Abstract
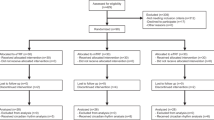
Intermittent fasting appears an equivalent alternative to calorie restriction (CR) to improve health in humans. However, few trials have considered applying meal timing during the ‘fasting’ day, which may be a limitation. We developed a novel intermittent fasting plus early time-restricted eating (iTRE) approach. Adults (N = 209, 58 ± 10 years, 34.8 ± 4.7 kg m−2) at increased risk of developing type 2 diabetes were randomized to one of three groups (2:2:1): iTRE (30% energy requirements between 0800 and 1200 hours and followed by a 20-h fasting period on three nonconsecutive days per week, and ad libitum eating on other days); CR (70% of energy requirements daily, without time prescription); or standard care (weight loss booklet). This open-label, parallel group, three-arm randomized controlled trial provided nutritional support to participants in the iTRE and CR arms for 6 months, with an additional 12-month follow-up. The primary outcome was change in glucose area under the curve in response to a mixed-meal tolerance test at month 6 in iTRE versus CR. Glucose tolerance was improved to a greater extent in iTRE compared with CR (−10.10 (95% confidence interval −14.08, −6.11) versus −3.57 (95% confidence interval −7.72, 0.57) mg dl−1 min−1; P = 0.03) at month 6, but these differences were lost at month 18. Adverse events were transient and generally mild. Reports of fatigue were higher in iTRE versus CR and standard care, whereas reports of constipation and headache were higher in iTRE and CR versus standard care. In conclusion, incorporating advice for meal timing with prolonged fasting led to greater improvements in postprandial glucose metabolism in adults at increased risk of developing type 2 diabetes. ClinicalTrials.gov identifier NCT03689608.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
209,00 € per year
only 17,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
Anonymized data from this study are available on request from the corresponding author for 36 months from date of publication with a full research plan for academic use only. The data are not publicly available as they contain information that could compromise research participant consent.
Code availability
No unique software or computational code was created for this study.
References
Diabetes Prevention Program Research Group et al. 10-Year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 374, 1677–1686 (2009).
Uusitupa, M. et al. Prevention of type 2 diabetes by lifestyle changes: a systematic review and meta-analysis. Nutrients 11, 2611 (2019).
Evert, A. B. et al. Nutrition therapy for adults with diabetes or prediabetes: a consensus report. Diabetes Care 42, 731–754 (2019).
Patikorn, C. et al. Intermittent fasting and obesity-related health outcomes: an umbrella review of meta-analyses of randomized clinical trials. JAMA Netw. Open 4, e2139558 (2021).
Harvie, M. et al. The effect of intermittent energy and carbohydrate restriction v. daily energy restriction on weight loss and metabolic disease risk markers in overweight women. Br. J. Nutr. 110, 1534–1547 (2013).
Harvie, M. N. et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: a randomized trial in young overweight women. Int J. Obes. (Lond.) 35, 714–727 (2011).
Di Francesco, A. et al. A time to fast. Science 362, 770–775 (2018).
Lawson, C. A. et al. Animal models of GM2 gangliosidosis: utility and limitations. Appl. Clin. Genet. 9, 111–120 (2016).
Tiribuzi, R. et al. Lysosomal β-galactosidase and β-hexosaminidase activities correlate with clinical stages of dementia associated with Alzheimer’s disease and type 2 diabetes mellitus. J. Alzheimers Dis. 24, 785–797 (2011).
Hultberg, B. et al. beta-Hexosaminidase isoenzymes A and B in middle-aged and elderly subjects: determinants of plasma levels and relation to vascular disease. Ann. Clin. Biochem. 33, 432–437 (1996).
Kim, H. K. et al. Renal tubular damage marker, urinary N-acetyl-β-d-glucosaminidase, as a predictive marker of hepatic fibrosis in type 2 diabetes mellitus. Diabetes Metab. J. 46, 104–116 (2022).
Montgomery, M. K. et al. Hexosaminidase A (HEXA) regulates hepatic sphingolipid and lipoprotein metabolism in mice. FASEB J. 35, e22046 (2021).
Teong, X. T. et al. Evidence gaps and potential roles of intermittent fasting in the prevention of chronic diseases. Exp. Gerontol. 153, 111506 (2021).
Acosta-Rodriguez, V. et al. Circadian alignment of early onset caloric restriction promotes longevity in male C57BL/6J mice. Science 376, 1192–1202 (2022).
Pak, H. H. et al. Fasting drives the metabolic, molecular and geroprotective effects of a calorie-restricted diet in mice. Nat. Metab. 3, 1327–1341 (2021).
Froy, O. et al. Effect of intermittent fasting on circadian rhythms in mice depends on feeding time. Mech. Ageing Dev. 130, 154–160 (2009).
Regmi, P. et al. Time-restricted eating: benefits, mechanisms, and challenges in translation. iScience 23, 101161 (2020).
Liu, D. et al. Calorie restriction with or without time-restricted eating in weight loss. N. Engl. J. Med. 386, 1495–1504 (2022).
Thomas, E. A. et al. Early time-restricted eating compared with daily caloric restriction: a randomized trial in adults with obesity. Obesity (Silver Spring) 30, 1027–1038 (2022).
Jamshed, H. et al. Effectiveness of early time-restricted eating for weight loss, fat loss, and cardiometabolic health in adults with obesity: a randomized clinical trial. JAMA Intern. Med. 182, 953–962 (2022).
Anton, S. D. et al. Flipping the metabolic switch: understanding and applying the health benefits of fasting. Obesity (Silver Spring) 26, 254–268 (2018).
Trepanowski, J. F. et al. Effect of alternate-day fasting on weight loss, weight maintenance, and cardioprotection among metabolically healthy obese adults: a randomized clinical trial. JAMA Intern. Med. 177, 930–938 (2017).
Sundfor, T. M. et al. Effect of intermittent versus continuous energy restriction on weight loss, maintenance and cardiometabolic risk: a randomized 1-year trial. Nutr. Metab. Cardiovasc. Dis. 28, 698–706 (2018).
Shankar, S. S. et al. Standardized mixed-meal tolerance and arginine stimulation tests provide reproducible and complementary measures of beta-cell function: results from the Foundation for the National Institutes of Health Biomarkers Consortium Investigative Series. Diabetes Care 39, 1602–1613 (2016).
Berry, S. E. et al. Human postprandial responses to food and potential for precision nutrition. Nat. Med. 26, 964–973 (2020).
Lind, M. et al. The association between HbA1c, fasting glucose, 1-hour glucose and 2-hour glucose during an oral glucose tolerance test and cardiovascular disease in individuals with elevated risk for diabetes. PLoS ONE 9, e109506 (2014).
Cavalot, F. et al. Postprandial blood glucose is a stronger predictor of cardiovascular events than fasting blood glucose in type 2 diabetes mellitus, particularly in women: lessons from the San Luigi Gonzaga Diabetes Study. J. Clin. Endocrinol. Metab. 91, 813–819 (2006).
Antoni, R. et al. Intermittent v. continuous energy restriction: differential effects on postprandial glucose and lipid metabolism following matched weight loss in overweight/obese participants. Br. J. Nutr. 119, 507–516 (2018).
Templeman, I. et al. A randomized controlled trial to isolate the effects of fasting and energy restriction on weight loss and metabolic health in lean adults. Sci. Transl. Med. 13, eabd8034 (2021).
Gao, Y. et al. Effects of intermittent (5:2) or continuous energy restriction on basal and postprandial metabolism: a randomised study in normal-weight, young participants. Eur. J. Clin. Nutr. 76, 65–71 (2022).
Jones, R. et al. Two weeks of early time-restricted feeding (eTRF) improves skeletal muscle insulin and anabolic sensitivity in healthy men. Am. J. Clin. Nutr. 112, 1015–1028 (2020).
Karpe, F. et al. Fatty acids, obesity, and insulin resistance: time for a reevaluation. Diabetes 60, 2441–2449 (2011).
Phillips, D. I. W. et al. Understanding oral glucose tolerance: comparison of glucose or insulin measurements during the oral glucose tolerance test with specific measurements of insulin resistance and insulin secretion. Diabet. Med. 11, 286–292 (1994).
Horowitz, M. et al. Relationship between oral glucose tolerance and gastric emptying in normal healthy subjects. Diabetologia 36, 857–862 (1993).
Hutchison, A. T. et al. Time-restricted feeding improves glucose tolerance in men at risk for type 2 diabetes: a randomized crossover trial. Obesity (Silver Spring) 27, 724–732 (2019).
Hemmingsen, B. et al. Diet, physical activity or both for prevention or delay of type 2 diabetes mellitus and its associated complications in people at increased risk of developing type 2 diabetes mellitus. Cochrane Database Syst. Rev. 12, CD003054 (2017).
Dashti, H. S. et al. Late eating is associated with cardiometabolic risk traits, obesogenic behaviors, and impaired weight loss. Am. J. Clin. Nutr. 113, 154–161 (2020).
Jamshed, H. et al. Early time-restricted feeding improves 24-hour glucose levels and affects markers of the circadian clock, aging, and autophagy in humans. Nutrients 11, 1234 (2019).
Sutton, E. F. et al. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab. 27, 1212–1221 (2018).
Vujović, N. et al. Late isocaloric eating increases hunger, decreases energy expenditure, and modifies metabolic pathways in adults with overweight and obesity. Cell Metab. 34, 1486–1498 (2022).
Wehrens, S. M. T. et al. Meal timing regulates the human circadian system. Curr. Biol. 27, 1768–1775 (2017).
Van Namen, M. et al. Supervised lifestyle intervention for people with metabolic syndrome improves outcomes and reduces individual risk factors of metabolic syndrome: a systematic review and meta-analysis. Metabolism 101, 153988 (2019).
Keirns, B. H. et al. Fasting, non-fasting and postprandial triglycerides for screening cardiometabolic risk. J. Nutr. Sci. 10, e75 (2021).
Hultberg, B. et al. Pattern of serum beta-hexosaminidase in liver cirrhosis. Scand. J. Gastroenterol. 18, 877–880 (1983).
Poon, P. Y. et al. Plasma N-acetyl-beta-d-glucosaminidase activities and glycaemia in diabetes mellitus. Diabetologia 24, 433–436 (1983).
Whiting, P. H. et al. Serum and urine N-acetyl-beta-d-glucosaminidase in diabetics on diagnosis and subsequent treatment, and stable insulin dependent diabetics. Clin. Chim. Acta 92, 459–463 (1979).
Lipina, C. et al. Ganglioside GM3 as a gatekeeper of obesity-associated insulin resistance: evidence and mechanisms. FEBS Lett. 589, 3221–3227 (2015).
Jensen, M. D. et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults. Circulation 129, S102–S138 (2014).
Teong, X. T. et al. Rationale and protocol for a randomized controlled trial comparing daily calorie restriction versus intermittent fasting to improve glycaemia in individuals at increased risk of developing type 2 diabetes. Obes. Res Clin. Pract. 14, 176–183 (2020).
Teong, X. T. et al. An update to the study protocol for a randomized controlled trial comparing daily calorie restriction versus intermittent fasting to improve glycaemia in individuals at increased risk of developing type 2 diabetes. Obes. Res Clin. Pract. 15, 306 (2021).
Institute of Medicine. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (The National Academies Press, 2005).
Matsuda, M. & DeFronzo, R. A. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 22, 1462–1470 (1999).
Leaback, D. H. & Walker, P. G. Studies on glucosaminidase. 4. fluorimetric assay. N.-acetyl-beta-glucosaminidase. Biochem J. 78, 151–156 (1961).
Whyte, L. S. et al. Lysosomal gene Hexb displays haploinsufficiency in a knock-in mouse model of Alzheimer’s disease. IBRO Neurosci. Rep. 12, 131–141 (2022).
Acknowledgements
This work was supported by the National Health and Medical Research Council Project Grant (APP1143092). X.T.T. was supported by an Australian Government Research Training Program Scholarship from The University of Adelaide. This work was supported by a Diabetes Australia Research Program Grant (Y21G-SART) awarded to T.J.S., J.B. and L.K.H. The funder had no role in the design of this study and the interpretation of the study results. We thank all the trial participants.
Author information
Authors and Affiliations
Contributions
L.K.H., A.T.H., C.F.-B., A.D.V. and G.A.W. designed the study. A.D.V. wrote the statistical analysis plan, performed randomization and statistical analysis. X.T.T., K.L., B.L., L.Z. and A.T.H. collected the blood samples. G.A.W. provided clinical support and supervision. X.T.T., K.L., A.D.V. and L.K.H. analyzed the data. J.B., K.J.H. and T.J.S. measured the β-hexosaminidase activity. All authors critically revised the draft and approved the final manuscript. L.K.H. had full access to the data and had primary responsibility for the final publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Medicine thanks Jonathan Little, Krista Varady, Luke Ouma and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Jennifer Sargent, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Comparisons of (a) iTRE vs CR, and (b) iTRE + CR vs SC for joint change in postprandial glucose (mg/dL/min) and HbA1c (%).
Points indicate prior (blue), posterior (red) and observed means (black). The ellipses indicate 95% central prior (blue) and posterior (red) probabilities. We note that our prior belief was for a correlation of 0.7 in change of the two outcomes, which was not observed. iTRE, intermittent time-restricted diet at 70% of calculated energy requirements; CR, calorie restriction diet at 70% of calculated daily energy requirements; SC: standard care diet.
Extended Data Fig. 2 Weight loss (kg) relative to baseline.
Presented are means and 95% CIs (calculated as \(\overline x \pm 1.96\,{{{\mathrm{SEM}}}}\)) of the non-fasting weight change by group during each face to face check-in visit. Treatment group trajectories were compared using linear mixed effects regression assuming piecewise linear effects assumed for the interventions over two time periods: month 0–6 and month 7–18, and both random intercepts and slopes for individuals. iTRE, intermittent time-restricted diet at 70% of calculated energy requirements; CR, calorie restriction diet at 70% of calculated daily energy requirements; SC: standard care diet.
Extended Data Fig. 3 Weight loss percentage from baseline to month 6 in iTRE (a), CR (b), SC (c).
iTRE, intermittent time-restricted diet at 70% of calculated energy requirements; CR, calorie restriction diet at 70% of calculated daily energy requirements; SC: standard care diet.
Supplementary information
Supplementary Information
Supplementary Tables 1–7.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Teong, X.T., Liu, K., Vincent, A.D. et al. Intermittent fasting plus early time-restricted eating versus calorie restriction and standard care in adults at risk of type 2 diabetes: a randomized controlled trial. Nat Med 29, 963–972 (2023). https://doi.org/10.1038/s41591-023-02287-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-023-02287-7
This article is cited by
-
Does breakfast skipping alter the serum lipids of university students?
BMC Nutrition (2025)
-
Efficacy of intermittent fasting on improving liver function in individuals with metabolic disorders: a systematic review and meta-analysis
Nutrition & Metabolism (2025)
-
The cyclic metabolic switching theory of intermittent fasting
Nature Metabolism (2025)
-
Adverse events profile associated with intermittent fasting in adults with overweight or obesity: a systematic review and meta-analysis of randomized controlled trials
Nutrition Journal (2024)
-
Effects of DASH diet with or without time-restricted eating in the management of stage 1 primary hypertension: a randomized controlled trial
Nutrition Journal (2024)