Relative levels of reduced glutathione (GSH) by LC-MS/MS after cystine (Cys2) pre-conditioning (1, 2.5 mM or vehicle for 6h) in WT and GLO2 KO cells. Mean values ± SD (n = 4) are shown. P-values for WT cells (P = 0.2 for 1 mM Cys vs vehicle and P = 0.5 for 2.5 mM vs vehicle), and for GLO2 KO (P = 0.23 and P = 0.56). (b) MG-H1 and (c) CEA levels after exhaustive enzymatic hydrolysis of protein material from cells challenged with MG (0.5 mM for 6 hours) following a 6-hour pre-conditioning period in cystine. P-values for testing MG-H1 levels (b) in cystine vs vehicle (WT: p = 0.7 (1 mM), p = 0.56 (2.5 mM), and for GLO2 KO: p = 0.02 (1 mM), p = 0.09 (2.5 mM). P-values for testing CEA MG-H1 levels (c) in cystine vs vehicle (WT: p = 0.75 (1 mM), p = 0.92 (2.5 mM), and for GLO2 KO: p = 0.22 (1 mM), p = 0.14 (2.5 mM). Data shown in (b-c) are means ± SD from 5 cell cultures (n = 5). Test of means (a-c) based on a one-way ANOVA using a Dunnet´s post-hoc test; ns p > 0.05, *p < 0.05. (d) and (e) Kinetics of formation (24-hour period) of stable isotope labelled D-Lac-Cys (d) and L-Lac-Cys (e) after a pulse of 0.5 mM 13C3-MG in WT and GLO2 KO cells. Data from two pulse cell experiments shown fitted with a line.
Extended Data Fig. 7 Abundance of L- and D-Lac-AAs in cells and human plasma.
(a) Basal levels of MG/LGSH and L-lactate derived metabolites in different human cell lines. The lactoylated metabolites were quantified in lysates generated from the same number of cells from each cell line, data are presented as mean values +/− SD from 5 cell cultures (n = 5). (b and c) LC-MS/MS measurements and chromatograms from three different MRM transitions of D-Lac-Phe (b) and L-Lac-Phe (c) authentic standards (top left) and their basal levels in four human plasma samples. See also Supplementary Fig. 7.
Extended Data Fig. 8
Cysteine amides react with MG and LGSH to generate Lac-Cys or lactate in GLO1 and 2 like reactions.
Supplementary information
Supplementary Information
Supplementary Figs. 1–8, Notes 1–3 and Tables 1–3.
Supplementary Data 1
Source data for Supplementary Fig. 5.
Supplementary Data 2
Source data for Supplementary Fig. 6.
Supplementary Data 3
Source data for Supplementary Note 3.
Supplementary Data 4
Source data for Supplementary Note 2 and Supplementary Fig. 2.
Supplementary Data 5
Source data for Supplementary Note 2 and Supplementary Fig. 1.
Source data
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Daniel Opfermann, M., Bøgelund Søndergård, M., Vase Bech, L. et al. Reactivity-based metabolomics reveal cysteine has glyoxalase 1-like and glyoxalase 2-like activities. Nat Chem Biol (2025). https://doi.org/10.1038/s41589-025-01909-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41589-025-01909-0