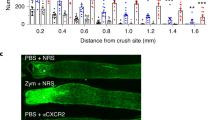
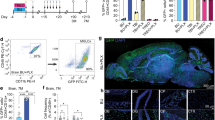
Abstract
The adult central nervous system (CNS) possesses a limited capacity for self-repair. Severed CNS axons typically fail to regrow. There is an unmet need for treatments designed to enhance neuronal viability, facilitate axon regeneration and ultimately restore lost neurological functions to individuals affected by traumatic CNS injury, multiple sclerosis, stroke and other neurological disorders. Here we demonstrate that both mouse and human bone marrow neutrophils, when polarized with a combination of recombinant interleukin-4 (IL-4) and granulocyte colony-stimulating factor (G-CSF), upregulate alternative activation markers and produce an array of growth factors, thereby gaining the capacity to promote neurite outgrowth. Moreover, adoptive transfer of IL-4/G-CSF-polarized bone marrow neutrophils into experimental models of CNS injury triggered substantial axon regeneration within the optic nerve and spinal cord. These findings have far-reaching implications for the future development of autologous myeloid cell-based therapies that may bring us closer to effective solutions for reversing CNS damage.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
209,00 € per year
only 17,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
Bulk RNA-seq data have been deposited in NCBI’s Gene Expression Omnibus (GEO) under accession code GSE244934. Source data are provided with this paper.
Code availability
All scripts are publicly available at: https://github.com/lahammond/2024_Jerome_Axon_Regeneration.
References
Chinetti-Gbaguidi, G., Colin, S. & Staels, B. Macrophage subsets in atherosclerosis. Nat. Rev. Cardiol. 12, 10–17 (2015).
Tourki, B. & Halade, G. Leukocyte diversity in resolving and nonresolving mechanisms of cardiac remodeling. FASEB J. 31, 4226–4239 (2017).
Willenborg, S., Injarabian, L. & Eming, S. A. Role of macrophages in wound healing. Cold Spring Harb. Perspect. Biol. 14, a041216 (2022).
Mantovani, A., Biswas, S. K., Galdiero, M. R., Sica, A. & Locati, M. Macrophage plasticity and polarization in tissue repair and remodelling. J. Pathol. 229, 176–185 (2013).
Leon, S., Yin, Y., Nguyen, J., Irwin, N. & Benowitz, L. I. Lens injury stimulates axon regeneration in the mature rat optic nerve. J. Neurosci. 20, 4615–4626 (2000).
Ng, L. G., Ostuni, R. & Hidalgo, A. Heterogeneity of neutrophils. Nat. Rev. Immunol. 19, 255–265 (2019).
Shim, H. B., Deniset, J. F. & Kubes, P. Neutrophils in homeostasis and tissue repair. Int. Immunol. 34, 399–407 (2022).
Cuartero, M. I. et al. N2 neutrophils, novel players in brain inflammation after stroke: modulation by the PPARγ agonist rosiglitazone. Stroke 44, 3498–3508 (2013).
Ma, Y. Role of neutrophils in cardiac injury and repair following myocardial infarction. Cells 10, 1676 (2021).
Jerome, A. D. et al. Characterization of zymosan-modulated neutrophils with neuroregenerative properties. Front. Immunol. 13, 912193 (2022).
Sas, A. R. et al. A new neutrophil subset promotes CNS neuron survival and axon regeneration. Nat. Immunol. 21, 1496–1505 (2020).
Baldwin, K. T., Carbajal, K. S., Segal, B. M. & Giger, R. J. Neuroinflammation triggered by β-glucan/dectin-1 signaling enables CNS axon regeneration. Proc. Natl Acad. Sci. USA 112, 2581–2586 (2015).
Yin, Y. et al. Macrophage-derived factors stimulate optic nerve regeneration. J. Neurosci. 23, 2284–2293 (2003).
Van Dyken, S. J. & Locksley, R. M. Interleukin-4- and interleukin-13-mediated alternatively activated macrophages: roles in homeostasis and disease. Annu. Rev. Immunol. 31, 317–343 (2013).
Wermeling, F., Anthony, R. M., Brombacher, F. & Ravetch, J. V. Acute inflammation primes myeloid effector cells for anti-inflammatory STAT6 signaling. Proc. Natl Acad. Sci. USA 110, 13487–13491 (2013).
Panda, S. K. et al. IL-4 controls activated neutrophil FcγR2b expression and migration into inflamed joints. Proc. Natl Acad. Sci. USA 117, 3103–3113 (2020).
Evrard, M. et al. Developmental analysis of bone marrow neutrophils reveals populations specialized in expansion, trafficking, and effector functions. Immunity 48, 364–379 e368 (2018).
da Rocha, J. F. et al. APP binds to the EGFR ligands HB-EGF and EGF, acting synergistically with EGF to promote ERK signaling and neuritogenesis. Mol. Neurobiol. 58, 668–688 (2021).
Jin, K. et al. Heparin-binding epidermal growth factor-like growth factor: hypoxia-inducible expression in vitro and stimulation of neurogenesis in vitro and in vivo. J. Neurosci. 22, 5365–5373 (2002).
Oyagi, A. & Hara, H. Essential roles of heparin-binding epidermal growth factor-like growth factor in the brain. CNS Neurosci. Ther. 18, 803–810 (2012).
de Figueiredo, C. S. et al. Insulin-like growth factor-1 stimulates retinal cell proliferation via activation of multiple signaling pathways. Curr. Res Neurobiol. 4, 100068 (2023).
Neumann, S. & Woolf, C. J. Regeneration of dorsal column fibers into and beyond the lesion site following adult spinal cord injury. Neuron 23, 83–91 (1999).
Attwell, C. L., van Zwieten, M., Verhaagen, J. & Mason, M. R. J. The dorsal column lesion model of spinal cord injury and its use in deciphering the neuron-intrinsic injury response. Dev. Neurobiol. 78, 926–951 (2018).
Ferrari-Toninelli, G., Bonini, S. A., Bettinsoli, P., Uberti, D. & Memo, M. Microtubule stabilizing effect of notch activation in primary cortical neurons. Neuroscience 154, 946–952 (2008).
White, R. E., Yin, F. Q. & Jakeman, L. B. TGF-α increases astrocyte invasion and promotes axonal growth into the lesion following spinal cord injury in mice. Exp. Neurol. 214, 10–24 (2008).
Kamei, N. et al. Endothelial progenitor cells promote astrogliosis following spinal cord injury through Jagged1-dependent Notch signaling. J. Neurotrauma 29, 1758–1769 (2012).
Rajkovic, I., Denes, A., Allan, S. M. & Pinteaux, E. Emerging roles of the acute phase protein pentraxin-3 during central nervous system disorders. J. Neuroimmunol. 292, 27–33 (2016).
Xian, C. J. & Zhou, X. F. Roles of transforming growth factor-α and related molecules in the nervous system. Mol. Neurobiol. 20, 157–183 (1999).
Edvardsson, L., Dykes, J., Olsson, M. L. & Olofsson, T. Clonogenicity, gene expression and phenotype during neutrophil versus erythroid differentiation of cytokine-stimulated CD34+ human marrow cells in vitro. Br. J. Haematol. 127, 451–463 (2004).
Nadeau, S. et al. Functional recovery after peripheral nerve injury is dependent on the pro-inflammatory cytokines IL-1β and TNF: implications for neuropathic pain. J. Neurosci. 31, 12533–12542 (2011).
Hou, Y. et al. N2 neutrophils may participate in spontaneous recovery after transient cerebral ischemia by inhibiting ischemic neuron injury in rats. Int. Immunopharmacol. 77, 105970 (2019).
Veglia, F., Sanseviero, E. & Gabrilovich, D. I. Myeloid-derived suppressor cells in the era of increasing myeloid cell diversity. Nat. Rev. Immunol. 21, 485–498 (2021).
Fridlender, Z. G. et al. Polarization of tumor-associated neutrophil phenotype by TGF-β: ‘N1’ versus ‘N2’ TAN. Cancer Cell 16, 183–194 (2009).
Ogawa, K. et al. Frontline Science: Conversion of neutrophils into atypical Ly6G+ SiglecF+ immune cells with neurosupportive potential in olfactory neuroepithelium. J. Leukoc. Biol. 109, 481–496 (2021).
Serger, E. et al. The gut metabolite indole-3 propionate promotes nerve regeneration and repair. Nature 607, 585–592 (2022).
Dupraz, S. et al. The insulin-like growth factor 1 receptor is essential for axonal regeneration in adult central nervous system neurons. PLoS ONE 8, e54462 (2013).
Kermer, P., Klocker, N., Labes, M. & Bahr, M. Insulin-like growth factor-I protects axotomized rat retinal ganglion cells from secondary death via PI3-K-dependent Akt phosphorylation and inhibition of caspase-3 In vivo. J. Neurosci. 20, 2–8 (2000).
Chen, H., Liu, B. & Neufeld, A. H. Epidermal growth factor receptor in adult retinal neurons of rat, mouse, and human. J. Comp. Neurol. 500, 299–310 (2007).
Wang, S. et al. The upregulation of EGFR in the dorsal root ganglion contributes to chronic compression of dorsal root ganglions-induced neuropathic pain in rats. Mol. Pain. 15, 1744806919857297 (2019).
Xu, M. F. et al. The mechanisms of EGFR in the regulation of axon regeneration. Cell Biochem. Funct. 32, 101–105 (2014).
Yamada, M., Ikeuchi, T. & Hatanaka, H. The neurotrophic action and signalling of epidermal growth factor. Prog. Neurobiol. 51, 19–37 (1997).
Sun, Z. et al. IGF-1R stimulation alters microglial polarization via TLR4/NF-κB pathway after cerebral hemorrhage in mice. Brain Res. Bull. 164, 221–234 (2020).
White, R. E. et al. Transforming growth factor α transforms astrocytes to a growth-supportive phenotype after spinal cord injury. J. Neurosci. 31, 15173–15187 (2011).
Liu, B. & Neufeld, A. H. Activation of epidermal growth factor receptors directs astrocytes to organize in a network surrounding axons in the developing rat optic nerve. Dev. Biol. 273, 297–307 (2004).
Chan, S. J. et al. Promoting neuro-supportive properties of astrocytes with epidermal growth factor hydrogels. Stem Cells Transl. Med. 8, 1242–1248 (2019).
Liu, B., Chen, H., Johns, T. G. & Neufeld, A. H. Epidermal growth factor receptor activation: an upstream signal for transition of quiescent astrocytes into reactive astrocytes after neural injury. J. Neurosci. 26, 7532–7540 (2006).
Harrington, A. W. & Ginty, D. D. Long-distance retrograde neurotrophic factor signalling in neurons. Nat. Rev. Neurosci. 14, 177–187 (2013).
Donahue, R. J., Fehrman, R. L., Gustafson, J. R. & Nickells, R. W. BCLXL gene therapy moderates neuropathology in the DBA/2J mouse model of inherited glaucoma. Cell Death Dis. 12, 781 (2021).
Hirokawa, T. et al. Regulation of axonal regeneration by the level of function of the endogenous Nogo receptor antagonist LOTUS. Sci. Rep. 7, 12119 (2017).
Wang, X. et al. Intravitreal delivery of human NgR-Fc decoy protein regenerates axons after optic nerve crush and protects ganglion cells in glaucoma models. Invest. Ophthalmol. Vis. Sci. 56, 1357–1366 (2015).
Muzumdar, M. D., Tasic, B., Miyamichi, K., Li, L. & Luo, L. A global double-fluorescent Cre reporter mouse. Genesis 45, 593–605 (2007).
Weigert, M. et al. Content-aware image restoration: pushing the limits of fluorescence microscopy. Nat. Methods 15, 1090–1097 (2018).
Berg, S. et al. ilastik: interactive machine learning for (bio)image analysis. Nat. Methods 16, 1226–1232 (2019).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Zhao, Z. et al. The International Conference on Intelligent Biology and Medicine (ICIBM) 2019: bioinformatics methods and applications for human diseases. BMC Bioinformatics 20, 676 (2019).
Kim, D., Langmead, B. & Salzberg, S. L. HISAT: a fast spliced aligner with low memory requirements. Nat. Methods 12, 357–360 (2015).
Liao, Y., Smyth, G. K. & Shi, W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930 (2014).
Acknowledgements
We thank A. Sepeda and S. Atkins for technical assistance. This study was supported by NIH-NEI grants R01EY029159 (B.M.S.), R01EY028350 (B.M.S.) and K08EY029362 (A.R.S.).
Author information
Authors and Affiliations
Contributions
B.M.S. designed the study and supervised the study, data interpretation and manuscript editing. A.D.J. and A.R.S. designed the study and contributed to data interpretation under the supervision of B.M.S. A.D.J. and A.R.S. performed optic nerve crush experiments, tracing of optic nerve neurons, tissue clearance, immunohistochemical staining, optic nerve imaging, quantification of regenerating axons and intraocular injections. A.D.J. performed RGC neurite outgrowth assays, retinal whole mounts, RGC imaging and quantification, cytospins and Giemsa–Wright staining. A.D.J. and J.R.A. performed flow cytometry and analysis. Y.W. performed dorsal spinal cord injury experiments, tracing of spinal cord neurons, tissue clearance, immunohistochemical staining, imaging and quantification of regenerating axons. Y.W. performed dorsal root ganglia neurite outgrowth assays under the supervision of A.D.J. L.A.H. conducted postimage acquisition processing and analyses of spinal cord, DRG and sciatic nerve specimens. T.L. isolated RNA and prepared DNA libraries. J.W. performed western blot assays. A.W. analyzed RNA-seq data. B.M.S. and A.D.J. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
B.M.S., A.R.S., T.L. and A.D.J. are inventors on a patent titled ‘Engineered cells and uses thereof’, licensed by the Ohio State University. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Immunology thanks Richard Zigmond and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available. Laurie A. Dempsey was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Kinetics of gene expression in BMN during polarization. Polarized and unpolarized Ly6G+ BMN express canonical markers of granulopoiesis.
a, RNA was extracted from Ly6G+ BM cells at serial time points during culture in the presence or absence of polarizing factors. Levels of transcripts encoding IL-4Ra (IL4r), arginase-1 (Arg1), mannose receptor (Mrc1), and F4/80 (Adgre1), were measured by RT-qPCR and normalized to Actb. Data are shown as fold increase over mean normalized levels in unpolarized BMN. Each symbol represents data derived from an individual mouse. Data are presented as mean values ± SEM. b, Normalized levels of myeloperoxidase (Mpo) and neutrophil elastase (Ela, also known as Elane) mRNA, extracted from Ly6G+ BM cells, following a 24 hour polarization under the indicated conditions (n = 5). Transcript levels were also measured in mature peripheral blood neutrophils, isolated from naïve mice, for an additional control. (n = 5). Each symbol represents an individual mouse. Data are presented as mean values ± SEM. c, Polarized and unpolarized BMN were analyzed by flow cytometry. Histograms showing MFI of MPO (left) and Elastase (right), gating on CD11b+Ly6G+ cells (Representative of n = 5 mice per group). a, Data shown from one experiment, representative of two independent experiments. b, c, Data are shown from one experiment, representative of four independent experiments. d, Flow cytometric gating strategy for assessing the purity of MACS purified Ly6G+ BM cells.
Extended Data Fig. 2 GFAP expression in retina from mice subjected to ONC injury and intraocular injection of unpolarized or polarized BMN versus PBS.
Retina were harvested on day 4 following ONC injury and intraocular injection of either IL-4/G-CSF polarized BMN, unpolarized BMN, or PBS. Each panel shows a representative retinal cross-section obtained from an individual mouse, stained with antibodies against GFAP (green). Scale bar, 100 μm. Data shown from one experiment, representative of two independent experiments.
Extended Data Fig. 3 IL-4/G-CSF-polarized BMNs produce HB-EGF protein.
Representative western blot of HB-EGF protein in BMN lysates Representative western blot, analyzing lysates from unpolarized BMN (left) and IL-4/G-CSF polarized BMN (right), displaying total protein (stain-free gel) and HB-EGF band on a PVDF membrane. Data shown from one experiment, representative of two independent experiments.
Extended Data Fig. 4 IL-4/G-CSF polarized Ly6G+ BMN accumulate in the sciatic nerve at 4 hours, and the spinal cord at 24 hours, following SCI and i.n. BMN injection.
a, Representative section of a sciatic nerve harvested 4 hours after SCI injury and i.n. injection of IL-4/G-CSF polarized BMN that were derived from tdTomato-reporter mice. Donor BMN are identified as tdTomato+ (red) and Ly6G+ (white) (scale bar, 50 μm). b, Representative flow cytometric pseudocolored dot plot of mononuclear cells isolated from sciatic nerves 4 hours after SCI injury and i.n. injection of either IL-4/G-CSF polarized tdTomato+ BMN (left) or PBS (right), gating on CD11b+Ly6G+ cells. c, Representative flow cytometric pseudocolored dot plot of spinal cord mononuclear cells isolated 24 hours following SCI injury and i.n. injection of either IL-4/G-CSF polarized tdT+ BMN (left) or PBS (right), gating on CD11b+Ly6G+ cells. a-c, Data shown from one experiment, representative of two independent experiments.
Extended Data Fig. 5 IL-4/G-CSF polarized CD34+ bone marrow cells, derived from individual donors, reproducibly induce neurite outgrowth of human cortical neurons.
a, Gating strategy for flow cytometric analysis and sorting of IL-4/G-CSF polarized CD34+ human BM cells. b, Length of the longest neurite grown by human primary cortical neurons following 24 hour culture with IL-4/G-CSF polarized, single cytokine polarized, or unpolarized human CD34+ BM cells. Some neurons were cultured in media alone (negative control) or with NGF (positive control). Each plot represents an independent experiment performed using BM cells derived from a unique BM donor. c, Length of the longest neurite grown by human primary cortical neurons following 24 hour culture with CD34+ or CD33+CD15+ cells, FACS sorted from IL-4/G-CSF polarized human BM stem cells. Some neurons were cultured in media alone (negative control) or with NGF (positive control). Each plot represents an independent experiment performed using BM cells derived from a unique BM donor. b, c, Data are presented as mean values ± SEM. Each symbol represents a single neuron with the n value listed under each experimental group. Statistical significance was determined by one-way ANOVA followed by Dunnett’s post hoc test.
Supplementary information
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jerome, A.D., Sas, A.R., Wang, Y. et al. Cytokine polarized, alternatively activated bone marrow neutrophils drive axon regeneration. Nat Immunol 25, 957–968 (2024). https://doi.org/10.1038/s41590-024-01836-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41590-024-01836-7
This article is cited by
-
A stromal inflammasome Ras safeguard against Myc-driven lymphomagenesis
Nature Immunology (2025)
-
Immune drivers of pain resolution and protection
Nature Immunology (2024)
-
Critical Care of Spinal Cord Injury
Current Neurology and Neuroscience Reports (2024)