Abstract
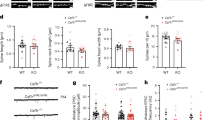
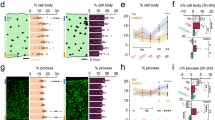
To test the hypothesized crucial role of microglia in the developmental refinement of neural circuitry, we depleted microglia from mice of both sexes with PLX5622 and examined the experience-dependent maturation of visual circuitry and function. We assessed retinal function, receptive field tuning of visual cortex neurons, acuity and experience-dependent plasticity. None of these measurements detectibly differed in the absence of microglia, challenging the role of microglia in sculpting neural circuits.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
209,00 € per year
only 17,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The deposited data listed in the Methods table were deposited to Mendeley data at https://data.mendeley.com/datasets/4x96trx9tv/1.
Code availability
No new code was generated by this study.
References
Wilton, D. K., Dissing-Olesen, L. & Stevens, B. Neuron–glia signaling in synapse elimination. Annu. Rev. Neurosci. 42, 107–127 (2019).
Elmore, M. R. P. et al. Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron 82, 380–397 (2014).
Spangenberg, E. et al. Sustained microglial depletion with CSF1R inhibitor impairs parenchymal plaque development in an Alzheimer’s disease model. Nat. Commun. 10, 1–21 (2019).
Velez, D. X. F., Arreola, M., Huh, C. Y. L., Green, K. & Gandhi, S. P. Juvenile depletion of microglia reduces orientation but not high spatial frequency selectivity in mouse V1. Sci. Rep. 12, 1–12 (2022).
Jung, S. et al. Analysis of fractalkine receptor CX3CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol. Cell. Biol. 20, 4106–4114 (2000).
Hilla, A. M., Diekmann, H. & Fischer, D. Microglia are irrelevant for neuronal degeneration and axon regeneration after acute injury. J. Neurosci. 37, 6113–6124 (2017).
Dana, H. et al. Thy1-GCaMP6 transgenic mice for neuronal population imaging in vivo. PLoS ONE 9, e108697 (2014).
Wang, X. et al. Requirement for microglia for the maintenance of synaptic function and integrity in the mature retina. J. Neurosci. 36, 2827–2842 (2016).
Godement, P., Salaün, J. & Imbert, M. Prenatal and postnatal development of retinogeniculate and retinocollicular projections in the mouse. J. Comp. Neurol. 230, 552–575 (1984).
Jaubert-Miazza, L. et al. Structural and functional composition of the developing retinogeniculate pathway in the mouse. Vis. Neurosci. 22, 661–676 (2005).
Stevens, B. et al. The classical complement cascade mediates CNS synapse elimination. Cell 131, 1164–1178 (2007).
Schafer, D. P. et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 74, 691–705 (2012).
Guido, W. Refinement of the retinogeniculate pathway. J. Physiol. 586, 4357–4362 (2008).
Wekselblatt, J. B., Flister, E. D., Piscopo, D. M. & Niell, C. M. Large-scale imaging of cortical dynamics during sensory perception and behavior. J. Neurophysiol. 115, 2852–2866 (2016).
Tan, L., Tring, E., Ringach, D. L., Zipursky, S. L. & Trachtenberg, J. T. Vision changes the cellular composition of binocular circuitry during the critical period. Neuron 108, 735–747 (2020).
Brown, T. C. & McGee, A. W. Monocular deprivation during the critical period alters neuronal tuning and the composition of visual circuitry. PLoS Biol. 21, e3002096 (2023).
Hoy, J. L. & Niell, C. M. Layer-specific refinement of visual cortex function after eye opening in the awake mouse. J. Neurosci. 35, 3370–3383 (2015).
Tan, L., Ringach, D. L. & Trachtenberg, J. T. The development of receptive field tuning properties in mouse binocular primary visual cortex. J. Neurosci. 42, 3546–3556 (2022).
Wang, B. S., Feng, L., Liu, M., Liu, X. & Cang, J. Environmental enrichment rescues binocular matching of orientation preference in mice that have a precocious critical period. Neuron 80, 198–209 (2013).
Sarnaik, R., Wang, B. S. & Cang, J. Experience-dependent and independent binocular correspondence of receptive field subregions in mouse visual cortex. Cereb. Cortex 24, 1658–1670 (2014).
Wang, B. S., Sarnaik, R. & Cang, J. Critical period plasticity matches binocular orientation preference in the visual cortex. Neuron 65, 246–256 (2010).
Stephany, C. É. et al. Plasticity of binocularity and visual acuity are differentially limited by nogo receptor. J. Neurosci. 34, 11631–11640 (2014).
Prusky, G. T., West, P. W. R. & Douglas, R. M. Behavioral assessment of visual acuity in mice and rats. Vis. Res. 40, 2201–2209 (2000).
Prusky, G. T. & Douglas, R. M. Developmental plasticity of mouse visual acuity. Eur. J. Neurosci. 17, 167–173 (2003).
Gordon, J. A. & Stryker, M. P. Experience-dependent plasticity of binocular responses in the primary visual cortex of the mouse. J. Neurosci. 16, 3274–3286 (1996).
Sekar, A. et al. Schizophrenia risk from complex variation of complement component 4. Nature 530, 177–183 (2016).
Cheadle, L. et al. Sensory experience engages microglia to shape neural connectivity through a non-phagocytic mechanism. Neuron 108, 451–468 (2020).
Sipe, G. O. et al. Microglial P2Y12 is necessary for synaptic plasticity in mouse visual cortex. Nat. Commun. 7, 10905 (2016).
Cong, Q., Soteros, B. M., Wollet, M., Kim, J. H. & Sia, G. M. The endogenous neuronal complement inhibitor SRPX2 protects against complement-mediated synapse elimination during development. Nat. Neurosci. 23, 1067–1078 (2020).
Cong, Q. et al. C1q and SRPX2 regulate microglia mediated synapse elimination during early development in the visual thalamus but not the visual cortex. Glia 70, 451–465 (2022).
Welsh, C. A., Stephany, C.-É., Sapp, R. W. & Stevens, B. Ocular dominance plasticity in binocular primary visual cortex does not require C1q. J. Neurosci. 40, 769–783 (2020).
Schecter, R. W. et al. Experience-dependent synaptic plasticity in V1 occurs without microglial CX3CR1. J. Neurosci. 37, 10541–10553 (2017).
Lowery, R. L., Tremblay, M. E., Hopkins, B. E. & Majewska, A. K. The microglial fractalkine receptor is not required for activity-dependent plasticity in the mouse visual system. Glia 65, 1744–1761 (2017).
Császár, E. et al. Microglia modulate blood flow, neurovascular coupling and hypoperfusion via purinergic actions. J. Exp. Med. 219, e20211071 (2022).
Ma, X. et al. Depletion of microglia in developing cortical circuits reveals its critical role in glutamatergic synapse development, functional connectivity and critical period plasticity. J. Neurosci. Res. 98, 1968–1986 (2020).
Frantz, M. G., Kast, R. J., Dorton, H. M., Chapman, K. S. & McGee, A. W. Nogo receptor 1 limits ocular dominance plasticity but not turnover of axonal boutons in a model of amblyopia. Cereb. Cortex 26, 1975–1985 (2016).
Stephany, C.-E., Ikrar, T., Nguyen, C., Xu, X. & McGee, A. W. Nogo receptor 1 confines a disinhibitory microcircuit to the critical period in visual cortex. J. Neurosci. 36, 11006–11012 (2016).
Stephany, C. É. et al. Distinct circuits for recovery of eye dominance and acuity in murine amblyopia. Curr. Biol. 28, 1914–1923 (2018).
Frantz, M. G. et al. Layer 4 gates plasticity in visual cortex independent of a canonical microcircuit. Curr. Biol. 30, 2962–2973 (2020).
Mayford, M. et al. Control of memory formation through regulated expression of a CaMKII transgene. Science 274, 1678–1683 (1996).
Hasan, N. et al. LRIT3 is required for nyctalopin expression and normal ON and OFF pathway signaling in the retina. eNeuro 7, ENEURO.0002-20.2020 (2020).
Depiero, V. J. & Borghuis, B. G. Phase advancing is a common property of multiple neuron classes in the mouse retina. eNeuro 9, ENEURO.0270-22.2022 (2022).
Ringach, D. L. et al. Spatial clustering of tuning in mouse primary visual cortex. Nat. Commun. 7, 1–9 (2016).
Berens, P. et al. Community-based benchmarking improves spike rate inference from two-photon calcium imaging data. PLoS Comput. Biol. 14, 1–13 (2018).
Jimenez, L. O., Tring, E., Trachtenberg, J. T. & Ringach, D. L. Local tuning biases in mouse primary visual cortex. J. Neurophysiol. 120, 274–280 (2018).
Yaeger, C. E., Ringach, D. L. & Trachtenberg, J. T. Neuromodulatory control of localized dendritic spiking in critical period cortex. Nature 567, 100–104 (2019).
Salinas, K. J., Velez, D. X. F., Zeitoun, J. H., Kim, H. & Gandhi, S. P. Contralateral bias of high spatial frequency tuning and cardinal direction selectivity in mouse visual cortex. J. Neurosci. 37, 10125–10138 (2017).
Gu, Y. & Cang, J. Binocular matching of thalamocortical and intracortical circuits in the mouse visual cortex. eLife 5, 1–14 (2016).
Acknowledgements
This work was supported by a Karl Kirchgessner award (to B.G.B.), a grant from the E. Matilda Ziegler Foundation for the Blind (to S.W.M. and B.G.B.), grants from the NIH (EY035138, to A.W.M.; EY028188, to B.G.B.) and a Jewish Heritage Fund for Excellence Research Enhancement Grant (A.W.M.). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the paper.
Author information
Authors and Affiliations
Contributions
B.G.B., A.W.M. and T.C.B. conceptualized and designed the study. T.C.B., E.C.C., C.A.A., D.K.O., S.W.M., B.G.B. and A.W.M. performed experiments and analyzed the data. T.C.B., B.G.B. and A.W.M. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Neuroscience thanks Michael Stryker and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
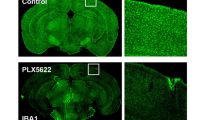
Extended Data Fig. 1 Timeline of PLX treatment and representative coronal images of control and PLX-treated brains.
(a) The PLX treatment was initiated at P14. Experiments to examine the refinement of visual circuitry and plasticity began after P24 as indicated. (b) Representative images of coronal sections of brains from control mice and PLX-treated mice at P18, P21, P28 stained with antibodies directed at Iba-1.
Supplementary information
Supplementary Video 1
Calcium imaging of V1 in control mouse at neuronal resolution. Images were collected at 15.5 fps. Images are presented at 100 fps (~7× speed). The video is downsampled to 480p.
Supplementary Video 2
Calcium imaging of V1 in PLX-treated mouse at neuronal resolution. Images were collected at 15.5 fps. Images are presented at 100 fps (~7× speed). The video is downsampled to 480p.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Brown, T.C., Crouse, E.C., Attaway, C.A. et al. Microglia are dispensable for experience-dependent refinement of mouse visual circuitry. Nat Neurosci 27, 1462–1467 (2024). https://doi.org/10.1038/s41593-024-01706-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-024-01706-3
This article is cited by
-
Recent Advances in the Molecular Mechanisms of Ocular Dominance Plasticity in the Visual Cortex
Neuroscience Bulletin (2025)
-
Typical development of synaptic and neuronal properties can proceed without microglia in the cortex and thalamus
Nature Neuroscience (2025)
-
Circuit refinement without microglia
Nature Reviews Neuroscience (2024)