Abstract
The two effectors LnaB and MavL of Legionella pneumophila coordinate the conversion of phosphoribosyl ubiquitin (PR-Ub) released by reversal of ubiquitination induced by members of the SidE effector family into functional Ub. LnaB acts as an actin-dependent phosphoryl AMPylase that converts PR-Ub into ADP-ribosylated (ADPR)-Ub. Catalysis by LnaB requires the conserved SHE motif present in a large family of bacterial toxins. Here we describe a series of structures of LnaB in complex with the cofactor actin and the substrate PR-Ub and ATP. LnaB harbors both adenylyltransferase and ATPase activities, which reveal an adenylylation mechanism involved in a two-step catalytic process. Actin performs a unique activation mechanism that promotes the recruitment of PR-Ub by LnaB to activate LnaB’s ATPase activity through interacting with LnaB and PR-Ub. Mechanisms derived from this series of structures covering the process of LnaB action establish an important biochemical basis for protein AMPylation.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
269,00 € per year
only 22,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Coordinates and structure factors for the complexes included in this study were deposited to the PDB under accession numbers 8K6E and 8K6I for LnaB19–371–actin–PR-Ub, 8K6R for LnaB19–371–ANP–actin–PR-Ub and 8K6V for LnaB19–371–actin–ADPR-Ub. Other protein structures used in the study were also obtained from the PDB under accession codes 8J9B, 3DAW, 4PKG and 1UBQ. The MS proteomics data were deposited to the ProteomeXchange Consortium through the iProX partner repository55,56 with the dataset identifier PXD062291. Data are also available from the authors upon request. Source data are provided with this paper.
References
Maculins, T., Fiskin, E., Bhogaraju, S. & Dikic, I. Bacteria–host relationship: ubiquitin ligases as weapons of invasion. Cell Res. 26, 499–510 (2016).
Mondino, S. et al. Legionnaires’ disease: state of the art knowledge of pathogenesis mechanisms of Legionella. Annu. Rev. Pathol. 15, 439–466 (2020).
Bhogaraju, S. et al. Phosphoribosylation of ubiquitin promotes serine ubiquitination and impairs conventional ubiquitination. Cell 167, 1636–1649 (2016).
Qiu, J. et al. Ubiquitination independent of E1 and E2 enzymes by bacterial effectors. Nature 533, 120–124 (2016).
Wan, M. et al. Deubiquitination of phosphoribosyl-ubiquitin conjugates by phosphodiesterase-___domain-containing Legionella effectors. Proc. Natl Acad. Sci. USA 116, 23518–23526 (2019).
Shin, D. et al. Regulation of phosphoribosyl-linked serine ubiquitination by deubiquitinases DupA and DupB. Mol. Cell 77, 164–179 (2020).
Wang, T. et al. Legionella effector LnaB is a phosphoryl-AMPylase that impairs phosphosignalling. Nature 631, 393–401 (2024).
Fu, J. et al. Legionella maintains host cell ubiquitin homeostasis by effectors with unique catalytic mechanisms. Nat. Commun. 15, 5953 (2024).
Porta, J. C. & Borgstahl, G. E. Structural basis for profilin-mediated actin nucleotide exchange. J. Mol. Biol. 418, 103–116 (2012).
Safer, D., Elzinga, M. & Nachmias, V. T. Thymosin β4 and Fx, an actin-sequestering peptide, are indistinguishable. J. Biol. Chem. 266, 4029–4032 (1991).
Paavilainen, V. O., Oksanen, E., Goldman, A. & Lappalainen, P. Structure of the actin-depolymerizing factor homology ___domain in complex with actin. J. Cell Biol. 182, 51–59 (2008).
Chavali, S. S. et al. Cryo-EM structures reveal how phosphate release from Arp3 weakens actin filament branches formed by Arp2/3 complex. Nat. Commun. 15, 2059 (2024).
Rao, J. N., Madasu, Y. & Dominguez, R. Mechanism of actin filament pointed-end capping by tropomodulin. Science 345, 463–467 (2014).
Hundt, N. et al. Molecular mechanisms of disease-related human β-actin mutations p.R183W and p.E364K. FEBS J. 281, 5279–5291 (2014).
Kanematsu, Y. et al. Structures and mechanisms of actin ATP hydrolysis. Proc. Natl Acad. Sci. USA 119, e2122641119 (2022).
Dikic, I., Wakatsuki, S. & Walters, K. J. Ubiquitin-binding domains—from structures to functions. Nat. Rev. Mol. Cell Biol. 10, 659–671 (2009).
Pei, J., Kim, B. H. & Grishin, N. V. PROMALS3D: a tool for multiple protein sequence and structure alignments. Nucleic Acids Res. 36, 2295–2300 (2008).
Oosterheert, W., Klink, B. U., Belyy, A., Pospich, S. & Raunser, S. Structural basis of actin filament assembly and aging. Nature 611, 374–379 (2022).
Zhang, Z., Patel, R., Luo, Z. Q. & Das, C. Cryo-EM detection of AMPylated histidine implies covalent catalysis in AMPylation mediated by a bacterial effector. J. Mol. Biol. 437, 168917 (2024).
Morera, S., Chiadmi, M., LeBras, G., Lascu, I. & Janin, J. Mechanism of phosphate transfer by nucleoside diphosphate kinase: X-ray structures of the phosphohistidine intermediate of the enzymes from Drosophila and Dictyostelium. Biochemistry 34, 11062–11070 (1995).
Teplyakov, A. et al. Structure of phosphorylated enzyme I, the phosphoenolpyruvate:sugar phosphotransferase system sugar translocation signal protein. Proc. Natl Acad. Sci. USA 103, 16218–16223 (2006).
van Nuland, N. A., Boelens, R., Scheek, R. M. & Robillard, G. T. High-resolution structure of the phosphorylated form of the histidine-containing phosphocarrier protein HPr from Escherichia coli determined by restrained molecular dynamics from NMR-NOE data. J. Mol. Biol. 246, 180–193 (1995).
Lima, C. D., Klein, M. G. & Hendrickson, W. A. Structure-based analysis of catalysis and substrate definition in the HIT protein family. Science 278, 286–290 (1997).
Lahiri, S. D., Zhang, G., Dunaway-Mariano, D. & Allen, K. N. The pentacovalent phosphorus intermediate of a phosphoryl transfer reaction. Science 299, 2067–2071 (2003).
Qiu, J. et al. A unique deubiquitinase that deconjugates phosphoribosyl-linked protein ubiquitination. Cell Res. 27, 865–881 (2017).
Gan, N. et al. Regulation of phosphoribosyl ubiquitination by a calmodulin-dependent glutamylase. Nature 572, 387–391 (2019).
Bhogaraju, S. et al. Inhibition of bacterial ubiquitin ligases by SidJ–calmodulin catalysed glutamylation. Nature 572, 382–386 (2019).
Black, M. H. et al. Bacterial pseudokinase catalyzes protein polyglutamylation to inhibit the SidE-family ubiquitin ligases. Science 364, 787–792 (2019).
Liu, Y. & Luo, Z. Q. The Legionella pneumophila effector SidJ is required for efficient recruitment of endoplasmic reticulum proteins to the bacterial phagosome. Infect. Immun. 75, 592–603 (2007).
Song, L. et al. The Legionella effector SdjA is a bifunctional enzyme that distinctly regulates phosphoribosyl ubiquitination. mBio 12, e0231621 (2021).
Sheedlo, M. J. et al. Structural basis of substrate recognition by a bacterial deubiquitinase important for dynamics of phagosome ubiquitination. Proc. Natl Acad. Sci. USA 112, 15090–15095 (2015).
Kotewicz, K. M. et al. Sde proteins coordinate ubiquitin utilization and phosphoribosylation to establish and maintain the Legionella replication vacuole. Nat. Commun. 15, 7479 (2024).
Casey, A. K. & Orth, K. Enzymes involved in AMPylation and deAMPylation. Chem. Rev. 118, 1199–1215 (2018).
Sreelatha, A. et al. Protein AMPylation by an evolutionarily conserved pseudokinase. Cell 175, 809–821 (2018).
Muller, M. P. et al. The Legionella effector protein DrrA AMPylates the membrane traffic regulator Rab1b. Science 329, 946–949 (2010).
Hou, Y. X., Cui, L., Riordan, J. R. & Chang, X. B. ATP binding to the first nucleotide-binding ___domain of multidrug resistance protein MRP1 increases binding and hydrolysis of ATP and trapping of ADP at the second ___domain. J. Biol. Chem. 277, 5110–5119 (2002).
Ghosh, A., Praefcke, G. J., Renault, L., Wittinghofer, A. & Herrmann, C. How guanylate-binding proteins achieve assembly-stimulated processive cleavage of GTP to GMP. Nature 440, 101–104 (2006).
Li, H. et al. A novel and unique ATP hydrolysis to AMP by a human Hsp70 binding immunoglobin protein (BiP). Protein Sci. 31, 797–810 (2022).
Alphonse, N. et al. A family of conserved bacterial virulence factors dampens interferon responses by blocking calcium signaling. Cell 185, 2354–2369 (2022).
Peng, T. et al. Pathogen hijacks programmed cell death signaling by arginine ADPR-deacylization of caspases. Mol. Cell 82, 1806–1820 (2022).
Li, X., Anderson, D. E., Chang, Y. Y., Jarnik, M. & Machner, M. P. VpdC is a ubiquitin-activated phospholipase effector that regulates Legionella vacuole expansion during infection. Proc. Natl Acad. Sci. USA 119, e2209149119 (2022).
Chai, Q. et al. A bacterial phospholipid phosphatase inhibits host pyroptosis by hijacking ubiquitin. Science 378, eabq0132 (2022).
Wang, J. et al. Mycobacterium tuberculosis suppresses innate immunity by coopting the host ubiquitin system. Nat. Immunol. 16, 237–245 (2015).
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Adams, P. D. et al. PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr. D Biol. Crystallogr. 58, 1948–1954 (2002).
Pollard, T. D. Rate constants for the reactions of ATP- and ADP-actin with the ends of actin filaments. J. Cell Biol. 103, 2747–2754 (1986).
Ali, R., Zahm, J. A. & Rosen, M. K. Bound nucleotide can control the dynamic architecture of monomeric actin. Nat. Struct. Mol. Biol. 29, 320–328 (2022).
Schuck, P. Size-distribution analysis of macromolecules by sedimentation velocity ultracentrifugation and lamm equation modeling. Biophys. J. 78, 1606–1619 (2000).
Philo, J. S. SEDNTERP: a calculation and database utility to aid interpretation of analytical ultracentrifugation and light scattering data. Eur. Biophys. J. 52, 233–266 (2023).
Xu, L. et al. Inhibition of host vacuolar H+-ATPase activity by a Legionella pneumophila effector. PLoS Pathog. 6, e1000822 (2010).
Shevchenko, A., Tomas, H., Havlis, J., Olsen, J. V. & Mann, M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat. Protoc. 1, 2856–2860 (2006).
Morris, G. M. et al. AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J. Comput. Chem. 30, 2785–2791 (2009).
Morris, G. M. et al. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comput. Chem. 19, 1639–1662 (1998).
Ma, J. et al. iProX: an integrated proteome resource. Nucleic Acids Res. 47, D1211–D1217 (2019).
Chen, T. et al. iProX in 2021: connecting proteomics data sharing with big data. Nucleic Acids Res. 50, D1522–D1527 (2022).
Acknowledgements
This work was supported in part by the National Natural Science Foundation of China grants 82225028 (S.O.), 82172287 (S.O.) and 32370185 (J.F.), the National Key Research and Development Program of China 2021YFC2301403 (S.O.), the high-level personnel introduction grant of Fujian Normal University (Z0210509) (S.O.), the National Institutes of Health grant R01AI127465 (Z.Q.L.) and the startup fund for scientific research, Fujian Medical University 2023QH1028 (J.C.). The diffraction data were collected at beamlines BL-02U1 and BL-10U2 of SSRF. MS analysis was performed in the core facility of the First Hospital of Jilin University.
Author information
Authors and Affiliations
Contributions
S.O. and Z.-Q.L. conceptualized the study. T.-T.C. performed the crystal collection, structural determination and biochemical activity analysis. J.T. performed the structural refinement. Q.L. performed the protein purification, crystallization and mutagenesis. S.-R.Z. and J. Luo performed the cellular experiments. L.K. and X.L. performed the protein expression. J.C. and S.W. performed the NMR experiments. J.F. and Siying Li performed the bacterial infection experiments and HPLC assays. J.W. and J. Li performed the molecular docking. T.-T.C. analyzed the data and wrote the paper. Y.F. and Shan Li reviewed the paper. Z.-Q.L. and S.O. analyzed the data and revised the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemical Biology thanks Dmitri Kudryashov, Yuxin Mao, Minhajuddin Sirajuddin and the other, anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
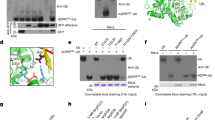
Extended Data Fig. 1 LnaB or Ub within the LnaB-Ub fusion protein is functional.
a, Western blotting analysis of the AMPylase activity of LnaB. Residues S261, H305 and E309 of the SHE motif are highlighted in red. The data represent three independent experiments. b, c, Ub (b) and LnaB profile (c) in fusion protein LnaB-Ub are functional. The purified LnaB-Ub protein was incubated with SdeA mART and NAD+ for 30 min at 37 °C and then added SdeA PDE for another 30 min (b). The prepared LnaB-PR-Ub protein was incubated with actin and ATP for 30 min at 37 °C (c). The data represent three independent experiments.
Extended Data Fig. 2 Actin interacts with PR-Ub.
a, Evaluation of the binding of actin to PR-Ub by pull-down. The data represent three independent experiments. b, c, Interaction between actin and PR-Ub is required for the activity of LnaB. The data represent three independent experiments. d, The original results of the binding affinity of PR-Ub to actin, LnaB or LnaB: actin binary complex determined by ITC. These were the original data for results summarized in Fig. 1f. The binding affinity are also shown within. Data present mean ± s.d. The data represent three independent experiments.
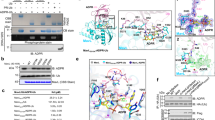
Extended Data Fig. 3 Helix α14 of LnaB is important for its interaction with actin.
a–c, Detailed views of LnaB interacting with actin as shown in Fig. 2a. Hydrogen bonds are shown in blue dashed lines. d, Structural superposition of LnaB: actin binary complex with other actin complexes by αC atoms from actin. Actin is showed in grey ribbon and its subdomains are numbered. The actin-binding helixes are shown as cylindrical cartoon. e, Sequence alignment of the LxER motif in helix α14 of LnaB with other actin-binding proteins. Identities and similarities are highlighted in red, in green, respectively. f, Region of residues 361–369 in helix α14 is required for LnaB activation. The data represent three independent experiments. g, Detailed view of the ATP-binding pocket within actin. The ATP is depicted in gray stick with an annealing omit map encountered at 2.0σ. The residues Q137 and D154 that are necessary to ATPase activity were displayed by yellow sticks. h, Evaluation of effect of the ATPase activity of actin on its activation of LnaB. The data represent three independent experiments.
Extended Data Fig. 4 Interaction between LnaB and ATP.
a, Two views of overall structure of LnaB (cyan): actin (green): PR-Ub (orange) ternary complex, which contains an ANP molecule in LnaB. Proteins are depicted in cylindrical cartoon. b, ATP is docked in the positively charged cavity of LnaB. LnaB is depicted in cartoon and surface, colored according to the electrostatic surface potential. AMPPNP(ANP) molecule is shown in stick. c, Zoom-in view shows the ATP-binding pocket where by the anti-parallel β-sheet β1/β2 is colored in light blue. The PR moiety and ANP is depicted in stick, respectively. d, Detail view of weak interactions between LnaB and adenylyl portion of ANP. Residues involved in reaction are depicted in cyan sticks. Black dashed lines indicate distances between 3.5 Å and 4 Å.
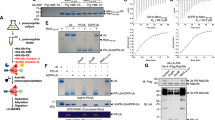
Extended Data Fig. 5 Conformation change of LnaB upon PR-Ub binding.
a, Two views of the structural superimposition of LnaB in binary complex (green) and ternary complex (pink) by αC atoms. LnaB and actin are depicted in cylindrical cartoon. PR-Ub is shown in orange ribbon. The RMSD was 0.83 Å. b, Evaluation of the role of residues involved in the activation loop in LnaB’s activity. The data represent three independent experiments. ΔAA: Alanine-substitutions in residues containing a side chain of the activation loop; Δloop: deletion of the activation loop. Residue E309 in the SHE motif is highlighted in red. c, Multiple sequence alignment of LnaB with other SHE family proteins by PROMALS3D. Listed from left to right are species, accession number, and amino acids. Identities of residues important for LnaB activity are highlighted in red. Residues composing of the activation loop are underlined. The species listed are Legionella pneumophila (L.p.), Francisella sp. W12-1067 (F.s.), Burkholderia ambifaria (B.a.), Xenorhabdus bovienii (X.b.), Photorhabdus temperate (P.t.), and Pseudomonas protegens (P.p.). d, Evaluation of the M217 mutation activities. The data represent three independent experiments.
Extended Data Fig. 6 LnaB hydrolyzes ATP into ADP and free phosphates.
a, Evaluation of LnaB’s ATP hydrolysis capacity. Data represent mean values ± s.d. n = 3 biological replicates, p values were generated using a Student’s t- test. ns, no significant. b, Model of the proposed LnaB-mediated ATP hydrolysis. c, ATP analogs with a cleavable α-phosphate support LnaB activity. The data represent three independent experiments. Note that CPP or AMP is uncleavable at the α-position did not support the activity of LnaB. (ANP: AMPPNP). d, Enzyme kinetics of LnaB measured using a concentration gradient of ATP or ADP. The kinetic parameters shown are derived by fitting to the Michaelis-Menten equation. Results of Km and Vmax represent mean ± s.d., from three independent experiments.
Extended Data Fig. 7 Residue H305 in LnaB engages in transfer of the AMP moiety.
a, Two views of overall structure of the LnaB: actin: ADPR-Ub complex. The ADPR moiety is depicted in stick within orange box. b, Sectional view of a potential ADP-binding pocket formed upon PR-Ub binding. Position of the potential ADP-binding pocket is highlighted in blue circle. c, Docking models of ADP binding to the LnaB ternary complex. d, Close-up views of interactions between LnaB (cyan) and ADP. Selected hydrogen bonds are shown as blue dashed lines. e, Residue H305 of LnaB is places at a position similar to the catalytic His residue within canonical FIC proteins. Distances are shown in black dotted lines with the specific value. Residues H305 and E309 of the SHE motif from LnaB are highlighted in red. f, Evaluation of LnaB’s ATP hydrolysis capacity by different reaction time durations. Data represent mean values ± s.d. n = 3 biological replicates, p values were generated using a Student’s t- test. ns, no significant.
Supplementary information
Supplementary Information
Supplementary Figs. 1–7, Tables 1–3 and source data for supplementary figures.
Source data
Source Data Fig. 1
Unprocessed western blots.
Source Data Fig. 2
Unprocessed western blot and gel.
Source Data Fig. 3
Unprocessed western blot and gel.
Source Data Fig. 4
Raw data.
Source Data Fig. 5
Unprocessed western blot and gel.
Source Data Fig. 5
Raw data.
Source Data Extended Data Fig. 1
Unprocessed western blot and gel.
Source Data Extended Data Fig. 2
Unprocessed western blot and gel.
Source Data Extended Data Fig. 3
Unprocessed western blot and gel.
Source Data Extended Data Fig. 5
Unprocessed western blot and gel.
Source Data Extended Data Fig. 6
Unprocessed western blot and gel.
Source Data Extended Data Fig. 6
Raw data.
Source Data Extended Data Fig. 7
Raw data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, TT., Lu, Q., Zheng, SR. et al. Structure and mechanism of an actin-dependent bacterial phosphoryl AMPylase. Nat Chem Biol (2025). https://doi.org/10.1038/s41589-025-01945-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41589-025-01945-w